Abstract
Gustatory stimuli have at least 2 kinds of function: They can support immediate, reflexive responses (such as substrate choice and feeding) and they can drive internal reinforcement. We provide behavioral analyses of these functions with respect to sweet taste in larval Drosophila. The idea is to use the dose–effect characteristics as behavioral “fingerprints” to dissociate reflexive and reinforcing functions. For glucose and trehalose, we uncover relatively weak preference. In contrast, for fructose and sucrose, preference responses are strong and the effects on feeding pronounced. Specifically, larvae are attracted to, and feeding is stimulated most strongly for, intermediate concentrations of either sugar: Using very high concentrations (4 M) results in weakened preference and suppression of feeding. In contrast to such an optimum function regarding choice and feeding, an asymptotic dose–effect function is found for reinforcement learning: Learning scores reach asymptote at 2 M and remain stable for a 4-M concentration. A similar parametric discrepancy between the reflexive (choice and feeding) and reinforcing function is also seen for sodium chloride (Niewalda T, Singhal S, Fiala A, Saumweber T, Wegener S, Gerber B, in preparation). We discuss whether these discrepancies are based either on inhibition from high-osmolarity sensors upon specifically the reflexive pathways or whether different sensory pathways, with different effective dose–response characteristics, may have preferential access to drive either reflex responses or modulatory neurons mediating internal reinforcement, respectively.
Keywords: Drosophila, feeding, gustation, learning, olfaction, sugar
Introduction
The sense of taste enables animals to prefer the edible and avoid the non-nutritious or toxic, an unquestionably vital faculty. In addition, gustatory stimuli are effective reinforcers; that is, they can induce memories for those stimuli or actions that repeatedly precede them, such that animals can yield good and avoid bad food, respectively. Gustatory stimuli thus support both immediate, reflexive behavior toward food (such as choice and ingestion) and, by virtue of their association with predictive stimuli or instrumental actions, the search for food. These 2 functions, that is, the reflex releasing and the reinforcing function of tastants, obviously need to be dissociated neuronally. Although at the level of gustatory interneurons such dissociation can clearly be found (e.g., in terms of the sufficiency of octopaminergic signaling for reinforcement, but not for ingestive behavior: Hammer 1997; Hammer and Menzel 1998; Menzel et al. 1999), it is unknown whether different sets of sensory neurons may trigger reflex behavior and instruct reinforcement, respectively (for an interesting study of this issue in mice, see de Araujo et al. 2008). Here, we want to take a first step into such an analysis, by behaviorally “fingerprinting” choice, feeding, and the reinforcing function for their respective dose–effect characteristics. We do so with respect to sweet taste in larval Drosophila.
The larva is the feeding and growth stage of the fly life cycle and as such lends itself to studies of gustation. Substrate choice, feeding, and reinforcement learning can be tackled by simple, well-defined behavioral assays; furthermore, the larval gustatory system is relatively simple and reasonably well described at the anatomical, cellular, and to some extent also the molecular level (for a review, see Gerber and Stocker 2007; Gerber et al. 2008). We focus on sweet taste, aiming to relate parametrically the reflex releasing and the reinforcing function of various kinds of sugar. Specifically, we ask:
How does sugar concentration affect choice between sugary and tasteless substrates?
How do these different sugar concentrations affect feeding behavior?
How potent are they in inducing learning?
How do the dose–effect curves for choice, feeding, and learning relate?
We find that the dose–effect curves of the reflexive (choice and feeding) function of both fructose and sucrose are shifted by one order of magnitude relative to the reinforcing function; we discuss whether inhibition from high-osmolarity sensors upon specifically the reflexive pathways is responsible for this parametric dissociation. Alternatively, we suggest that this dissociation is based on a dissociation already at the sensory level, such that different sensory pathways, with different effective dose–response characteristics, may have preferential access to either reflex pathways or to modulatory neurons mediating internal reinforcement.
Materials and methods
Larvae
We use feeding-stage third-instar larvae of the wild-type Canton-S strain, aged 5 days after egg laying. Flies are maintained on standard medium, in mass culture at 25 °C, 60–70% relative humidity and a 14:10 h light:dark cycle. Before each experiment, we remove a spoonful of medium from a food vial, collect the desired number of larvae, briefly rinse them in distilled water, and start the experiment. All experiments are performed under a fume hood in a regularly lit room, at approximately 23 °C ambient temperature.
Choice
The day before experiments, we prepare the petri dishes (55 mm inner diameter; Sarstedt, Nümbrecht, Germany): We split them into 2 halves with a piece of overhead transparency, fill one side with 1% agarose (electrophoresis grade; Roth, Karlsruhe, Germany) (PURE) and then the other side with 1% agarose containing a given sugar (SUGAR). As sugar we use glucose, trehalose, fructose, or sucrose (each with 99% purity; all from Roth) at various concentrations. Once the agarose has solidified, we remove the overhead transparency, cover the dishes with their lids, and leave them at room temperature until the following day.
We place 15 larvae in the middle of the dish and close the lid. The SUGAR side is in half of the cases to the right and in the other half to the left. We record the number of larvae on either side of the dish and calculate a gustatory preference index (PREFGustatory) as
| (1) |
In this equation, # indicates the number of larvae on the respective half of the dish. Thus, PREFGustatory values are constrained between 1 and −1, positive values indicating a preference for SUGAR and negative values aversion. These scores are taken at various time points after the animals are placed onto the dish (for details, see Results).
