Abstract
There is a widely held view that hunger prompts feeding to ensure energy needs are met, while thirst cues drinking to address hydration requirements. However, recent changes in the nature of the food supply and eating patterns have raised questions about the functionality of these relationships with respect to maintaining energy balance. The increasing consumption of energy-yielding beverages and foods with diluted energy density, through the use of ingredients such as high intensity sweeteners and fat replacers, pose new challenges to presumed homeostatic energy regulatory mechanisms. This review draws on findings from a recent observational study and other published evidence to explore whether shifts of food composition and use patterns may be disrupting relationships between thirst, hunger, drinking, and eating resulting in positive energy balance (e.g., drinking low satiety, energy-yielding beverages in response to hunger). The observational study entailed collecting hourly appetitive ratings and dietary recalls from 50 adults for seven consecutive days. These data reveal a clear bimodal daily hunger pattern whereas thirst is stronger and more stable throughout the day. Further, approximately 75% of fluid intake occurs peri-prandially, with the majority derived from energy-yielding beverages. While there is published evidence that drinking is responsive to feeding, support for the view that drinking is the more tightly regulated behavior is stronger. Our data indicates that, due to a number of plausible factors, neither absolute values nor changes of hunger or thirst are strong predictors of energy intake. However, it is proposed that stable, high thirst facilitates drinking, and with the increased availability and use of energy-yielding beverages that have low satiety properties, this can promote positive energy balance. There are marked individual differences in mean daily hunger and thirst ratings with unknown implications for energy balance.
Keywords: hunger, thirst, feeding, drinking, energy balance, human, beverage, intake, appetite
Introduction
While definitions may vary in different contexts, hunger is often defined as a sensation that promotes food seeking and ingestive behaviors [1-5]. Although not always the case (e.g., eating in response to boredom or desire for sensory stimulation) [6], a primary function is to ensure energy needs are met. Similarly, thirst, in this context, generally refers to sensations that initiate the identification and ingestion of fluids to meet hydration needs [7]. Though parallel in many ways, there are important distinctions between the physiological systems these sensations activate. Key among them are the health implications of deficits or surfeits of their respective substrates. With regard to eating, life-threatening health consequences of inadequate intake are not manifest for weeks to months, allowing behavioral responses to hunger to be deferred for extended periods. For drinking, the time frame is reduced to days, so thirst is a particularly salient signal and less likely to be ignored. In contrast, eating to excess may pose a greater health risk as it results in increased stored energy as body fat that may progress to overweight /obesity with a host of co-morbidities [8,9]. However, drinking in excess, save extreme instances, poses little health risk due to efficient excretion. These asymmetries have been highlighted in animal models where weaker stimulation is required to induce drinking than eating [10]. They hold important implications in the current environment for humans where fluids such as sodas, fruit drinks, sports beverages, gourmet coffees and teas now supply energy and, through the use of high intensity sweeteners and fat replacers, foods can contribute little energy. Thus, hunger and thirst may now prompt ingestive behaviors with unintended consequences, such as increasing energy intake while drinking to relieve thirst. Further, current trends may alter learned associations between either hunger or thirst and the post-ingestive consequences of eating and drinking. The reduced veracity or reliability of such a sensory signal may compromise assumed homeostatic physiological systems [11] (see Levitsky for a challenge to this view [12]), resulting in weight loss or, more commonly, weight gain. The present paper explores these issues, drawing on the literature and a recent observational study from our laboratory [13].
Our study explored the relationships between hunger, thirst, eating, and drinking patterns in fifty, weight-stable, euhydrated, free-living US adults (39F; 11M; age 30±11 yrs; BMI 26.3±5.9). Participants were recruited through public advertisements, with eligibility criteria including weight stable (<3 kg change within the previous 3 months), male or female, 18-60 years of age, in good health, and not taking medication known to influence appetite. The initial pool included 122 individuals, but 72 were excluded based on calculations [14] suggesting their diet records were incomplete due to under-recording or under eating. The final sample had a higher proportion of females (39F vs. M11), but there were no significant gender differences with respect to age, BMI, or appetitive sensations. Self-reported physical activity level was stable over the observational period and extreme athletes were excluded. Data on habitual food and beverage intake were collected for 7 consecutive days by telephone-administered 24-hour diet recalls using multi-pass software (NDS-R; version 2005; Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN). Thirst and hunger ratings were recorded using a Personal Digital Assistant (PDA, PalmZire 21, Palm, Inc., Sunnyvale, CA) every waking hour over the same 7-d period. These scales required the participant to respond to a series of appetitive questions by placing a mark on a line scale with opposing end anchors, such as “not at all hungry” and “as hungry as I have ever felt”. Subsequently, hourly thirst and hunger sensations were correlated with energy consumed during the subsequent hour.
Appetitive Patterns
Thirst sensations oscillate throughout the day, with peaks coincident with midday and evening meals (Figure 1). However, the peak to nadir excursions are small; about 10% of the scale relative to 20-30% changes for hunger ratings observed in the same individuals on comparable scales (Figure 2). The stability of daily patterns, as assessed by correlations between all days of the week, is high for thirst (Figure 3) and only moderate for hunger (Figure 4). The mean correlation coefficient for thirst was 0.78±0.02, while the value was 0.52±0.02 for hunger. A paired-test revealed these distributions of correlations were significantly different (t=11.67, P<0.001). Thirst ratings also appear to be more stable following interventions with loads varying in rheology (Figure 5 (thirst) and Figure 6 (hunger)) [15]. Correlations range from 0.64 – 0.82 for thirst ratings after ingestion of apple juice, applesauce or whole apples, but were 0.43 – 0.76 for hunger ratings.
Figure 1.
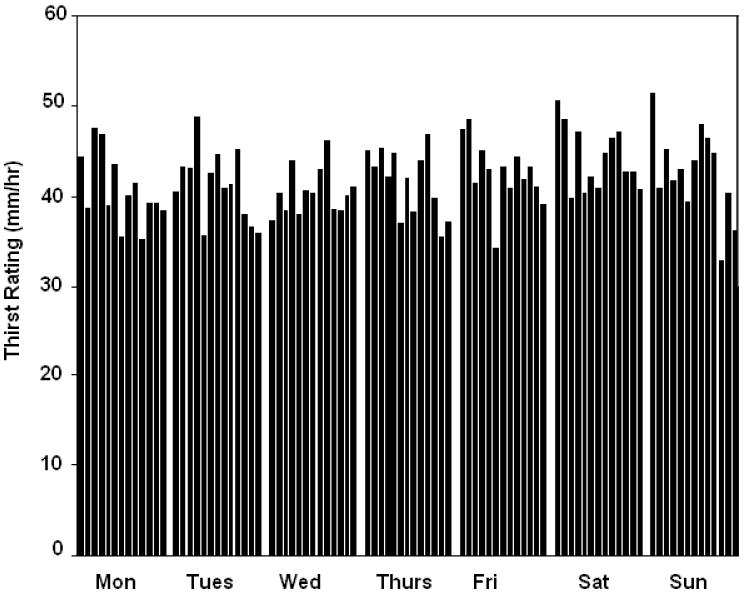
Group mean hourly thirst ratings across a 7-day period, from 09:00-21:00h (n = 50).
Figure 2.
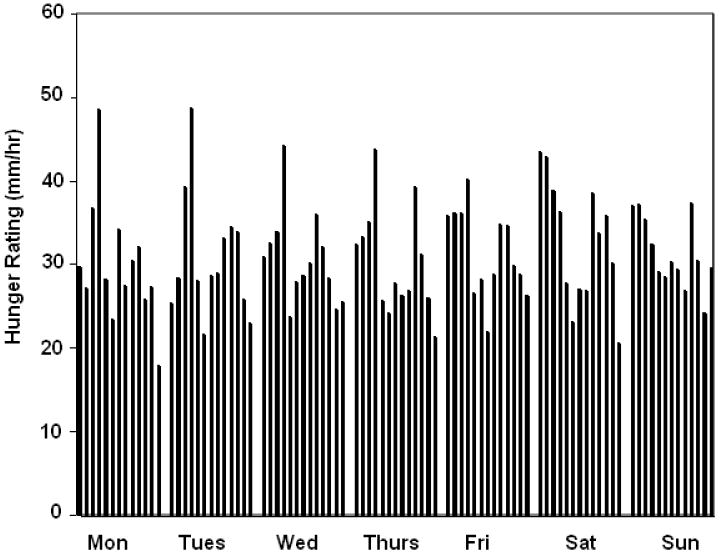
Group mean hourly hunger ratings across a 7-day period, from 09:00-21:00h (n = 50).
Figure 3.
