Abstract
The neurotrophin brain-derived neurotrophic factor (BDNF) has been implicated in the generation and differentiation of new olfactory sensory neurons (OSNs) and in the regulation of branching of OSN axons in their target glomeruli. However, previous reports of BDNF mRNA and protein expression in olfactory epithelium and olfactory bulb (OB) have been inconsistent, raising questions on the proposed roles for BDNF. Here, we report on β-galactosidase (β-gal) expression in adult gene-targeted mice where the BDNF promoter drives expression of the Escherichia coli lacZ gene (BDNFlacZneo mice). We find that β-gal is expressed in a small subset of OSNs with axons that reach the olfactory nerve layers throughout the OB. In the OB, we find expression of β-gal in γ-aminobutyric acidergic but not dopaminergic periglomerular cells and external tufted cells and in interneurons located in the mitral cell layer. Our results are inconsistent with the regulation of generation and differentiation of new OSNs elicited by the release of BDNF from horizontal basal cells. The results are consistent with a role for BDNF in competitive branching of OSN axons within the glomeruli of the OB.
Keywords: BDNF, neurotrophin, olfactory bulb, olfactory sensory neurons, periglomerular cells
Introduction
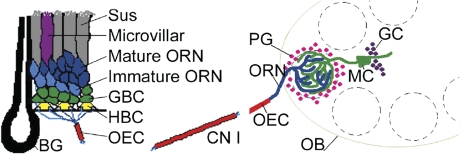
Brain-derived neurotrophic factor (BDNF) is a member of the neurotrophin family implicated in the survival and differentiation of neurons and in the outgrowth and pruning of neuronal processes (Lewin and Barde 1996; Lindsay 1996; Horch 2004). BDNF has been implicated in 2 important aspects of olfactory sensory neuron (OSN) cell biology. With a lifespan averaging 3 months, OSNs are among the few neuron types that have the ability to regenerate and differentiate to mature neurons in the adult animal (Graziadei and Metcalf 1971; Weiler and Farbman 1997). Based on expression of this neurotrophin in horizontal basal cells (HBCs) located adjacent to the lamina propria underlying the olfactory epithelium (OE) (Figure 1), BDNF has been postulated to play a role in the differentiation/generation of new OSNs (Buckland and Cunningham 1999). In addition, changes in axonal branching in heterozygous BDNF mice have led to the hypothesis that this neurotrophin participates in regulation of activity-dependent competition in branching of OSN axons within the glomerulus where they synapse with dendrites of mitral, tufted, and periglomerular (PG) cells (Cao et al. 2007).
Figure 1.
Cell populations of the OE and OB. Left panel: Diagrammatic representation of the OE. BG, Bowman's gland; CN I, cranial nerve I, the olfactory nerve; OEC, olfactory ensheathing cell; Sus, sustentacular cell. Right panel: Cartoon of the OB. GC, granule cell; MC, mitral cell. This figure was modified from a previously published image (Huard et al. 1998) reproduced with permission of Wiley-Liss Inc., a subsidiary of John Wiley and Sons, Inc.
Although these studies highlight the potential importance of BDNF in the cell biology of the OSN, inconsistent reports on BDNF expression call into question the proposed roles for this neurotrophin. Table 1 illustrates the discrepant results of past surveys of expression of BDNF protein and/or mRNA in the OE and olfactory bulb (OB). Whereas Buckland and Cunningham (1999) report strong HBC-specific BDNF protein expression, Takami et al. (2005) localize BDNF to mature OSNs by immunohistochemistry as well as in situ hybridization. In contrast, Nef et al. do not detect expression of BDNF mRNA in neonatal OE. Furthermore, they report that genetic ablation of BDNF expression does not affect the number of mature OSNs at birth (Nef et al. 2001). Reports on BDNF expression in the OB are similarly inconsistent. Whereas some investigators report BDNF expression in PG cells, consistent with a role for this neurotrophin in OSN axon branching within the glomerulus, other investigators either do not mention or report the absence of expression of BDNF in PG cells (Table 1). These inconsistencies may have arisen from the well-known problematic nature of BDNF immunohistochemistry or from the fact that BDNF is generated as a pro-protein from multiple RNA splice variants. In an effort to circumvent these issues, we report here on BDNF promoter–driven expression of the Escherichia coli lacZ gene (BDNFlacZneo mice) in adult gene-targeted mice. These mice express the lacZ marker irrespective of the final BDNF product and thus provide a high-resolution window into the gene expression pattern of BDNF without the putative pitfalls arising from the posttranslational processing of the BDNF product.