Feeding
To measure feeding behavior on substrates containing sugars at different concentrations, 30 larvae are placed on a petri dish filled with 1% agarose containing the chosen concentration of the respective sugar (either fructose or sucrose, at either 0.02-, 0.2-, 2-, or 4-M concentration) and 30% red food dye (RU9805; backfun.de, Uhingen, Germany). The animals are allowed to feed on this substrate for 15 min; then, they are washed in tap water and, as a group, homogenized in 80 μl of distilled water. The homogenate is centrifuged for 30 s at 13 200 rpm and 50 μl of the supernatant is loaded into single wells of a 96-well plate (Hartenstein, Würzburg, Germany). Then, using a “Sunrise” spectrophotometer (Tecan AG, Männedorf, Switzerland), absorbance at 500 nm is measured. On each experimental day, we measure the absorbance of homogenate from animals that have been feeding on a plate containing no sugar but dye. From 4 to 6 independent samples of this condition, we calculate a median absorbance which we take as baseline. This baseline is subtracted from all spectrophotometer readings on that experimental day to yield the feeding scores. Thus, if larvae feed as much in the presence of a given sugar concentration as they do in its absence, feeding scores are zero; if they eat more or less than in the absence of sugar, respectively positive and negative feeding scores result. Per experimental day, 3 to 12 independent samples of 30 larvae each are measured per sugar concentration.
Learning
Preparation and treatment of petri dishes for the learning experiments are as detailed above, except that we use petri dishes of approximately 90 mm diameter (Sarstedt), filled uniformly either with 1% agarose only or with 1% agarose containing the reinforcer (+). As reinforcer, we use fructose or sucrose at the indicated concentrations.
Prior to the learning experiments, odor containers are prepared: 10 μl of odor substance is filled into each custom-made Teflon odor container (5 mm inner diameter with a lid perforated with seven 0.5-mm diameter holes). As odors, we use N-amyl acetate (AM, 99%; Merck, Hohenbrunn, Germany) and 1-octanol (OCT, 99%; Fluka/Sigma-Aldrich, Steinheim, Germany). We dilute AM 1:250 in paraffin oil (Merck).
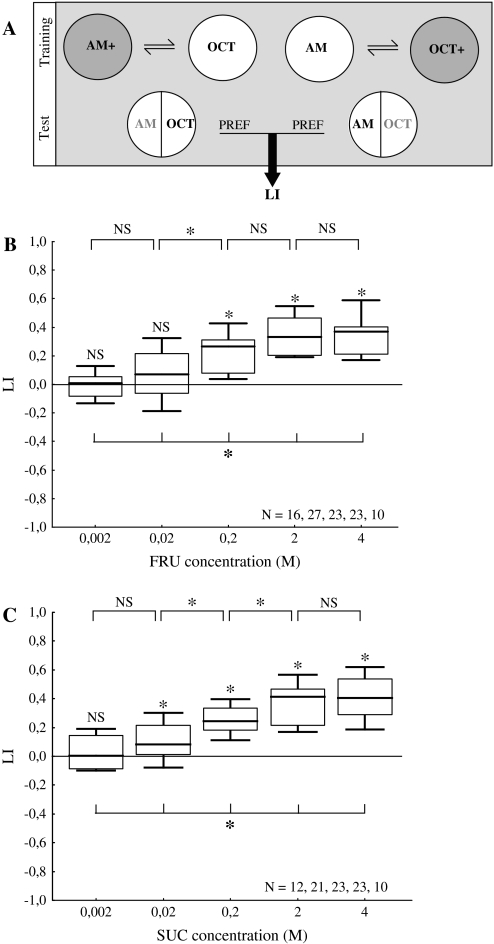
Immediately before the experiment starts, dishes are covered with modified lids perforated in the center by 15 holes with 1 mm diameter to improve aeration. To start training, 30 larvae are placed in the middle of a reinforcer-added dish with 2 odor containers on opposite sides (7 mm from the edges), both filled with AM. After 5 min, larvae are displaced onto an agarose-only dish with 2 odor containers, this time both filled with OCT, where they also spend 5 min. Three such AM+/OCT training cycles are performed, each using fresh dishes. Along repetitions of the experiment, in half of the cases training starts with a reinforcer-added dish (AM+/OCT for all three training cycles) and in the other half with an agarose-only dish (OCT/AM+ for all three training cycles). Consequently, in half of the cases AM is present in the first trial, whereas in the other half the first trial involves OCT. Once this AM+/OCT training is completed, larvae are transferred to the middle of a fresh agarose-only dish with 2 odor containers, this time filled with OCT on one side and AM on the opposite side to create a choice situation. After 3 min, the number of larvae on each half of the dish is recorded and an olfactory preference (PREF) is calculated as
| (2) |
Again, # indicates the number of larvae observed on the respective half of the dish. PREF values are bound between 1 and −1, positive values indicating preference for and negative values avoidance of AM.
For each group of larvae trained AM+/OCT, a second group is trained reciprocally: AM/OCT+. Associative learning shall result in a stronger preference for AM after AM+/OCT training than after AM/OCT+ training. This difference is quantified by the learning index (LI) as
| (3) |
Here, PREFAM+/OCT is the AM preference of the AM+/OCT group and PREFAM/OCT+ is that of the reciprocally trained AM/OCT+ group. The LI is a pure measure of associative learning because it measures the difference in preference between 2 groups trained reciprocally, but otherwise treated the same (i.e., with respect to handling, exposure to odors, and the reinforcer). LI values are bound between 1 and −1, positive values indicating approach toward the reinforcer-paired odor (appetitive learning) and negative values avoidance from the reinforced odor (aversive learning).
Statistical analysis
All statistical analyses are performed with Statistica on a PC. Preference values, feeding scores, and learning indices from multiple experimental groups are compared with Kruskal–Wallis tests. For subsequent pairwise comparisons, Mann–Whitney U tests are used. To test whether values of a given group differ from zero, we use 1-sample sign tests. When multiple 1-sample sign tests or Mann–Whitney U tests are performed within one experiment, we adjust significance levels by a Bonferroni correction to keep the experiment-wide error rate at 5%. This is done by dividing the critical P value 0.05 by the number of tests; that is, if e.g. four 1-sample sign tests are performed within one experiment, we present statements of significance as P <>0.05/4. We present our data as box plots which represent the median as the middle line and 25/75% and 10/90% as box boundaries and whiskers, respectively. In all cases, sample sizes are presented exclusively within the figures.