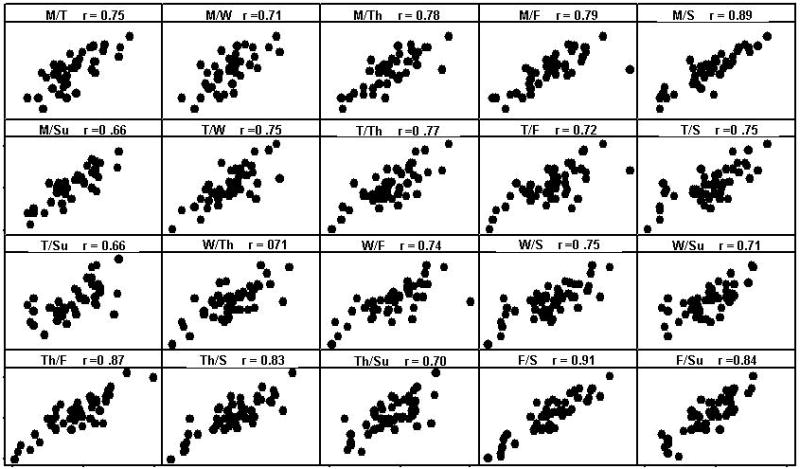
Group correlation coefficients between days of the week for thirst across a 7-day period, from 09:00-21:00h (n = 50).
Figure 4.
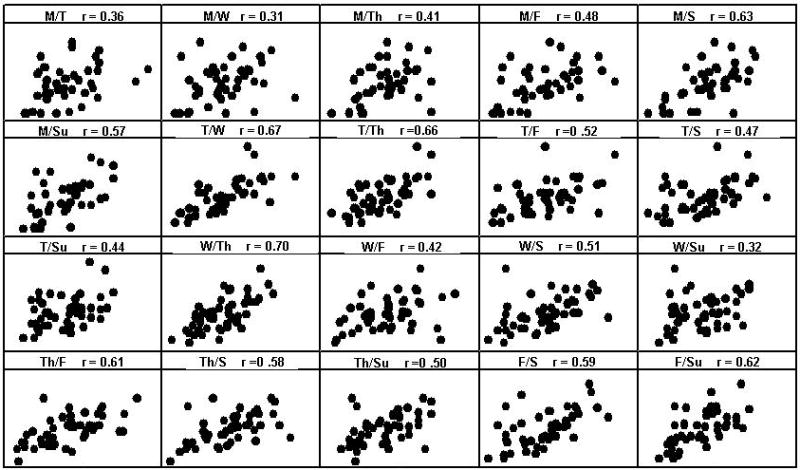
Group correlation coefficients between days of the week for hunger across a 7-day period, from 09:00-21:00h (n = 50).
Figure 5.
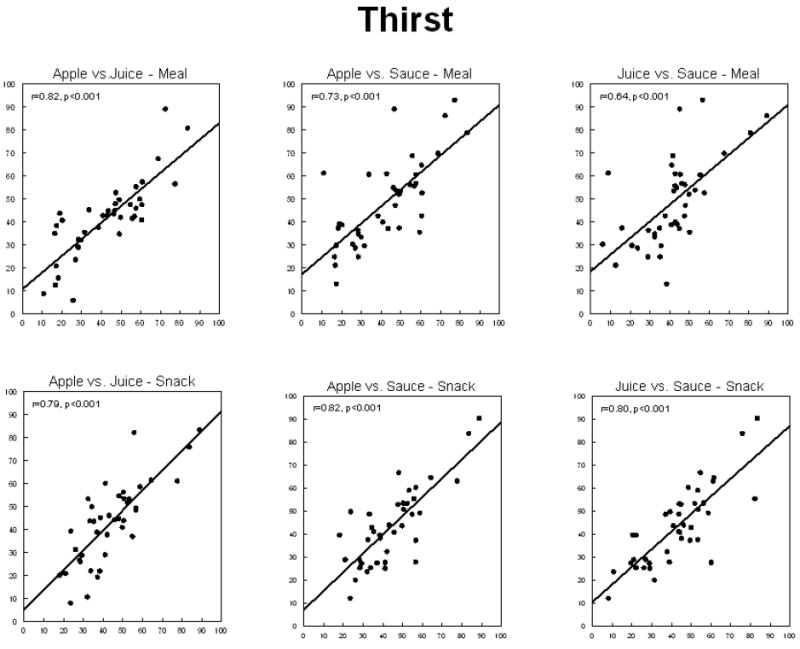
Correlations between mean self-reported thirst following ingestion of 300kcals of apple juice, applesauce, or whole apple as a meal component or alone as a snack by 20 (10 male, 10 female) lean and 20 (10 male, 10 female) obese adults.
Figure 6.
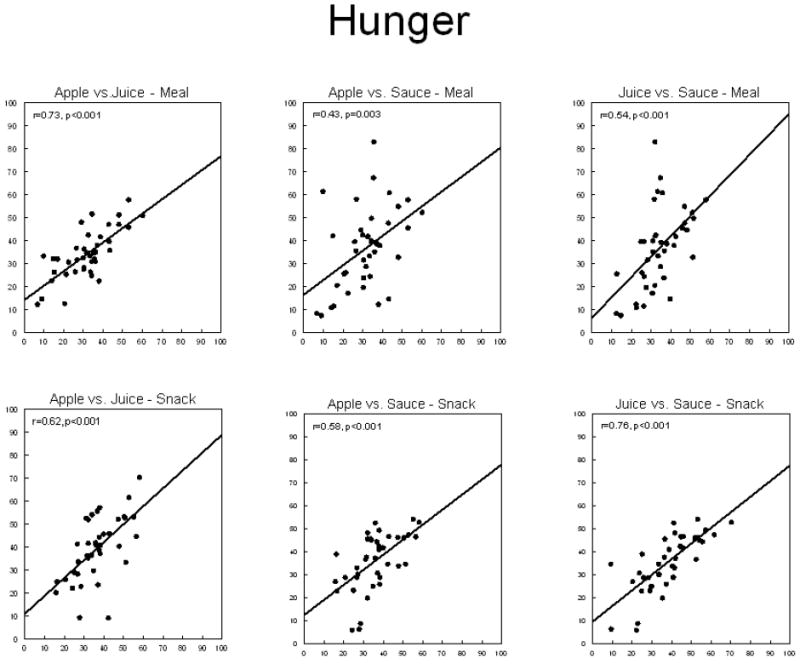
Correlations between mean self-reported hunger following ingestion of 300kcals of apple juice, applesauce, or whole apple as a meal component or alone as a snack by 20 (10 male, 10 female) lean and 20 (10 male, 10 female) obese adults.
Although distinct in some regards, the daily patterns of variability in reported thirst and hunger sensations have important commonalities. The intra-individual variability for daily thirst and hunger sensations is markedly greater than the inter-individual variability (Figure 7 and Figure 8) (P<0.05). This is evidenced by the consistently wide ranges of individual daily thirst, and especially hunger, sensations (i.e., large intra-individual variance) that overlap extensively (i.e., small inter-individual variability). In contrast, the intra-individual ranges of daily mean hunger and thirst sensations are markedly reduced and there is less overlap of individual ranges. This pattern has been observed previously for hunger ratings [16]. The implications of customarily experiencing high or low intensities of these sensations are not known. Possibilities include: (1) high sensation intensities reflect higher motivation to eat or drink and result in higher BMI if sensations evoke ingestive responses in excess of energy need, or (2) high sensation intensities grow because they are ignored (purposeful (e.g., high cognitive restraint) or otherwise) and result in lower BMI. However, exploration of inter-individual differences of mean daily thirst and hunger ratings in our study revealed no disparities between groups based on dietary restraint, as measured by the Three-Factor Eating Questionnaire [17]. Further, no disparities were noted between groups based on age, gender, BMI, or total daily energy intake. However, the limited sample size (n=50) hampers definitive conclusions on the importance of these characteristics. As susceptibility to weight gain may be due, in part, to variability in appetite sensations [18], further exploration of the dietary and health implications of inter-individual differences in thirst and hunger ratings is warranted. There is substantial evidence that thirst, and perhaps a corresponding drinking response, is problematic for energy balance. Access to energy-yielding beverages is ubiquitous, and is associated with weight gain [19-21] and high BMI [21], suggesting that particular attention should be focused on thirst and drinking.
Figure 7.