Table 1.
Published reports of BDNF expression in OE and OB
| Study | Method | BDNF expression in OE | BDNF expression in OB |
| Buckland and Cunningham (1999) | IHC in adult Wistar rats | Expression in HBCs | No expression of BDNF |
| Cao et al. (2007) | IHC in postnatal mice | Not reported | Expression in mitral and juxtaglomerular cells |
| Conner et al. (1997) | IHC and in situ in adult rat | Not reported | Mitral cells and periglomerular cells. |
| Deckner et al. (1993) | In situ in adult rat and cat | Not reported | Expression in granule cell layer, mitral cell layer, and periglomerular cells |
| Ernfors et al. (1990) | In situ adult rat | Not reported | No expression detected |
| Guthrie and Gall (1991) | In situ in adult rat | Not reported | Expression in the granule cell layer |
| Hofer et al. (1990) | In situ in adult mouse | Not reported | Expression in granule cell layer, mitral cell layer, and periglomerular cells |
| Kawamoto et al. (1996) | IHC in adult rat | Not reported | Labeling in glomerular and granule cell layer |
| McLean et al. (2001) | Western blot and IHC in P30–40 rats | Not reported | Expression in the internal part of the mitral cell layer in Cajal and horizontal cells (but not in mitral cells). Expression is mentioned—but not shown—in some cells in the granule cell layer and in the external plexiform layer |
| Nef et al. (2001) | In situ in mouse neonates | No mRNA expression | Expression in the granule cell layer |
| Phillips et al. (1990) | In situ adult rat | Not reported | No expression detected |
| Takami et al. (2005) | IHC and in situ in adult rats | Expression in mature OSNs | Not reported |
IHC, immunohistochemistry.
Methods
Animals
Adult male mice 2–8 months old were used for all experiments. Animals were housed in groups of no more than 5 animals per cage, maintained in a 12:12 light:dark cycle, and given food and water ad libitum. BDNFlacZneo mice are animals in which the BDNF allele has been replaced by the E. coli lacZ gene (Bennett et al. 1999; Yee et al. 2003). These BDNFlacZneo mice were generated by inserting the lacZ gene into the BDNF protein–coding exon such that the β-galactosidase (β-gal) translation initiation codon precisely substitutes for the BDNF initiation codon. Thus, this lacZ insertion reports expression from all the BDNF promoters that drive expression of the BDNF-coding exon. We used heterozygous BDNFlacZneo (+/−) mice and their BDNFlacZneo negative siblings (+/+) (as controls) in all experiments. BDNFlacZneo mice were maintained by crossing BDNFlacZneo heterozygous male mice to C57BL/6 female mice (Jax Mice, Bar Harbor, ME). The C57BL/6 inbred strain was chosen because it was the background strain for the BDNFlacZneo mice. Genotyping was performed by amplification of genomic DNA by polymerase chain reaction. All procedures were performed under protocols approved by the animal care and use committee of the University of Colorado Denver.
Immunohistochemistry
Mice were anesthetized with 20% chloral hydrate in 0.9% saline and perfused transcardially with saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer (PB), pH 7.4. The brain and OE were dissected and postfixed for 3 h, followed by overnight cryoprotection in 0.1 M PB containing 20% sucrose. Tissue was embedded in optimum cutting temperature (OCT) embedding compound and cut in 14 μm sections directly onto slides or in 30 μm floating sections. Tissue sections were incubated in blocking serum (0.3% Triton X-100, 1% bovine serum albumin, and 1% normal horse serum) for 60 min at room temperature. Antibodies, listed in Table 2, were diluted to the appropriate concentration using blocking serum, and the tissue was incubated with primary antibodies overnight at 4 °C. Sections were washed for 3 × 10 min in phosphate-buffered saline. Tissue was incubated with secondary antibodies for 1 h at room temperature. Sections were then washed for 3 × 10 min in 0.1 M PB, mounted on slides if appropriate, and coverslipped with fluoromount (SouthernBiotech, Birmingham, AL).