Results
Experiment 1: optimizing the duration of the choice assay
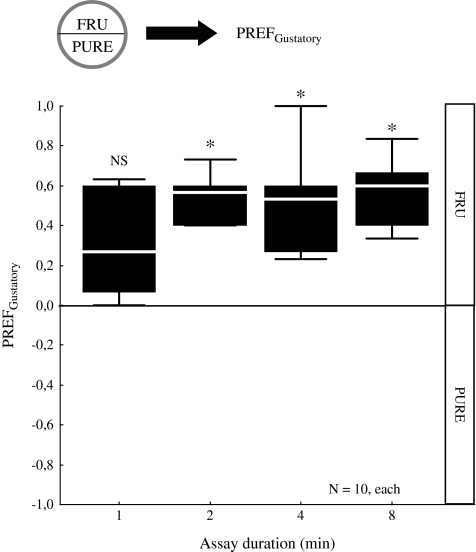
First, we want to find an appropriate assay duration for testing the sugar preference of experimentally naive larvae; this seems warranted because here we use assay plates with smaller diameter (ca. 55 mm) than in previous studies (ca. 90 mm; see review by Gerber and Stocker 2007). We use fructose at 2-M concentration because in previous work this concentration has been used as gustatory reinforcer in larval learning experiments (reviewed in Gerber and Stocker 2007). We allow the larvae to choose between pure agarose (PURE) and agarose in addition containing 2 M fructose (FRU) and recurrently score for the gustatory preference index (PREFGustatory) at 1, 2, 4, and 8 min. Positive PREFGustatory values indicate a preference for fructose.
We observe a preference for 2 M fructose over pure agarose beginning from 2 min after assay onset (Figure 1; 1-sample sign tests: P > 0.05/4 for 1 min; P < 0.05/4 for 2, 4, and 8 min). The larval response to fructose seems to saturate at 2 min. We choose 90 s as assay duration for the following experiments in order to be able to detect both higher and lower preference scores.
Figure 1.
Optimizing the duration of the choice assay. Groups of 15 larvae are allowed to distribute between pure agarose (PURE) on one side and agarose containing 2 M fructose (FRU) on the other. A gustatory preference (PREFGustatory) is calculated based on their distribution at different time points after the experiment has started. Positive PREFGustatory values indicate a preference for fructose. At each time point, larvae seem to prefer fructose; this response is statistically significant from 2 min on; it seems to saturate already at 2 min after choice onset. NS, P > 0.05/4; *P< 0.05/4 in 1-sample sign tests, keeping the experiment-wide error rate at 5% (i.e., Bonferroni correction). Box plots represent median as the middle line and 25/75% and 10/90% as box boundaries and whiskers, respectively.
Experiment 2: choice
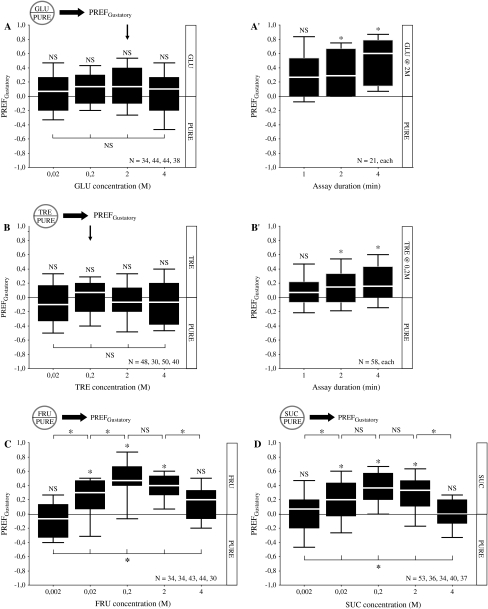
We next test the choice response of experimentally naive larvae between pure agarose (PURE) and agarose containing different types of sugar, at various concentrations. Specifically, we study the preferences for different concentrations of glucose (GLU), trehalose (TRE), fructose (FRU), and sucrose (SUC), scoring the larvae 90 s after the start of the assay.
Scores for glucose (GLU) are indistinguishable from random level for all tested concentrations (Figure 2A; 1-sample sign tests: P > 0.05/4 for each concentration) and are uniform across concentrations within the range tested (Figure 2A; Kruskal–Wallis test: P > 0.05; H = 2.16; degrees of freedom [df] = 3). However, maybe the larvae just need more time to “make up their minds”? Given the trend for highest preference scores for 2 M glucose (arrow in Figure 2A), we repeat the experiment for 2 M glucose, but this time recurrently score at 1, 2, and 4 min after the start of the assay. As expected from the previous experiment, larvae appear indifferent after 1 min, but after 2 min and in particular after 4 min, a substantial preference for glucose is apparent (Figure 2A′; 1-sample sign tests: P > 0.05/3 for 1 min, P < 0.05/3 for 2 and 4 min).
Figure 2.
Choice. (A) Gustatory preference scores (PREFGustatory) for glucose (GLU) calculated based on the distribution of larvae at 90 s after assay onset. The larvae are indifferent toward glucose at each of the tested concentrations; a comparison across groups reveals no effect of glucose concentration on behavior. The arrow indicates the concentration chosen for the follow-up experiment in A′. (A′) Recurrently scoring 1, 2, or 4 min after assay onset reveals a delayed, appetitive response toward glucose. (B) Larval preference scores for trehalose (TRE) are not different from random for either of the tested concentrations; behavior does not differ between groups. The arrow indicates the concentration chosen for the follow-up experiment in B′. (B′) Recurrently scoring 1, 2, or 4 min after assay onset reveals a delayed and weak appetitive response toward trehalose. (C) Larvae respond to fructose (FRU) depending on concentration. Intermediate concentrations of fructose are attractive, whereas larvae are indifferent toward low and high concentrations. (D) Also to sucrose (SUC), larval responses are concentration dependent. Intermediate concentrations of sucrose are attractive, whereas low and high concentrations remain without apparent effect. We use Kruskal–Wallis tests for all-group comparisons at P < 0.05; if applicable, follow-up pairwise comparisons between groups use the Mann–Whitney U test at P < 0.05/4; for single-group comparisons against zero, 1-sample sign tests are used at P < 0.05/3 (A′,B′), at P < 0.05/4 (A,B) or at P < 0.05/5 (C,D). For details concerning the box plots, see legend of Figure 1.