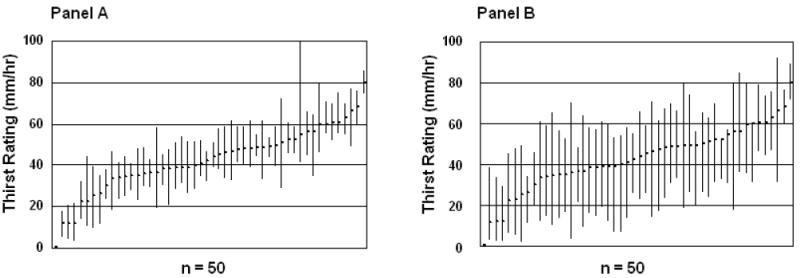
Panel A - Range of mean daily thirst ratings (between 09:00 and 21:00 h) for each individual (n=50). Each plotted bar represents the highest and lowest daily mean thirst rating experienced over a consecutive 7-day period. Panel B - Mean of the ranges of daily thirst ratings (between 09:00 and 21:00 h) for each individual (n = 50). Each plotted bar represents the mean highest and lowest daily thirst rating experienced over a consecutive 7-day period.
Figure 8.
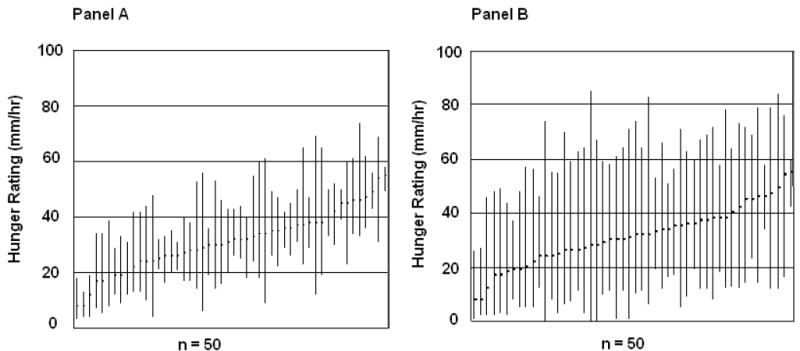
Panel A - Range of mean daily hunger ratings (between 09:00 and 21:00 h) for each individual (n=50). Each plotted bar represents the highest and lowest daily mean hunger rating experienced over a consecutive 7-day period. Panel B - Mean ranges of daily hunger ratings (between 09:00 and 21:00 h) for each individual over a consecutive 7-day period (n = 50). Each plotted bar represents the mean highest and lowest daily hunger rating experienced over a consecutive 7-day period.
Drinking patterns
Drinking is not always necessary for survival. On a succulent diet, many animals, including arboreal primates [22-24], can meet their fluid needs by the free water present in food, plus water formed from the oxidation of foodstuffs. Long-term historical human data on drinking are lacking. However, recent trend data [25], indicate there is decreasing reliance on foods for meeting fluid needs. US adults obtain a mean of only 19% of total daily fluid from foods [26]. Conversely, 81% of total water intake is derived from beverages. Water constitutes only about 10.7% of beverage consumption. Other substantive contributors include carbonated soft drinks (28.3%), beer (11.7%), milk (10.9%), fruit beverages (4.78%), sports drinks (2.3%), wine (1.2%), and distilled spirits (0.7%) [27]. Thus, over 60% of beverage consumption also supplies dietary energy. Current estimates are that about 21% of total dietary energy is derived from beverages, with the highest percent in the 19-39 age group (about 24%) and the lowest in the 60+ age group (about 16%) [28]. Between 1977-2001, with all ages combined, the total energy increase from beverages was approximately 278 kilocalories. Obtaining energy from beverages constitutes a recent and marked shift of dietary practice. Similar trends have been reported in other subgroups in other developed countries [e.g., 29,30], with an indication this applies globally [31].
In animals, approximately 70% of fluid intake occurs peri-prandially (e.g., [32]). This drinking pattern appears to hold in humans as well, although assessments have been based on very limited samples. In one 48-hour controlled feeding trial, where 20 male adult participants had ad lib access to tap water, 68% of water intake was consumed in association with eating [33]. The same result was reported in a study of five adult males monitored in their workplace for one day [34]. Under free-living conditions, where fluid intake was assessed for seven consecutive days in 36 healthy adults, 87% of fluid water intake occurred peri-prandially [35]. More recent data from our laboratory supports similar beverage consumption patterns, where 75% of total fluid intake was associated with eating. Our data also revealed that energy-yielding beverages are now the main contributor to fluid intake during peri-prandial events, accounting for 56% of total fluid intake. Further, energy-yielding beverages are the predominant source of fluid (55%) during ‘drink-only’ events. Fruit juices/drinks and soda were large contributors to fluid intake during these occasions. In contrast, water accounted for 32% of ‘drink-only’ consumption and 33% of peri-prandial consumption. Of note, while there were no significant differences between age, gender, or BMI groups and consumption of these types of beverages at either occasion, mean fluid intake from energy-yielding beverages was higher among the overweight group compared to the normal weight group (61% vs. 49%). This is similar to previous findings of higher energy-yielding beverage consumption among overweight individuals [36]. Moreover, a higher BMI was associated with lower proportional water consumption. This may be due to the inverse association reported between water and energy-yielding beverage consumption [37]. This observation is consistent with a predisposing role of energy-yielding beverage consumption in weight gain.
In our study sample, consumption of energy-yielding beverages and water led to similar reductions of thirst. However, consumption of energy-yielding beverages to relieve thirst may disrupt homeostatic associative learning patterns, with the resulting loss of an important signal for energy intake regulation. Evidence indicates the orosensory properties of foods serve as conditioned predictors of the consequences of consumption on energy balance [38]. However, as energy delivered in a clear fluid medium appears to have weak satiety value compared to solids [39], the conditioned response to energy intake may be disrupted or degraded. Consequently, energy-yielding beverages are associated with thirst reduction but not the equally appropriate association of hunger reduction. The high palatability of many energy-yielding beverages may further facilitate the formation of disruptive associations through the enhancement of rewarding sensory associations. Flavor strength [40], sweetness [41], and temperature [41,42] can all be manipulated to enhance non-homeostatic ingestive behaviors such as drinking highly palatable energy-yielding beverages to satisfy thirst in the absence of energy need. In our study sample, individuals responded “appropriately” by consuming water in response to thirst, in the absence of hunger, only 2% of the time. They responded “inappropriately” (i.e., thirsty and hungry but did not drink or eat; not thirsty and not hungry but drank and/or ate; not thirsty but hungry and drank but did not eat; thirsty but not hungry and did not drink but ate) 62% of the time. Acknowledging that this is a very rigid cut-off for classification that ignores the fact that water is obtained from food, these drinking patterns, often apparently uncoupled from hydration status, may increase health risk. These drinking patterns and behaviors appear to have deviated from an assumed link to hydration status, with uncertain health consequences.
Eating Patterns
Similar to changes in fluid consumption patterns over time, shifts have occurred in eating patterns that may also be disruptive to energy balance [43-45]. Historically, food availability was not constant. Body fat served as a storage compartment during times of surfeit and as a reserve during times of low energy availability. As documented in rural Gambian women, and indicative of broader historical patterns in humans, there has been an annual cycle of weight gain and loss [46]. Generally, weight gain occurred during and immediately following harvest season and weight loss occurred during planting and when the food supply was limited. The cycles linking body weight and food availability may ultimately lead to long-term energy balance. However, in developed nations, agricultural, processing and distribution advancements have secured a constant, inexpensive food supply that requires little personal energy expenditure to acquire. It may also facilitate increased energy consumption. Data from the Continuing Survey of Food Intake by Individuals suggests there was a 268 kcal increase in intake for men and 143 kcal increase for women between the 1977-1978 and 1994-1996 [43]. This increase has contributed, at least partly, to the high and increasing prevalence of overweight/obesity.