Table 2.
Primary and secondary antibodies
| Fluorophore | Target | Host | Concentration | Source |
| CGRP | Rabbit | 1:1000 | Peninsula Laboratories | |
| β-gal | Guinea pig | 1:1000 | Drs Tom Finger and Cindy Yee (Yee et al. 2003) | |
| β-gal | Rabbit | 1:1000 | Cappel | |
| GAD67 | Rabbit | 1:1000 | Chemicon | |
| GFAP | Mouse | 1:400 | Sigma-Aldrich | |
| PGP 9.5 | Rabbit | 1:1000 | Biogenesis | |
| TH | Rabbit | 1:2000 | Calbiochem | |
| Fluorescein Isothiocyanate (FITC) | Chicken IgY | Donkey | 1:200 | Jackson Laboratories |
| Rhodamine | Chicken IgY | Donkey | 1:200 | Jackson Laboratories |
| Rhodamine | Goat | Donkey | 1:200 | Jackson Laboratories |
| Alexa 488 | Goat | Donkey | 1:200 | Molecular Probes |
| Rhodamine | Guinea pig | Donkey | 1:200 | Jackson Laboratories |
| Alexa 488 | Guinea pig | Goat | 1:200 | Molecular Probes |
| Alexa 568 | Mouse | Goat | 1:200 | Molecular Probes |
| Alexa 488 | Rabbit | Goat | 1:200 | Molecular Probes |
| Alexa 568 | Rabbit | Goat | 1:200 | Molecular Probes |
TH, tyrosine hydroxylase.
Three sets of immunohistochemistry control experiments were completed. First, primary antibody was omitted to determine background fluorescence of the tissue and nonspecific binding of secondary antibodies. Second, in order to ensure that the β-gal antibodies did not bind to cells in which no β-gal was expressed, we demonstrated no endogenous β-gal immunoreactivity in OE and OB in BDNFlacZneo negative mice (data not shown). Third, we demonstrated ubiquitous β-gal immunoreactivity in mice in which the ROSA26 promoter drives expression of the lacZ gene (Jax Mice) (data not shown).
Finally, although one β-gal antibody is commercially produced and the other's specificity has been published (Yee et al. 2003), we performed an additional control experiment in which we analyzed β-gal immunoreactivity using both the rabbit and the guinea pig antibodies on the same BDNFlacZneo tissue to demonstrate precise correlation of the immunoreactivity for the 2 antibodies (data not shown). Because we showed precise correlation between the 2 β-gal antibodies, all figures included below are images using the guinea pig antibody. This was done for consistency, and the guinea pig antibody was chosen over the rabbit antibody because most of the antibodies used for double-labeling experiments were also developed in rabbits.
Colorimetric detection of β-gal
For whole-mount colorimetric detection of β-gal (Figure 8), BDNFlacZneo mice and BDNFlacZneo negative sibling controls were euthanized by CO2 inhalation. The brain and OE were quickly isolated, and β-gal was detected with the X-gal assay kit from Specialty Media (Phillipsburg, NJ), which results in the cleavage of 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-gal) to produce a blue reaction product. Briefly, tissue was placed in tissue fixative solution for 45 min on ice. Following a brief rinse in rinse solution A, tissue was incubated in rinse solution A for 30 min at room temperature. It was then rinsed briefly in rinse solution B, followed by a 5-min incubation with solution B. After rinsing, tissue was placed in β-gal tissue stain solution (1:40 X-gal stock solution in tissue stain base solution) for 5 h at 37 °C.
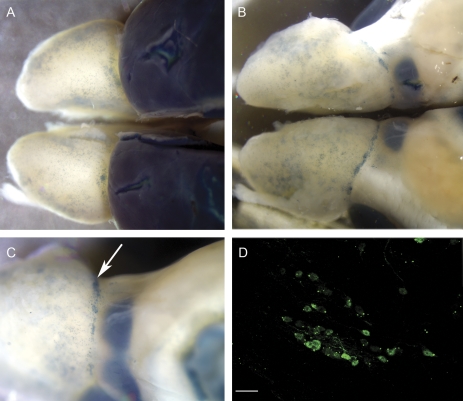
Figure 8.