For trehalose (TRE), we find that preference values scored after 90 s are indistiguishable from random for all tested concentrations (Figure 2B; 1-sample sign tests: P > 0.05/4 for each concentration) and are independent of concentration within the range tested (Figure 2B; Kruskal–Wallis test: P > 0.05; H = 2.08; df = 3). To further probe this apparent lack of behavioral effect of trehalose, we repeat the experiment for 0.2 M trehalose (arrow in Figure 2B), this time, however, scoring recurrently at 1, 2, and 4 min after the start of the assay. Indeed, preferences for trehalose develop over time; we find no preference after 1 min; however, at 2 and 4 min after start of the test, a weak yet significant preference for trehalose is found (Figure 2B′; 1-sample sign tests: P > 0.05/3 for 1 min, P < 0.05/3 for 2 and 4 min).
Larval preferences for fructose (FRU) are clearly concentration dependent when scored at 90 s (Figure 2C; Kruskal–Wallis test: P < 0.05; H = 61.38; df = 4). Larvae prefer fructose at intermediate concentrations (Figure 2C; 1-sample sign tests: P < 0.05/5 for 0.02 M, 0.2 M, and 2 M) but are indifferent to it at lower and higher concentrations (Figure 2C; 1-sample sign tests: P > 0.05/5 for 0.002 M and 4 M). Based on pairwise comparisons, fructose seems to be most attractive to larvae at concentrations between 0.2 M and 2 M (Figure 2C; Mann–Whitney U tests: P < 0.05/4; U = 243.00 for 0.002 M vs. 0.02 M; P < 0.05/4; U = 390.50 for 0.02 M vs. 0.2 M; P > 0.05/4; U = 722.00 for 0.2 M vs. 2 M; P < 0.05/4; U = 350.00 for 2 M vs. 4 M).
Similarly, sucrose (SUC) is preferred by the larvae depending on its concentration (Figure 2D; Kruskal–Wallis test: P < 0.05; H = 38.72; df = 4). Larvae find sucrose attractive at intermediate concentrations (Figure 2D; 1-sample sign tests: P < 0.05/5 for 0.02 M, 0.2 M, and 2 M), whereas they do not respond to it at lower and at higher concentrations (Figure 2D; 1-sample sign tests: P > 0.05/5 for 0.002 M and 4 M). Sucrose has a relatively broad peak of attractiveness, spanning 2 orders of magnitude (between 0.02 M and 2 M), as is revealed by pairwise comparisons (Figure 2D; Mann–Whitney U tests: P < 0.05/4; U = 628.50 for 0.002 M vs. 0.02 M; P > 0.05/4; U = 448.00 for 0.02 M vs. 0.2 M; P > 0.05/4; U = 573.50 for 0.2 M vs. 2 M; P < 0.05/4; U = 361.00 for 2 M vs. 4 M).
To summarize, all sugars tested are preferred by the larvae when offered against a pure agarose substrate. Preference for glucose and trehalose is weak and/or delayed, whereas fructose and sucrose support fast and strong preference responses in a concentration-dependent way. The fast and strong preference responses toward fructose and sucrose prompt us to choose these 2 for an analysis of their potency as modulators of feeding and as reinforcers. Specifically, we are interested to see whether, concomitant with the loss of preference at very high concentrations of fructose and sucrose (Figure 2C,D), a loss of appetitive effect in feeding or learning assays would be observed.
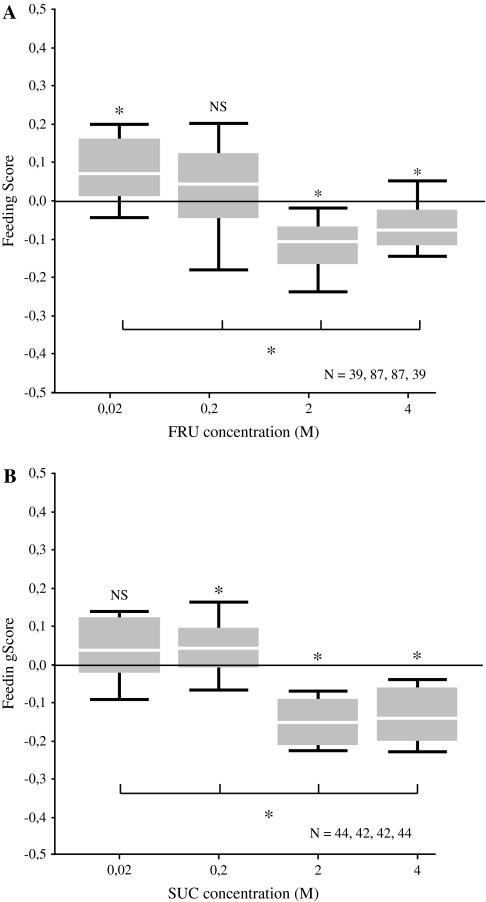
Experiment 3: feeding
We allow larvae 15-min access to a red-dyed assay plate with sugar added at various concentrations to then estimate photometrically the amount fed. Data are presented as feeding score, expressing the difference in feeding as compared with larvae offered a red-dyed assay plate with no sugar added.
For both fructose and sucrose, the concentration of the added sugar has an effect on feeding behavior (Figure 3A; for fructose: Kruskal–Wallis test: P < 0.05; H = 90.98; df = 3; Figure 3B; for sucrose: Kruskal–Wallis test: P < 0.05; H = 97.33; df = 3). Both sugars lead to increases in feeding, relative to the baseline condition with no sugar added, at low but to suppression of feeding at higher concentrations (Figure 3A; for fructose: 1-sample sign tests: P < 0.05/4 for 0.02 M, 2 M, and 4 M, P > 0.05/4 for 0.2 M; Figure 3B; for sucrose: 1-sample sign tests: P < 0.05/4 for 0.2 M, 2 M, and 4 M, P > 0.05/4 for 0.02 M). Thus, the dose–effect function concerning feeding is similar to the one for choice in the sense that both sugars lose their appetitive effect at high concentration; maybe surprisingly, both fructose and sucrose even suppress feeding at these concentrations.