The increase in energy intake is primarily due to an increase in snacking frequency and an increase in energy consumed during snacking occasions [43,44]. While the contribution of main meals to total dietary energy has remained relatively stable [43,45], the contribution of snacks to total dietary energy increased by 261 kcal for men and by 160 kcal for women between 1977 and 1996 [43]. Study of this trend and potential differences in lean and obese individuals is hampered by weak methodologies to quantify intake. Under-reporting of energy is high in all groups, but especially for snacks in the overweight and obese [47]. This likely partially explains the conflicting evidence in the literature. While some studies have reported a positive association between eating frequency and body weight or risk of obesity (e.g., [48,49]) others have not (e.g., [50,51]). Portion sizes have also increased over the past three decades [52-54] and preload and short-term trials indicate this leads to increased intake [55]. However, evidence of compensation across meals in children [56] and adults [57], and stability of meal sizes over time [43], suggest this may not be an important contributor to positive energy balance. However, lack of longer term studies precludes confirmation of this point. Changes of dietary energy density have also been implicated in trends for rising energy intake and weight gain. Interestingly, both increases and decreases are cited as problematic. The evidence for a role of increased energy density generally holds when particular beverages are omitted [58], especially when water is excluded from the analysis [59,60]. Given that beverages now contribute over 20% [28] of dietary energy, their exclusion is a questionable practice. In contrast, a direct association between ingestion of foods with diluted energy density and BMI has been reported [60-63]. The epidemiological findings may be explained by reverse causality (i.e., heavier individuals are more likely to use products that dilute energy density such as high intensity sweeteners and fat replacers to manage their weight) (e.g., [64]) or methodological errors [65]. However, there are acute feeding data that dilution of energy density through the use of high-intensity sweeteners and fat replacers [66-68] or perceived dilution [69] disrupts homeostatic eating in humans. Further, there are longer-term animal studies suggestive of a causal role in weight gain [70,71], though extrapolation of these findings to humans with highly varied diets is uncertain. Overall, it appears that eating patterns have changed substantively over a relatively short period of time and that increased eating frequency, in particular, is contributing to positive energy balance and weight gain. In that energy-yielding beverages may contribute to the frequency and size of ingestive events and often are not differentiated from solid food ingestion when calculating energy intake, they may play a heretofore underappreciated role in reported trends in eating patterns.
Eating and Drinking: Cause and Effect
One view holds that feeding is a determinant of drinking, and fluid intake is regulated secondarily to food intake [32,33,35]. This interpretation is based, in part, on the observations that approximately 75% of fluid intake is peri-prandial and there is a direct relationship between eating and drinking under ad lib conditions [33-35]. Significant positive correlations have been noted between meal size and the quantity of water consumed as well [32,33,72]. Further, the interval between fluid ingestion bouts is reportedly predicted by food intake, not the amount of fluid consumed or the level of self-reported thirst [35]. In one analysis, food intake accounted for over 50% of the variance in fluid intake [35]. The strongest relationship noted was between the amount of food energy consumed in an eating event and the amount of fluid consumed in the same event [35].
While these findings are consistent with the view that drinking is largely governed by feeding, other findings challenge this interpretation. We and others [72,73] have failed to observe a correlation between thirst or drinking and energy intake. The relationship between fluid and food intake may reflect concurrent responses to a third factor rather than a causal relationship between the two. Drinking may occur at meal times to facilitate chewing and swallowing [74,75], improve sensory stimulation and food palatability [72], reduce aversive sensations evoked by irritants such as spices [72], or in anticipation of physiological deprivation [34]. Availability and effort needed to obtain a beverage likely also partially account for the apparent association between fluid and food intake. Beverages may be more readily available, and consequently consumed, at mealtimes, compared to between meals. When greater effort is required to obtain water, the relationship between fluid and food intake dissipates [76]. Thus, questions remain about the degree to which feeding elicits drinking.
In contrast, another view holds that fluid requirements are of paramount importance and fluid intake will continue to ensure adequate hydration status independent of energy needs [77-79]. In our study, thirst ratings were higher and more stable than hunger ratings suggesting they are the more salient. While mean daily hunger was rated at 31±16, mean daily thirst was rated at 43±11 on a 100 unit scale. The observation that isocaloric beverages differing in macronutrient content can quench thirst similarly [80] is cited by Anderson [78] as implying that energy delivered in a fluid medium overrides satiety signals to quench thirst and maintain fluid homeostasis. As greater consequences are associated with acute fluid deficit and lesser consequences with small to moderate excesses of water consumption relative to the effects of deviations of energy balance, the view that fluid intake regulation is prepotent over energy balance is physiologically more plausible. However, with energy-yielding beverages substituting for water, and strong evidence indicating that energy delivered in a fluid medium does not elicit strong compensation relative to solids [81,82] and is associated with weight gain and risk of obesity [20,21,39,83], the consistent drive to maintain fluid balance in the current environment may be problematic for energy balance.
Appetite and Dietary Intake
The relationship between appetitive sensations and dietary intake is complex despite the existence of multiple elegant physiological regulatory systems. This stems not only from interactions between these systems, but probably more so to contributions of environmental factors such as cost, convenience, culture, and health beliefs [84]. De Castro [35] noted that thirst was not the strongest predictor of fluid intake during meals and was only weakly correlated with total fluid intake. This may be due to unmeasured shifts of hydration status if this regulates drinking; unmeasured anticipation of dehydration if this is a stronger influence [34], or a true weak association. The strongest predictor was food intake. Additionally, under controlled experimental conditions, subjective hunger ratings are only moderately associated with energy intake [85]. Moreover, under free-living conditions, the association between hunger and energy intake is even weaker [17,86]. In our recent study exploring relationships over the day, patterns of hunger were only weakly predictive of energy intake (r = 0.30) and patterns of thirst failed to predict energy intake or fluid intake (r = 0.08 and r = 0.03, respectively). Further, mean thirst and hunger ratings were not significantly different at the beginning of ‘drink-only’, ‘solid only’, or mixed eating and drinking events. De Castro [35] reported similar findings for thirst, with levels of thirst failing to differ significantly according to the type of ingestive occasion.
It may be argued that it is not the absolute thirst or hunger level that predicts intake, but; rather changes of sensation are the salient cue to initiate drinking or eating. However, changes in thirst and hunger 1 hour, 2 hours, or 3 hours prior to drinking or eating did not offer greater predictive power in our study (r = -0.06 to - 0.09). Similar findings were reported earlier [17].
The weak correlations noted in the literature between appetitive sensations and intake may reflect a weak coupling between biological needs and sensations presumed to elicit behaviors to remediate them, an inability of untrained (or perhaps even trained) individuals to report on such associations, or a low specificity and sensitivity of current measures of appetitive sensations and intake. A number of approaches have been used to quantify appetite, but none with great success, as gauged by expected changes of food intake. The most common method entails asking individuals to respond to a series of questions on visual analog or category scales with end anchors defined variously from “Not at all” and “Extremely” to questions such as: “How hungry are you?”; “How strong is your desire to eat?”; “How much could you eat?”; and “How full are you?”. There are several shortcomings with this approach. First, it assumes a common sensation across people or at least a common interpretation of the terms. However, open-ended surveys reveal marked individual variability in the nature and sites of these sensations [17,87]. For example, under standardized conditions, hunger may be experienced as a pain in the stomach, light-headedness or anxiety [88]. Moreover, the site and sensation change with time since the last eating episode [89]. Second, the common practice is to ask all four of these questions, and often several others, without a priori hypotheses or appropriate statistical evaluation of the findings. Presumably these four questions access unique information so, depending on the research question, the appropriate outcome variable should be specified a priori. This rarely occurs. Instead, a change in any one is viewed as evidence of an effect. Without correction for multiple testing, this increases the probability of a type I error and obscures potentially useful mechanistic insights. Third, the demand characteristics of the approach have not been adequately explored. Repeated questioning may stimulate thoughts of eating, and sampling just before or after eating could yield responses more linked to expectations than true sensation. A possible consequence of these methodological issues is that the association is presently poorly supported. Indeed, we conducted an unbiased search of the life sciences literature to identify papers published between 1995 and 2005 that contained the following terms: hunger, fullness, satiety, appetite, food intake, human. Excluding reports involving pharmacologic interventions and clinical populations, 39 papers were identified. In 25 reports (64%) no significant association was observed between any of these appetitive questions and food intake [90-114]. In 8 papers (21%) a significant association was reported between a subset of appetitive questions and intake, but there was no a priori hypothesis indicating the specific questions were the target outcome variables [115-122]. Six papers (15%) found consistent, significant associations [85,123-127]. Whether the latter indicates a robust effect or lack of independence of the questions is not clear. Publication bias against studies finding no effect compounds concern about the validity of appetite ratings as proxy measures of intake. Shortcomings in methods to characterize customary diets are also well recognized [128] and complicate efforts to link appetite to intake. These methodological issues may require fundamental re-thinking if the goal is to determine the predictive value of appetitive sensations for intake in free-living humans.
Conclusion
Our observational study, coupled with data from the literature, suggests thirst is a stronger and more stable sensation than hunger. Hunger may be a stronger predictor of eating than thirst, but both are weak. Neither hunger nor thirst are reliable predictors of drinking. Based on the observed high and sustained sensations of thirst throughout the day and hypothesized subordination of hunger and energy regulation to thirst and fluid regulation in motivating ingestive behavior, it is proposed that the high and increasing exposure to palatable, energy-yielding beverages facilitates drinking substantive quantities of energy. As energy from beverages elicits limited dietary compensation, beverages may contribute to positive energy balance. The marked inter-individual variation in self-reported thirst and hunger sensations indicates that certain individuals may be more at risk from this recent dietary trend than others.