Figure 3.
Feeding. Groups of 30 larvae are allowed to feed on petri dishes filled with dyed agarose which contains either fructose (FRU) or sucrose (SUC) at the indicated concentration; the amount fed then is quantified photospectrometrically. Values from a group which is allowed to feed on dyed agarose without any sugar added serves as baseline; absorbance values of this group are subtracted from the spectrometer readings of the experimental groups to yield the feeding score. Therefore, feeding scores greater than zero indicate that the larvae eat more than if sugar were absent, and feeding scores below zero indicate that larvae eat less than in the absence of sugar. (A) Fructose enhances feeding at low but suppresses feeding at higher concentration. (B) Sucrose also leads to increases in feeding at low but to decreased feeding at higher concentration. We use Kruskal–Wallis tests for all-group comparisons at P < 0.05; for single-group comparisons against zero, 1-sample sign tests are used at P < 0.05/4. For details concerning the box plots, see the caption of Figure 1.
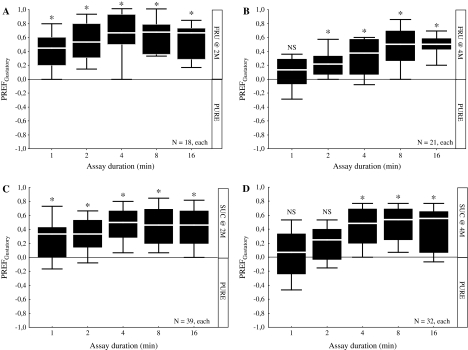
Experiment 4: choice revisited
Given that concentrations of fructose and sucrose which suppress feeding (Figure 3A,B) do not seem to induce aversion in a choice assay (Figure 2C,D), we return to the choice assay for both sugars and test whether, if more time is allotted, an aversion response may become apparent. This is not the case: We find for 2 M fructose that responses are appetitive already after 1 min and remain stably appetitive throughout the 16 min of the assay (Figure 4A; 1-sample sign tests: P < 0.05/5 for all time points). Concerning 4 M fructose, we find that at short assay duration, there is no significant preference (Figure 4B; 1-sample sign test for 1-min assay duration: P > 0.05/5); this is consistent with the results from Experiment 2 (Figure 2C) which had suggested that 2 M but not 4 M fructose supports preference at short (90 s in Figure 2C) assay durations. If 2 min or more time is allowed, however, the larvae eventually express a preference response for 4 M fructose as well (Figure 4B; 1-sample sign tests for 2-, 4-, 8-, and 16-min assay duration: P < 0.05/5), with no apparent decrement between 8 and 16 min.
Figure 4.
Choice revisited. Gustatory preference scores (PREFGustatory) for high concentrations of fructose (FRU) and sucrose (SUC) scored 1, 2, 4, 8, and 16 min after assay onset. (A) For 2 M fructose, preference scores remain stably appetitive throughout the testing period. (B) For 4 M fructose, preferences are uncovered for 2 min or longer assay durations. (C) Similar to the case of 2 M fructose, also for 2 M sucrose, preference scores are positive throughout the testing period. (D) For 4 M sucrose a similar pattern is found as for 4 M fructose; preferences are uncovered only with 4 min or longer assay durations. For single-group comparisons against zero, 1-sample sign tests are used at P < 0.05/5. For details concerning the box plots, see the caption of Figure 1.
Regarding sucrose, the same pattern of results is found: For a 2-M concentration, larvae express appetitive responses from 1 min on (Figure 4C; 1-sample sign tests: P < 0.05/5 for 1-, 2-, 4-, 8-, and 16-min testing times). Using 4 M sucrose, however, larvae remain indifferent for the first 2 min (Figure 4D; 1-sample sign tests: P > 0.05/5 for 1 and 2 min); only as time passes, the larvae start to express appetitive responses (Figure 4D; 1-sample sign tests: P < 0.05/5 for 4-, 8-, and 16-min testing times), without any trend for scores turning into aversion over time. The observation that preference responses to 4 M sucrose unfold between 2 and 4 min is consistent with the indifference of the larvae after 90 s as seen in Figure 2D.
Experiment 5: learning
We assess the reinforcing potency of fructose and sucrose in olfactory associative learning (reviewed in Gerber and Stocker 2007): larvae are trained with 2 odors, one of which is presented in the presence of a reinforcer. After such training, larvae are allowed to distribute between the reinforcer-paired odor and the other odor in a choice situation. The LI, which is a measure of associative learning, is based on the comparison between the odor preferences of 2 groups of larvae, trained reciprocally but otherwise handled the same (see Materials and methods and Figure 5A). Based on this experimental design, we train larvae with various concentrations of either fructose (FRU) or sucrose (SUC) as reinforcer. Specifically, we want to compare the strength of these sugars as reinforcers to their ability to govern choice as measured in Experiment 2 and to their effects as modulators of feeding behavior as measured in Experiment 3.
Figure 5.
Learning. (A) Groups of 30 larvae are trained with 2 odors (i.e., AM and OCT) and a reinforcer (i.e., either fructose: FRU or sucrose: SUC at the indicated concentration). One group of larvae receives AM while crawling on a reinforcer-containing agarose plate, whereas OCT is presented in the absence of the reinforcer (i.e., AM+/OCT training). Another group is trained reciprocally (AM/OCT+) (note that for half of the cases the sequence of trials is as indicated; in the other half, sequences are reversed: OCT/AM+ and OCT+/AM). After repeated training, both groups are tested for their preference between AM and OCT in a choice situation. Associative learning shows by higher preference scores for AM in the group trained AM+/OCT than in the reciprocally trained AM/OCT+ group. This difference is quantified by the LI. Positive LI values thus indicate appetitive learning. (B) The strength of fructose as a reinforcer depends on its concentration. Low concentrations of fructose do not support learning, whereas higher concentrations do. Learning gets stronger with increasing fructose concentration until it saturates between 0.2 M and 2 M. (C) Sucrose also has a concentration-dependent reinforcing effect. A low sucrose concentration does not support any learning, whereas higher concentrations do. Increasing sucrose concentration strengthens learning until an asymptote is reached at 2 M. For all-group comparisons, Kruskal–Wallis tests are used at P < 0.05; for follow-up pairwise comparisons, Mann–Whitney U tests are used at P < 0.05/4; for single-group comparisons against zero, 1-sample sign tests are used at P < 0.05/5. For details concerning the box plots, see the caption of Figure 1.
The concentration of fructose (FRU) matters for its reinforcing potency (Figure 5B; Kruskal–Wallis test: P < 0.05; H = 42.38; df = 4). Low concentrations of fructose apparently do not support learning (Figure 5B; 1-sample sign tests: P > 0.05/5 for 0.002 M and 0.02 M), whereas higher concentrations do (Figure 5B; 1-sample sign tests: P < 0.05/5 for 0.2 M, 2 M, and 4 M). As revealed by pairwise comparisons between learning indices, the reinforcing potency of fructose seems to saturate at concentrations between 0.2 M and 2 M (Figure 5B; Mann–Whitney U tests: P > 0.05/4; U = 169.00 for 0.002 M vs. 0.02 M; P < 0.05/4; U = 161.00 for 0.02 M vs. 0.2 M; P > 0.05/4; U = 165.00 for 0.2 M vs. 2 M; P > 0.05/4; U = 113.00 for 2 M vs. 4 M).
The reinforcing potency of sucrose (SUC) also depends on its concentration (Figure 5C; Kruskal–Wallis test: P < 0.05; H = 42.04; df = 4). Similar to fructose, a low concentration of sucrose does not support learning (Figure 5C; 1-sample sign test: P > 0.05/5 for 0.002 M), whereas higher concentrations do (Figure 5C; 1-sample sign tests: P < 0.05/5 for 0.02 M, 0.2 M, 2 M, and 4 M). The reinforcing ability of sucrose also increases with rising concentration until it reaches an asymptote at 2 M (Figure 5C; Mann–Whitney U tests: P > 0.05/4; U = 85.00 for 0.002 M vs. 0.02 M; P < 0.05/4; U = 122.00 for 0.02 M vs. 0.2 M; P < 0.05/4; U = 130.00 for 0.2 M vs. 2 M; P > 0.05/4; U = 108.00 for 2 M vs. 4 M).
Thus, the highest concentration of fructose and sucrose, although little potent in governing choice behavior (Figures 2C,D and 4B,D) and acting as suppressor of feeding (Figure 3A,B), nevertheless acts as a strong appetitive reinforcer (Figure 5B,C).
Discussion
We systematically analyze 4 natural sugars concerning choice behavior in experimentally naive Drosophila larvae. We then investigate 2 of these sugars in more detail to determine the relation between the dose dependencies of choice of these sugars versus their effect on feeding versus their reinforcing effect. Before discussing the results of these behavioral experiments, we want to briefly sketch the neurobiological organization of the larval taste system.
Neurobiology of taste processing
The neurobiology of taste processing in the larva is resolved partially and in principle conforms to what had been found in adults (see discussions in Python and Stocker 2002; Ishimoto and Tanimura 2004; Gerber and Stocker 2007; Gerber et al. 2008): Candidate gustatory sensory neurons are located in 2 types of sense organ (both of which likely include some nongustatory sensory neurons as well): external sensilla and internal sensilla. The external ones are the terminal (32 sensory neurons) and the ventral organ (7 sensory neurons) plus some gustatory sensory neurons in the bulge of the dorsal organ (9 sensory neurons). The internal sensilla are located along the pharynx and are organized into dorsal, ventral, and posterior sense organ (17, 16, and 6 sensory neurons, respectively). At present, the exact relation between cellular identity, expression of putative gustatory receptor gene of the Gr gene family (Clyne et al. 2000), and ligand profile of the neurons is largely unknown, except for the Gr5a and Gr64a genes (Dahanukar et al. 2007): In adult flies, both genes are concordantly expressed in a subset of gustatory sensory neurons. Deletions of Gr5a abolish electrophysiological responses to only 4 out of 14 tested sugars (trehalose, methyl-a-glucoside, glucose, and melezitose). In turn, deleting the Gr64a gene abolishes (maltotriose, stachyose, raffinose, leucrose, and fructose) or partially reduces (sucrose, maltose, turanose, maltitol, and palatinose) the Gr5a-independent activations. Behavioral analyses using the proboscis extension response generally conform to the complementary requirement of Gr5a and Gr64a for detecting different kinds of sugars and acting within the same set of cells. Note, however, that in the larva Gr5a and Gr64a may not be expressed, as judged from the lack of reporter expression seen in the respective Gr-Gal4 driver strains (Colomb et al. 2007; Tanimura T, Kyushu University, personal communication).
Regarding connectivity toward gustatory interneurons, projections of the Gr-expressing neurons typically bypass the brain and project to the subesophageal ganglion where multiple distinct areas receive innervations from distinct subsets of these neurons (Colomb et al. 2007). It is from these areas that both premotor commands as well as internal reinforcement signals likely originate. Although the exact connectivity of gustatory receptor neurons to their postsynaptic targets is not resolved in detail, neurons expressing a given Gr gene can be found in different sense organs and project to distinct target regions in the subesophageal ganglion (Colomb et al. 2007); this suggests that one and the same tastant can have access to different kinds of downstream effect, dependent on input site.