Acknowledgments
Supported by PHS grant #5 R01 DK63185 and USDA HATCH grant IND084055.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.LeMagnen J. Hunger and food palatability in the control of feeding behavior. In: Katsubi Y, Sato M, Tobogi SF, Oomura Y, editors. Food Intake and Chemical Senses. Baltimore: University Park Press; 1977. pp. 263–80. [Google Scholar]
- 2.Rosenzweig MR. The mechanisms of hunger and thirst. In: Toates FM, editor. Biological Foundations of Behaviour. Philadelphia: Open University Press; 1986. pp. 73–143. [Google Scholar]
- 3.Silverstone T, Goodall E. Measurement of hunger and food intake. In: Ferrari E, editor. Disorders of eating behaviour, a psychoendocrine approach. Oxford: Pergamon Press; 1986. pp. 129–34. [Google Scholar]
- 4.Nicolaidis S, Even P. Physiological determinant of hunger, satiation, and satiety. Am J Clin Nutr. 1985;42(5 Suppl):1083–92. doi: 10.1093/ajcn/42.5.1083. [DOI] [PubMed] [Google Scholar]
- 5.Castonguay TW, Applegate EA, Upton DE, Stern JS. Hunger and appetite: old concepts/new distinctions. Nutr Rev. 1983;41(4):R101–10. doi: 10.1111/j.1753-4887.1983.tb07163.x. [DOI] [PubMed] [Google Scholar]
- 6.Mattes RD, Friedman MA. Hunger. Dig Dis. 1993;11(2):R65–77. doi: 10.1159/000171402. [DOI] [PubMed] [Google Scholar]
- 7.Rolls BJ, Rolls ET. In: Thirst. Gray J, Gelder M, Gregory R, Hinde R, Jaspars J, Longuet-Higgins, editors. London: Cambridge University Press; 1982. pp. 1–8. [Google Scholar]
- 8.Allison DB, Saunders SE. Obesity in North America. An overview. Med Clin North Am. 2000;84(2):305–32. doi: 10.1016/s0025-7125(05)70223-6. [DOI] [PubMed] [Google Scholar]
- 9.Bray GA. Medical Consequences of Obesity. J Clin Endocrinol Metab. 2004;89(6):2583–9. doi: 10.1210/jc.2004-0535. [DOI] [PubMed] [Google Scholar]
- 10.Stricker EM. Biological bases of hunger and satiety: therapeutic implications. Nutr Rev. 1984;42(10):R333–40. doi: 10.1111/j.1753-4887.1984.tb02249.x. [DOI] [PubMed] [Google Scholar]
- 11.Frayn KN. Metabolic Regulation: A human perspective. 2. Oxford, UK: Blackwell Publishing; 2003. Energy balance and body weight regulation; pp. 300–19. [Google Scholar]
- 12.Levitsky DA. The control of food intake and the regulation of body weight in humans. In: Harris R, Mattes RD, editors. Appetite and Food Intake: Behavioral and Physiological Considerations. Oxford, UK: Taylor & Francis; 2008. pp. 21–42. [Google Scholar]
- 13.Mattes RD, Hollis J, McKiernan F. The relationship between human thirst and feeding. Appetite. 2007;49:A312. [Google Scholar]
- 14.Black AE. Critical evaluation of energy intake using the Goldberg cut-off for energy intake:basal metabolic rate. A practical guide to its calculation, use and limitations. Int J Obes Relat Metab Disord. 2000;24:1119–30. doi: 10.1038/sj.ijo.0801376. [DOI] [PubMed] [Google Scholar]
- 15.Mattes R. Appetitive effects of food form and timing of ingestion. Obesity. 2007;15(Suppl):A151–A152. [Google Scholar]
- 16.Mattes RD. Hunger ratings are not a reliable proxy measure of food intake in humans. Appetite. 1990;15:103–13. doi: 10.1016/0195-6663(90)90043-8. [DOI] [PubMed] [Google Scholar]
- 17.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29:71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 18.Blundell JE, Stubbs RJ, Golding C, Croden F, Alam R, Whybrow S, et al. Resistance and susceptibility to weight gain: individual variability in response to a high-fat diet. Physiol Behav. 2005;86(5):614–22. doi: 10.1016/j.physbeh.2005.08.052. [DOI] [PubMed] [Google Scholar]
- 19.Berkey CS, Rockett HR, Field AE, Gillman MW, Colditz GA. Sugar-added beverages and adolescent weight change. Obes Res. 2004;12:778–88. doi: 10.1038/oby.2004.94. [DOI] [PubMed] [Google Scholar]
- 20.Schulze MB, Manson JE, Ludwig DS, Colditz GA, Stampfer MJ, Willet WC, et al. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA. 2004;292(8):927–34. doi: 10.1001/jama.292.8.927. [DOI] [PubMed] [Google Scholar]
- 21.Malik VS, Schulze MB, Hu FB. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nut. 2006;84(2):274–88. doi: 10.1093/ajcn/84.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Roosmalen MGM, Klein LL. The spider monkeys, genus Ateles. In: Coimbra-Filho AF, Mittermeier RA, editors. Ecology and Behavior of Neotropical Primates. Vol. 2 Brasil, Ciencias, Rio de Janeiro: Acad.; 1988. pp. 455–537. [Google Scholar]
- 23.Gaulin SJC, Konner M. On the Natural Diet of Primates, including Humans. In: Wurtman RJ, Wurtman JJ, editors. Nutrition and the Brain. New York, NY: Raven Press; 1977. pp. 1–87. [Google Scholar]
- 24.Schmidt-Nielsen K. The Physiology of Wild Animals. Proc R Soc Lon B. 1977;199:345–60. doi: 10.1098/rspb.1977.0146. [DOI] [PubMed] [Google Scholar]
- 25.Beverage Digest Company. Fact book. New York, NY: Bedford Hill; 1998. pp. 54–5. [Google Scholar]
- 26.Food and Nutrition Board of the Institute of Medicine. Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate. Washington, DC: National Academies Press; 2004. pp. D2–D9. [Google Scholar]
- 27.Beverage Marketing Corporation. University of Michigan Annual Consumer Satisfaction Index. 2005 [Google Scholar]
- 28.Nielsen SJ, Popkin BM. Changes in Beverage intake between 1977 and 2001. Am J Prev Med. 2004;27(3):205–10. doi: 10.1016/j.amepre.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 29.Libuda L, Alexy U, Sichert-Hellert W, Stehle P, Karaolis-Danckert N, Buyken Ae, et al. Pattern of beverage consumption and long-term association with body-weight status in German adolescents - results from the DONALD study. Br J Nutr. 2007;23:1–10. doi: 10.1017/S0007114507862362. [DOI] [PubMed] [Google Scholar]
- 30.Zohouri FV, Rugg-Gunn AJ, Fletcher ES, Hackett AF, Moynihan PJ, Mathers JC, et al. Changes in water intake of Northumbrian adolescents 1980 to 2000. Br Dent J. 2004;196:547–52. doi: 10.1038/sj.bdj.4811226. [DOI] [PubMed] [Google Scholar]
- 31.Corporate Marketing Department of Canadean Ltd. Global Beverage Consumption, Looking to the Future. [12 February 2007];2006 http://www.canadean.com/richtext.asp?page_id=3&content_id=653.
- 32.Fitzsimons JT, Le Magnen J. Eating as a regulatory control of drinking in the rat. J Comp Physiol Psychol. 1969;67:273–83. doi: 10.1037/h0026772. [DOI] [PubMed] [Google Scholar]
- 33.Engell D. Interdependency of food and water intake in humans. Appetite. 1988;10(2):133–41. doi: 10.1016/0195-6663(88)90064-5. [DOI] [PubMed] [Google Scholar]
- 34.Phillips PA, Rolls BJ, Ledingham JG, Morton JJ. Body fluid changes, thirst and drinking in man during free access to water. Physiol Behav. 1984;33:357–63. doi: 10.1016/0031-9384(84)90154-9. [DOI] [PubMed] [Google Scholar]
- 35.De Castro JM. A microregulatory analysis of spontaneous fluid intake by humans: evidence that the amount of liquid ingested and its timing is mainly governed by feeding. Physio Behav. 1988;43(6):705–14. doi: 10.1016/0031-9384(88)90367-8. [DOI] [PubMed] [Google Scholar]
- 36.Storey ML, Forshee RA, Anderson PA. Beverage consumption in the US population. J Am Diet Assoc. 2006;106(12):1992–2000. doi: 10.1016/j.jada.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 37.Popkin BM, Barclay DV, Nielsen SJ. Water and food consumption patterns of U.S. adults from 1999 to 2001. Obes Res. 2005;13(12):2146–52. doi: 10.1038/oby.2005.266. [DOI] [PubMed] [Google Scholar]
- 38.Sclafani A. Learned controls of ingestive behavior. Appetite. 1997;29(2):R153–8. doi: 10.1006/appe.1997.0120. [DOI] [PubMed] [Google Scholar]
- 39.DiMeglio DP, Mattes RD. Liquid versus solid carbohydrate: effects on food intake and body weight. Int J Obes. 2000;24:794–800. doi: 10.1038/sj.ijo.0801229. [DOI] [PubMed] [Google Scholar]
- 40.Szlyk PC, Sils IV, Francesconi RP, Hubbard RW, Armstrong LE. Effects of water temperature and flavoring on voluntary dehydration in men. Physiol Behav. 1989;45(3):639–47. doi: 10.1016/0031-9384(89)90085-1. [DOI] [PubMed] [Google Scholar]
- 41.Rolls BJ, Fedoroff IC, Guthrie JF, Laster LJ. Effects of temperature and mode of presentation of juice on hunger, thirst and food intake in humans. Appetite. 1990;15(3):199–208. doi: 10.1016/0195-6663(90)90020-9. [DOI] [PubMed] [Google Scholar]
- 42.Boulze D, Montastruc P, Cabanac M. Water intake, pleasure and water temperature in humans. Physiol Behav. 1983;30(1):97–102. doi: 10.1016/0031-9384(83)90044-6. [DOI] [PubMed] [Google Scholar]
- 43.Cutler D, Glaeser E, Shapiro J. Why have Americans become more obese? J Econ Perspect. 2003;17:93–118. [Google Scholar]
- 44.Zizza C, Siega-Riz A, Popkin B. Significant increase in young adults’ snacking between 1977–19778 and 1994–1996 represents a cause for concern. Prev Med. 2001;32:303–10. doi: 10.1006/pmed.2000.0817. [DOI] [PubMed] [Google Scholar]
- 45.Lin B, Gutherie J, Frazao E. America’s eating habits: Changes and consequences. US Department of Agriculture, Economic Research Service. 1999:AIB–750. [Google Scholar]
- 46.Prentice A, Jebb S. Energy intake/physical activity interactions in the homeostasis of body weight regulation. Nutr Rev. 2004;62(7):S98–104. doi: 10.1111/j.1753-4887.2004.tb00095.x. [DOI] [PubMed] [Google Scholar]
- 47.Bellisle F, McDevitt R, Prentice AM. Meal frequency and energy balance. Br J Nutr. 1997;77:S57–70. doi: 10.1079/bjn19970104. [DOI] [PubMed] [Google Scholar]
- 48.Drummond S, Crombie N, Cursiter M, Kirk T. Evidence that eating frequency is inversely related to body weight status in male but not female, non-obese adults reporting valid dietary intakes. Int J Obes. 1998;22:105–12. doi: 10.1038/sj.ijo.0800552. [DOI] [PubMed] [Google Scholar]
- 49.Forslund HB, Torgerson JS, Sjostrom L, Lindroos AK. Snacking frequency in relation to energy intake and food choices in obese men and women compared to a reference population. Int J Obes. 2005;29:711–9. doi: 10.1038/sj.ijo.0802950. [DOI] [PubMed] [Google Scholar]
- 50.Bellisle F, Dalix A, Mennen L, Galan P, Herchberg S, de Castro J, et al. Contribution of snacks and meals in the diet of French adults: A diet-diary study. Physiol Behav. 2003;79:183–9. doi: 10.1016/s0031-9384(03)00088-x. [DOI] [PubMed] [Google Scholar]
- 51.Hampl JS, Heaton CL, Taylor CA. Snacking patterns influence energy and nutrient intakes but not body mass index. J Hum Nutr Diet. 2003;6(1):3–11. doi: 10.1046/j.1365-277x.2003.00417.x. [DOI] [PubMed] [Google Scholar]
- 52.Young LR, Nestle M. The contribution of expanding portion sizes to the US obesity epidemic. Am J Public Health. 2002;92(2):246–9. doi: 10.2105/ajph.92.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nielsen SJ, Popkin BM. Patterns and trends in food portion sizes, 1977-1998. JAMA. 2003;289(4):450–3. doi: 10.1001/jama.289.4.450. [DOI] [PubMed] [Google Scholar]
- 54.Smiciklas-Wright H, Mitchell DC, Mickle SJ, Goldman MA, Cook A. Foods commonly eaten in the United States, 1989-1991 and 1994-1996: Are portion sizes changing? J Am Diet Assoc. 2003;103:41–7. doi: 10.1053/jada.2003.50000. [DOI] [PubMed] [Google Scholar]
- 55.Levitsky DA, Youn T. The more food young adults are served, the more they overeat. J Nutr. 2004;134(10):2546–9. doi: 10.1093/jn/134.10.2546. [DOI] [PubMed] [Google Scholar]
- 56.Birch LL, Johnson SL, Andresen G, Peters JC, Schulte MC. The variability of young children’s energy intake. N Engl J Med. 1991;324:232–5. doi: 10.1056/NEJM199101243240405. [DOI] [PubMed] [Google Scholar]
- 57.McKiernan F, Hollis JH, Mattes RD. Short-term dietary compensation in free-living adults. Physiol Behav. 2008 doi: 10.1016/j.physbeh.2007.12.014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cox DN, Mela DJ. Determination of energy density of freely selected diets: methodological issues and implications. Int J Obes. 2000;24:49–54. doi: 10.1038/sj.ijo.0801084. [DOI] [PubMed] [Google Scholar]
- 59.Stubbs RJ, Johnstone AM, O’Reilly LM, Barton K, Reid C. The effect of covertly manipulating the energy density of mixed diets on ad libitum food intake in ‘pseudo free-living’ humans. Int J Obes. 1998;22:980–87. doi: 10.1038/sj.ijo.0800715. [DOI] [PubMed] [Google Scholar]
- 60.De Castro JM. Dietary energy density is associated with increased intake in free-living humans. J Nutr. 2004;134:335–41. doi: 10.1093/jn/134.2.335. [DOI] [PubMed] [Google Scholar]
- 61.Stellman SD, Garfinkel L. Artificial sweetener use and one-year weight change among women. Prev Med. 1986;15(2):195–202. doi: 10.1016/0091-7435(86)90089-7. [DOI] [PubMed] [Google Scholar]
- 62.Colditz GA, Willet WC, Stampfer MJ, London SJ, Segal MR, Speizer FE. Patterns of weight change and their relation to diet in a cohort of healthy women. Am J Clin Nutr. 1990;51(6):1100–5. doi: 10.1093/ajcn/51.6.1100. [DOI] [PubMed] [Google Scholar]
- 63.Westeerterp-Plantenga MS, Wijckmans-Duijsens NE, Verboeket-van de Venne WP, de Graaf K, van het Hof KH, Westrate JA. Energy intake and body weight effects of six months reduced or full fat diets, as a function of dietary restraint. Int J Obes. 1998;22:14–22. doi: 10.1038/sj.ijo.0800538. [DOI] [PubMed] [Google Scholar]
- 64.Bellisle F, Altenburg de Assis MA, Fieux B, Preziosi P, Galan P, Guy-Grand B, et al. Use of ‘light’ foods and drinks in French adults: biological, anthropometric and nutritional correlates. J Hum Nutr Diet. 2001;14:191–206. doi: 10.1046/j.1365-277x.2001.00289.x. [DOI] [PubMed] [Google Scholar]
- 65.Lavin PT, Sanders PG, Mackey MA, Kotsonis FN. Intense sweeteners use and weight change among women: A critique of the Stellman and Garfinkel study. J Am Coll Nutr. 1994;13(1):102–5. doi: 10.1080/07315724.1994.10718379. [DOI] [PubMed] [Google Scholar]