Choice
We show that glucose and trehalose support relatively weak and/or somewhat sluggish preference responses (Figures 2A,A′ and 2B,B′), whereas those 2 sugars with a ketose unit (fructose and sucrose) support fast and strong preference. This may suggest that those gustatory sensory neurons which support preference responses are particularly sensitive to sugars containing such a ketose unit, whereas the nature of a sugar as mono- versus disaccharide should be of minor importance. As mentioned above, in adult flies processing of glucose and trehalose on the one hand and of fructose and sucrose on the other hand requires the Gr5a and Gr64a genes, respectively (Dahanukar et al. 2007); neither of these genes, however, is apparently expressed in the larva (see section Neurobiology of taste processing), suggesting that the discrepancies in behavioral effectiveness between these 2 classes of sugar may have different neurobiological bases in either life stage. In any event, the parametrically concordant behavioral effects of fructose and sucrose in the larva would be consistent with both sugars being processed via concordant sets of sensory neurons.
The preference responses for fructose and sucrose show a clear concentration dependency: Larvae prefer fructose and sucrose at intermediate concentrations, whereas they are indifferent to both lower and higher concentrations (Figure 2C,D); for higher concentrations, preferences can be uncovered only by increasing assay duration (Figure 4). Intuitively, the relatively weak appetitive response to very high sugar concentrations makes sense as things also for us can be “too” sweet. Also, very high concentrations may, although energetically in principle attractive, make substrates unattractive for reasons of viscosity, stickiness, and/or because of osmotic properties; these kinds of effect may undergo some adaptation/habituation to allow uncovering an appetitive effect only with some delay (Figure 4B,D). We thus regard it as little surprising (yet to the best of our knowledge not previously reported) that preference responses of larval Drosophila toward sugars follow an optimum function.
Feeding
Fructose and sucrose dose-dependently modulate feeding behavior (Figure 3). This dose dependency is similar to the one seen for choice in that the “appetizing” effect exerted by low concentrations of these sugars is lost for higher concentrations. Strikingly, such higher concentrations even suppress feeding. Whether these feeding-suppressant effects are also mediated by gustatory sensory neurons or rather may come about by neurons sensitive to high viscosity, osmolarity, or “stickiness” remains to be investigated. In any event, similar to the case of the preference responses, it seems plausible that >2-M sugar concentrations can appear impalatably high to the larvae.
We also note that both the increases and the decreases in feeding are moderate; given that insect larvae are notorious and continuous feeders to begin with (Carle 1969), it seems plausible that up and downregulations of feeding may be relatively difficult to obtain experimentally.
Learning
Fructose and sucrose act dose dependently as reinforcers (Figure 5). The reinforcing effect of both sugars reaches a stable asymptote at 2-M concentration, a finding matching the previous report of Neuser et al. (2005) who had looked at the dose–effect function of fructose (FRU) reinforcement in a range from 0.25 to 2 M in an individual–animal version of our learning assay. As we show here, there clearly is no decrement in learning scores if sugar concentrations yet higher than 2 M are used, at least not for a 4-M concentration, which is the limit of solubility of fructose (FRU) and sucrose (SUC) in agarose.
It is currently unknown which gustatory sensory neurons drive internal reinforcement; actually, even the sense organ origin of the responsible neurons (i.e., external vs. internal), is unknown. What has been reported, however, is that artificially driving octopaminergic/tyraminergic neurons is sufficient to substitute for appetitive reinforcement in larval olfactory learning (Schroll et al. 2006). In bees, artificially driving even a single, identified octopaminergic neuron, the so-called VUMmx1 neuron, is sufficient to substitute for appetitive reinforcement but is not sufficient to trigger feeding reflexes (Hammer 1997; see also Hammer and Menzel 1998; Menzel et al. 1999); a homolog of this neuron is found in both adult (Tanimoto H, Universität Würzburg, personal communication) and larval Drosophila (Thum A, Université Fribourg, personal communication), as well as in moths (Dacks et al. 2005). Whether output from octopaminergic/tyraminergic neurons is necessary for appetitive learning in the larva, however, remains unknown. Interestingly, the octopaminergic/tyraminergic neurons do not seem to be directly postsynaptic to gustatory sensory neurons, as argued by light microscopical analyses of their branching patterns as well as from the site of expression of pre- and postsynaptic markers (Thum A, Université Fribourg, personal communication).
Relation between reflex responses and reinforcing capacity
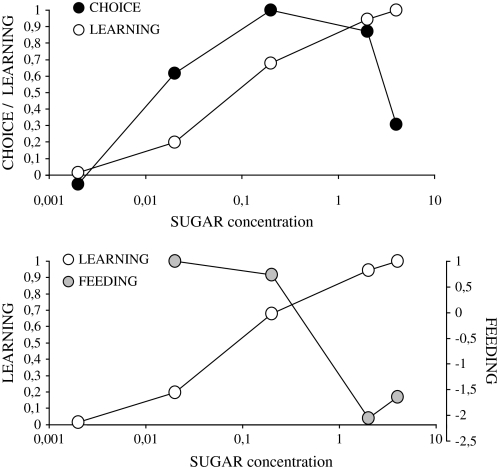
Obviously, while in the choice and the feeding assay both sugars lose their appetitive effect at high concentrations (Figures 2C,D and 3A,B), the reinforcing effect shows an asymptotic dose–effect function; notably, robust appetitive reinforcement is retained even at very high concentrations (Figure 5B,C). In Figure 6, we want to illustrate in a semischematic way the parametric relation between choice, feeding, and the reinforcing effect.
Figure 6.
Summary. Semischematic illustration of the relation between preference scores, feeding scores, and reinforcing effect. For details, see the last paragraph of the Discussion.
We take the median value of the fructose preference response for a given concentration (Figure 2C) as well as the corresponding value for the sucrose response (Figure 2D) and average these 2 values. Next, we do the same for all other concentrations. We then express these scores relative to the highest score thus obtained, such that the semischematic plot in Figure 6 shows the maximum “choice” score as “1.” Then, we do the same for the feeding scores and learning indices obtained for the different concentrations of fructose and sucrose (Figure 3A,B and 5B,C) and display them as “learning” and “feeding” values.