- 66.De Graaf C, Hulshof T, Weststrate JA, Hautvast J. Nonabsorbable fat (sucrose polyester) and the regulation of energy intake and body weight. Am J Physiol Reg Int Comp Physiol. 1996;39:R1386–93. doi: 10.1152/ajpregu.1996.270.6.R1386. [DOI] [PubMed] [Google Scholar]
- 67.Bray GA, Lovejoy JC, Most-Windhauser M, Smith SR, Volaufova J, Denkins Y, et al. A 9-mo randomized clinical trial comparing fat-substituted and fat-reduced diets in healthy obese men: the Ole Study. Am J Clin Nutr. 2002;76:928–34. doi: 10.1093/ajcn/76.5.928. [DOI] [PubMed] [Google Scholar]
- 68.Lovejoy JC, Bray GA, Lefevre M, Smith SR, Most MM, Denkins YM, et al. Consumption of a controlled low-fat diet containing olestra for 9 months improves health risk factors in conjunction with weight loss in obese men: the Ole’ Study. Int J Obes Relat Metab Disord. 2003;27:1242–9. doi: 10.1038/sj.ijo.0802373. [DOI] [PubMed] [Google Scholar]
- 69.Caputo FA, Mattes RD. Human dietary response to perceived manipulation of fat content in a midday meal. Int J Obes. 1993;17:237–40. [PubMed] [Google Scholar]
- 70.Davidson TL, Swithers SE. A Pavlovian approach to the problem of obesity. Int J Obes. 2004;28:933–5. doi: 10.1038/sj.ijo.0802660. [DOI] [PubMed] [Google Scholar]
- 71.Pierce WD, Heth CD, Owczarczyk JC, Russell JC, Proctor SD. Overeating by young obesity-prone and lean rats caused by tastes associated with low energy foods. Obesity (Silver Springs) 2007;15(8):1969–79. doi: 10.1038/oby.2007.235. [DOI] [PubMed] [Google Scholar]
- 72.Bellisle F, LeMagnen J. The structure of meals in humans: eating and drinking patterns in lean and obese subjects. Physiol Behav. 1981;27:649–58. doi: 10.1016/0031-9384(81)90237-7. [DOI] [PubMed] [Google Scholar]
- 73.Adolph EF, Wills JH. Adolph and Associates. Physiology of man in the desert. New York: Interscience; 1947. Thirst; pp. 241–53. [Google Scholar]
- 74.Kissileff HR. Non-homeostatic controls of drinking. In: Epstein AN, Kissileff HR, Stellar E, editors. Neuropsychology of thirst: new findings and advances in concepts. Washington DC: VH Winston; 1973. pp. 163–98. [Google Scholar]
- 75.Kissileff HR. Food associated drinking in the rat. J Comp Physiol Psychol. 1969;67:284–300. doi: 10.1037/h0026773. [DOI] [PubMed] [Google Scholar]
- 76.Engell D, Kramer M, Malafi T, Salomon M, Lesher L. Effects of effort and social modeling on drinking in humans. Appetite. 1996;26:129–38. doi: 10.1006/appe.1996.0011. [DOI] [PubMed] [Google Scholar]
- 77.Stricker EM, Verbalis JG. Fluid intake and homeostasis. In: Zigmond MJ, Bloom FE, Landis SC, Roberts JL, Squire LR, editors. Fundamental Neuroscience. San Diego, CA: Academia Press; 1999. pp. 1091–9. [Google Scholar]
- 78.Anderson GH. Sugars-containing beverages and post-prandial satiety and food intake. Int J Obes. 2006;30:S52–9. [Google Scholar]
- 79.Blundell JE, Stubbs RJ, Hughes DA, Whybrow S, King NA. Cross talk between physical activity and appetite control: does physical activity stimulate appetite? Proc Nutr Soc. 2003;62(3):651–61. doi: 10.1079/PNS2003286. [DOI] [PubMed] [Google Scholar]
- 80.Almiron-Roig E, Drewnowski A. Hunger, thirst, and energy intakes following consumption of energy-containing beverages. Physiol Behav. 2003;79:767–73. doi: 10.1016/s0031-9384(03)00212-9. [DOI] [PubMed] [Google Scholar]
- 81.De Castro JM. The effect of the spontaneous ingestion of particular foods or beverages on meal pattern and overall nutrient intake of humans. Physiol Behav. 1993;53(6):1133–44. doi: 10.1016/0031-9384(93)90370-u. [DOI] [PubMed] [Google Scholar]
- 82.Mattes RD. Dietary Compensation by Humans for Supplemental Energy Provided as Ethanol or Carbohydrate in Fluids. Physiol Behav. 1996;59(1):179–87. doi: 10.1016/0031-9384(95)02007-1. [DOI] [PubMed] [Google Scholar]
- 83.Striegel-Moore RH, Thompson D, Affentio SG, Franko Dl, Obarzanek E, Barton BA, et al. Correlates of beverage intake in adolescent girls: the National Heart, Lung, and Blood Institute Growth and Health Study. J Pediatr. 2006;148(2):183–7. doi: 10.1016/j.jpeds.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 84.Mattes RD, Hollis J, Hayes D, Stunkard AJ. Appetite: measurement and manipulation misgivings. J Am Diet Assoc. 2005;105:S87–97. doi: 10.1016/j.jada.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 85.Parker BA, Ludher AK, Loon TK, Horowitz M, Chapman IM. Relationships of ratings of appetite to food intake in healthy older men and women. Appetite. 2000;43(3):227–33. doi: 10.1016/j.appet.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 86.De Castro JM, Elmore DK. Subjective hunger relationships with meal patterns in the spontaneous feeding behavior of humans: evidence for a causal connection. Physiol Behav. 1988;43(2):159–65. doi: 10.1016/0031-9384(88)90232-6. [DOI] [PubMed] [Google Scholar]
- 87.Harris A, Wardle J. The feeling of hunger. Br J Clin Psychol. 1987;26:153–4. doi: 10.1111/j.2044-8260.1987.tb00745.x. [DOI] [PubMed] [Google Scholar]
- 88.Friedman MI, Ulrich P, Mattes RD. A figurative measure of subjective hunger sensations. Appetite. 1999;32(3):395–404. doi: 10.1006/appe.1999.0230. [DOI] [PubMed] [Google Scholar]
- 89.Lowe MR, Friedman MI, Mattes R, Kopyt D, Gayda C. Comparison of verbal and pictorial measures of hunger during fasting in normal weight and obese subjects. Obes Res. 2000;8(8):566–74. doi: 10.1038/oby.2000.73. [DOI] [PubMed] [Google Scholar]
- 90.Irvine P, Mouzet JB, Marteau C, Salle A, Genaitay M, Favreau AM, et al. Short-term effect of a protein load on appetite and food intake in diseased mildly undernourished elderly people. Clin Nutr. 2004;23:1146–52. doi: 10.1016/j.clnu.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 91.Mattes R. Soup and satiety. Physiol Behav. 2005;83:739–47. doi: 10.1016/j.physbeh.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 92.Boudville A, Bruce DG. Lack of meal intake compensation following nutritional supplements in hospitalised elderly women. Br J Nutr. 2005;93:879–84. doi: 10.1079/bjn20041359. [DOI] [PubMed] [Google Scholar]
- 93.Yeomans MR, Lartamo S, Procter EL, Lee MD, Gray RW. The actual, but not labelled, fat content of a soup preload alters short-term appetite in healthy men. Physiol Behav. 2001;73:533–40. doi: 10.1016/s0031-9384(01)00502-9. [DOI] [PubMed] [Google Scholar]
- 94.Gustafson DR, McMahon DJ, Morrey J, Nan R. Appetite is not influenced by a unique milk peptide: caseinomacropeptide (CMP) Appetite. 2001;36:157–63. doi: 10.1006/appe.2000.0392. [DOI] [PubMed] [Google Scholar]
- 95.Cecil JE, Francis J, Read NW. Comparison of the effects of a high-fat and high-carbohydrate soup delivered orally and intragastrically on gastric emptying, appetite, and eating behaviour. Physiol Behav. 1999;67:299–306. doi: 10.1016/s0031-9384(99)00069-4. [DOI] [PubMed] [Google Scholar]
- 96.Chapman IM, Goble EA, Wittert GA, Horowitz M. Effects of small-intestinal fat and carbohydrate infusions on appetite and food intake in obese and nonobese men. Am J Clin Nutr. 1999;69:6–12. doi: 10.1093/ajcn/69.1.6. [DOI] [PubMed] [Google Scholar]
- 97.Cecil JE, Castiglione K, French S, Francis J, Read NW. Effects of intragastric infusions of fat and carbohydrate on appetite ratings and food intake from a test meal. Appetite. 1998;30:65–77. doi: 10.1006/appe.1997.0109. [DOI] [PubMed] [Google Scholar]
- 98.Kaplan RJ, Greenwood CE. Influence of dietary carbohydrates and glycaemic response on subjective appetite and food intake in healthy elderly persons. Int J Food Sci Nutr. 2002;53:305–16. doi: 10.1080/09637480220138160. [DOI] [PubMed] [Google Scholar]
- 99.Holt SHA, Sandona N, Brand-Miller JC. The effects of sugar-free vs sugar-rich beverages on feelings of fullness and subsequent food intake. Int J Food Sci Nutr. 2000;51:59–71. doi: 10.1080/096374800100912. [DOI] [PubMed] [Google Scholar]
- 100.Doucet E, Imbeault P, St-Pierre S, Almeras N, Mauriege P, Richard D, et al. Appetite after weight loss by energy restriction and a low-fat diet-exercise follow-up. Int J Obes. 2000;24:906–14. doi: 10.1038/sj.ijo.0801251. [DOI] [PubMed] [Google Scholar]
- 101.Kovacs EMR, Westerterp-Plantenga MS, Saris WHM, Goossens I, Geurten P, Brouns F. The effect of addition of modified guar gum to a low-energy semisolid meal on appetite and body weight loss. Int J Obes. 2001;25:307–15. doi: 10.1038/sj.ijo.0801546. [DOI] [PubMed] [Google Scholar]
- 102.Green SM, Blundell JE. Subjective and objective indices of the satiating effect of foods: Can people predict how filling a food will be? Eur J Clin Nutr. 1996;50:798–806. [PubMed] [Google Scholar]
- 103.Rayner CK, MacIntosh CG, Chapman IM, Morley JE, Horowitz M. Effects of age on proximal gastric motor and sensory function. Scand J Gastroenterol. 2000;35:1041–7. doi: 10.1080/003655200451153. [DOI] [PubMed] [Google Scholar]
- 104.Russell AW, Horowitz M, Ritz M, MacIntosh C, Fraser R, Chapman IM. The effect of acute hyperglycaemia on appetite and food intake in Type 1 diabetes mellitus. Diabet Med. 2001;18:718–25. doi: 10.1046/j.1464-5491.2001.00545.x. [DOI] [PubMed] [Google Scholar]
- 105.Hetherington MM, Cameron F, Wallis DJ, Pirie LM. Stimulation of appetite by alcohol. Physiol Behav. 2001;74:283–9. doi: 10.1016/s0031-9384(01)00598-4. [DOI] [PubMed] [Google Scholar]
- 106.MacIntosh CG, Sheehan J, Davani N, Morley JE, Horowitz M, Chapman IM. Effects of aging on the opioid modulation of feeding in humans. J Am Geriatr Soc. 2001;49:1518–24. doi: 10.1046/j.1532-5415.2001.4911246.x. [DOI] [PubMed] [Google Scholar]
- 107.Lavin JH, French SJ, Ruxton CHS, Read NW. An investigation of the role of oro-sensory stimulation in sugar satiety. Int J Obes. 2002;26:384–8. doi: 10.1038/sj.ijo.0801829. [DOI] [PubMed] [Google Scholar]
- 108.Yeomans MR, Phillips MF. Failure to reduce short-term appetite following alcohol is independent of beliefs about the presence of alcohol. Nutr Neurosci. 2002;5:131–9. doi: 10.1080/10284150290019008. [DOI] [PubMed] [Google Scholar]
- 109.Hall WL, Millward DJ, Long SJ, Morgan LM. Casein and whey exert different effects on plasma amino acid profiles gastrointestinal hormone secretion and appetite. Br J Nutr. 2003;89:239–48. doi: 10.1079/BJN2002760. [DOI] [PubMed] [Google Scholar]
- 110.Gray RW, French SJ, Robinson TM, Yeomans MR. Increasing preload volume with water reduces rated appetite but not food intake in healthy men even with minimum delay between preload and test meal. Nutr Neurosci. 2003;6:29–37. doi: 10.1080/1028415021000056032. [DOI] [PubMed] [Google Scholar]
- 111.Kamphuis MMJW, Lejeune MPGM, Saris WHM, Westerterp-Plantenga MS. Effect of conjugated linoleic acid supplementation after weight loss on appetite and food intake in overweight subjects. Eur J Clin Nutr. 2003;57:1268–74. doi: 10.1038/sj.ejcn.1601684. [DOI] [PubMed] [Google Scholar]
- 112.Devitt AA, Mattes RD. Effects of food unit size and energy density on intake in humans. Appetite. 2004;42:213–20. doi: 10.1016/j.appet.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 113.Iyer SS, Boateng LA, Sales RL, Coelho SB, Lokko P, Monteiro JBR, et al. Effects of peanut oil consumption on appetite and food choice. Int J Obes. 2006;30:704–10. doi: 10.1038/sj.ijo.0803180. [DOI] [PubMed] [Google Scholar]
- 114.Norton GNM, Anderson AS, Hetherington MM. Volume and variety; relative effects on food intake. Physiol Behav. 2006;87:714–22. doi: 10.1016/j.physbeh.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 115.Chapman IM, Goble EA, Wittert GA, Morley JE, Horowitz M. Effect of intravenous glucose and euglycemic insulin infusions on short-term appetite and food intake. Am J Physiol. 1998;274:R596–603. doi: 10.1152/ajpregu.1998.274.3.R596. [DOI] [PubMed] [Google Scholar]
- 116.Cooling J, Blundell J. Are high-fat and low-fat consumers distinct phenotypes? Differences in the subjective and behavioural response to energy and nutrient challenges. Eur J Clin Nutr. 1998;52:193–201. doi: 10.1038/sj.ejcn.1600539. [DOI] [PubMed] [Google Scholar]
- 117.Holt SHA, Delargy HJ, Lawton CL, Blundell JE. The effects of high-carbohydrate vs high-fat breakfasts on feelings of fullness and alertness, and subsequent food intake. Int J Food Sci Nutr. 1999;50:13–28. doi: 10.1080/096374899101382. [DOI] [PubMed] [Google Scholar]
- 118.Caton SJ, Ball M, Ahern A, Hetherington MM. Dose-dependent effects of alcohol on appetite and food intake. Physiol Behav. 2004;81:51–8. doi: 10.1016/j.physbeh.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 119.Drapeau V, Blundell J, Therrien F, Lawton C, Richard D, Tremblay A. Appetite sensations as a marker of overall intake. Br J Nutr. 2005;93:273–80. doi: 10.1079/bjn20041312. [DOI] [PubMed] [Google Scholar]
- 120.King NA, Craig SAS, Pepper T, Blundell JE. Evaluation of the independent and combined effects of xylitol and polydextrose consumed as a snack on hunger and energy intake over 10 d. Br J Nutr. 2005;93:911–5. doi: 10.1079/bjn20051431. [DOI] [PubMed] [Google Scholar]
- 121.Doucet E, St-Pierre S, Almeras N, Tremblay A. Relation between appetite ratings before and after a standard meal and estimates of daily energy intake in obese and reduced obese individuals. Appetite. 2003;40:137–43. doi: 10.1016/s0195-6663(02)00143-5. [DOI] [PubMed] [Google Scholar]
- 122.Blom WAM, Stafleu A, DeGraaf C, Kok FJ, Schaafsma G, Hendriks HFJ. Ghrelin response to carbohydrate-enriched breakfast is related to insulin. Am J Clin Nutr. 2005;81:367–75. doi: 10.1093/ajcn.81.2.367. [DOI] [PubMed] [Google Scholar]
- 123.Gutzwiller J-P, Drewe J, Goke B, Schmidt H, Rohrer B, Lareida J, et al. Glucagon-like peptide-1 promotes satiety and reduces food intake in patients with diabetes mellitus type 2. Am J Physiol. 1999;276:R1541–4. doi: 10.1152/ajpregu.1999.276.5.R1541. [DOI] [PubMed] [Google Scholar]
- 124.Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes. 2000;24:38–48. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- 125.Parker BA, Sturm K, MacIntosh CG, Feinle C, Horowitz M, Chapman IM. Relation between food intake and visual analogue scale ratings of appetite and other sensations in healthy older and young subjects. Eur J Clin Nutr. 2004;58:212–8. doi: 10.1038/sj.ejcn.1601768. [DOI] [PubMed] [Google Scholar]
- 126.Strum K, Parker B, Wishart J, Feinle-Bisset C, Jones KL, Chapman I, et al. Energy intake and appetite are related to antral area in healthy young and older subjects. Am J Clin Nutr. 2004;80:656–67. doi: 10.1093/ajcn/80.3.656. [DOI] [PubMed] [Google Scholar]
- 127.Cani PD, Joly E, Horsmans Y, Delzenne NM. Oligofructose promotes satiety in healthy human: a pilot study. Eur J Clin Nutr. 2006;60:567–72. doi: 10.1038/sj.ejcn.1602350. [DOI] [PubMed] [Google Scholar]
- 128.Johnson RK. Dietary assessment and validation. In: Monsen ER, Horn LVH, editors. Research: Successful Approaches. 3. Am Dietet Assoc; 2008. pp. 187–204. [Google Scholar]