As can be seen in Figure 6, the dose–effect curve for learning is shifted to the “east,” that is toward higher concentrations relative to choice and feeding. Strikingly, a similar east shift is found for salt processing as well: The optimum for the choice responses and for feeding is at around 0.02 M NaCl, whereas the optimum for appetitive learning is shifted by an order of magnitude to around 0.4 M (Niewalda T, Singhal S, Fiala A, Saumweber T, Wegener S, Gerber B, in preparation). Obviously, the discrepancies between the dose–effect functions of tastants with regard to choice and feeding as compared with their reinforcing potency must reflect some dissociation along the respective chemosensory-to-motor pathways. For a start, we note that mere differences in gain between these pathways would leave the “topology” of the dose–effect curve unchanged. Rather, a first possible scenario is that the reduction of the choice and feeding scores for high sugar concentrations is caused by an inhibition from, for example, high-osmolarity sensors specifically upon the reflexive pathways. Such high-osmolarity sensors, however, remain to be characterized in the larva. Alternatively, these parametric dissociations may be based on a dissociation already at the sensory level: Different sensory neurons may have preferential access toward premotor neurons that support choice and feeding on one hand and toward neurons which drive internal reinforcement on the other hand (for a similar proposal with regard to mice, see de Araujo et al. 2008). For example, if the reflexive and reinforcing functions were originating from external and internal taste organs, respectively, and if secreted saliva would dilute the tastants 10-fold, one may indeed expect a shift between the reflexive and reinforcing dose–effect functions by one order of magnitude. This second scenario could explain the apparent generality of such shift (for salt, see this paragraph, above) and would be consistent with the huge salivary glands of larval Drosophila. However, if feeding was indeed organized according to the sensors within the external sense organs, high concentration tastants would suppress feeding and the tastants would not “reach” the internal sense organs to signal aversive reinforcement to begin with. As a third scenario, we contemplate whether the respective gustatory sensory neurons may be expressing different gustatory receptor genes which endow them with different dose–effect characteristics. This may at first sight seem little parsimonious but may partially explain why there are so many different gustatory receptor genes. In any event, all these 3 scenarios, certainly, now invite further experimentation.
Funding
Deutsche Forschungsgemeinschaft via SFB 554-A10 Arthropode Behaviour and a Heisenberg Fellowship to B.G.; PhD fellowship of the Boehringer Ingelheim Fonds to A.Y.
Acknowledgments
Thanks to K. Tschirner and K. Gerber for help with the experiments; to N. Singhal and A. Fiala for an introduction to the feeding assay; and to M. Heisenberg, B. Michels, and H. Tanimoto for discussions all along the project. Experiments comply with applicable law.
References
- Carle E. The very hungry caterpillar. New York: Penguin; 1969. [Google Scholar]
- Clyne PJ, Warr CG, Carlson JR. Candidate taste receptors in Drosophila. Science. 2000;287:1830–1834. doi: 10.1126/science.287.5459.1830. [DOI] [PubMed] [Google Scholar]
- Colomb J, Grillenzoni N, Ramaekers A, Stocker RF. Architecture of the primary taste center of Drosophila melanogaster larvae. J Comp Neurol. 2007;502:834–847. doi: 10.1002/cne.21312. [DOI] [PubMed] [Google Scholar]
- Dacks AM, Christensen TA, Agricola HJ, Wollweber L, Hildebrand JG. Octopamine-immunoreactive neurons in the brain and subesophageal ganglion of the hawkmoth Manduca sexta. J Comp Neurol. 2005;488:255–268. doi: 10.1002/cne.20556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahanukar A, Lei Y-T, Kwon JY, Carlson JR. Two Gr genes underlie sugar reception in Drosophila. Neuron. 2007;56:503–516. doi: 10.1016/j.neuron.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araujo IE, Oliveira-Maia AJ, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MA, Simon SA. Food reward in the absence of taste receptor signaling. Neuron. 2008;57:930–941. doi: 10.1016/j.neuron.2008.01.032. [DOI] [PubMed] [Google Scholar]
- Gerber B, Stocker RF. The Drosophila larva as a model for studying chemosensation and chemosensory learning: a review. Chem Senses. 2007;32:65–89. doi: 10.1093/chemse/bjl030. [DOI] [PubMed] [Google Scholar]
- Gerber B, Stocker RF, Tanimura T, Thum A. Forthcoming. Smelling, tasting, learning: Drosophila as a study case. In: Meyerhof W, Korsching S, editors. Chemosensory systems in mammals, fishes and insects. Heidelberg (Germany): Springer; [Google Scholar]
- Hammer M. The neural basis of associative reward learning in honeybees. Trends Neurosci. 1997;20:245–252. doi: 10.1016/s0166-2236(96)01019-3. [DOI] [PubMed] [Google Scholar]
- Hammer M, Menzel R. Multiple sites of associative odor learning as revealed by local brain microinjections of octopamine in honeybees. Learn Mem. 1998;5:146–156. [PMC free article] [PubMed] [Google Scholar]
- Ishimoto H, Tanimura T. Molecular neurophysiology of taste in Drosophila. Cell Mol Life Sci. 2004;61:10–18. doi: 10.1007/s00018-003-3182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel R, Heyne A, Kinzel C, Gerber B, Fiala A. Pharmacological dissociation between the reinforcing, sensitizing, and response-releasing functions of reward in honeybee classical conditioning. Behav Neurosci. 1999;113:744–754. [PubMed] [Google Scholar]
- Neuser K, Husse J, Stock P, Gerber B. Appetitive olfactory learning in Drosophila larvae: effects of repetition, reward strength, age, gender, assay type and memory span. Anim Behav. 2005;69:891–898. [Google Scholar]
- Python F, Stocker RF. Adult-like complexity of the larval antennal lobe of D. melanogaster despite markedly low numbers of odorant receptor neurons. J Comp Neurol. 2002;445:374–387. doi: 10.1002/cne.10188. [DOI] [PubMed] [Google Scholar]
- Schroll C, Riemensperger T, Bucher D, Ehmer J, Voller T, Erbguth K, Gerber B, Hendel T, Nagel G, Buchner E, et al. Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae. Curr Biol. 2006;16:1741–1747. doi: 10.1016/j.cub.2006.07.023. [DOI] [PubMed] [Google Scholar]