Whole mount showing β-gal-reactive cells in blue. Notice the variability of β-gal-positive juxtaglomerular cell density throughout the OB surface. (A) Dorsal view of BDNFlacZneo mouse OBs and rostral cortex processed with X-gal reaction. (B) Ventral view. (C) Ventromedial view. Arrow indicates putative necklace glomeruli. (D) β-gal immunohistochemistry of putative necklace glomerulus. Scale bar: 20 μm.
Imaging and determination of zonal expression patterns in the OE
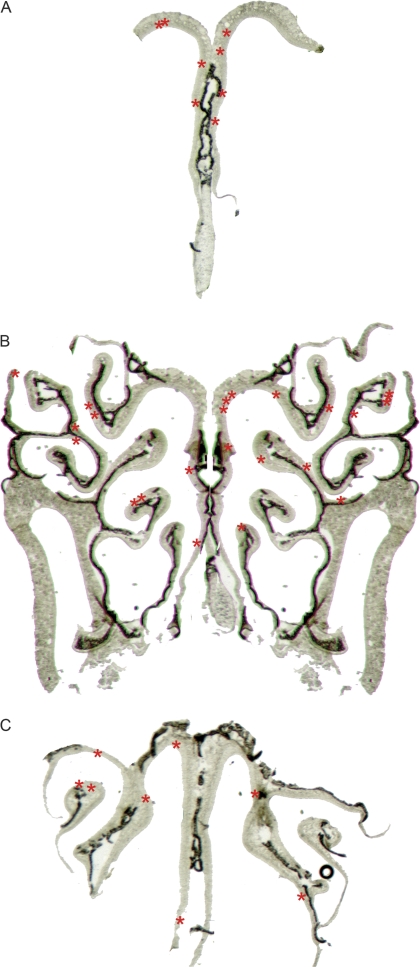
Light microscopic images of whole-mount OB and entire OE tissue sections were taken using a Spot RT camera in an Olympus SZX12 light microscope. Fluorescence images in tissue sections were acquired using an Olympus Fluoview laser-scanning confocal microscope. For double-labeling experiments, z-stack images were taken. In order to assess the presence of potential zonal expression patterns of BDNF in the OE, β-gal-positive cells were surveyed using epifluorescence in the Olympus Fluoview microscope in sections spaced 200 μm apart, and their location was drawn by hand onto low-magnification–transmitted light photographs encompassing the entire section (Figure 3).
Figure 3.
Diagram showing the distribution of β-gal-positive cells in the adult mouse OE. A red asterisk (*) denotes the location of a β-gal-positive cell. (A) Anterior OE. (B) Approximately 800 μm caudal to (A). (C) Posterior OE, approximately 800 μm caudal to (B).
Results
β-Gal expression in the OE of BDNFlacZneo mice
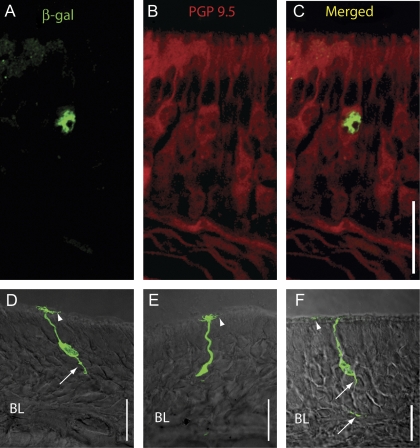
β-Gal immunoreactivity was assessed visually in every section throughout the OE. We did not detect β-gal immunoreactivity in HBCs or in a large number of OSNs. However, a small number of β-gal-immunoreactive cells were found sparsely scattered throughout the OE (Figure 2). In order to identify the types of OE cells that express β-gal, we relied on cell morphology, location of the cell soma, and double labeling for cell-specific markers. Most β-gal-positive cells were identified as mature OSNs using one or more of the following markers: 1) the location of the soma in the middle of the OE, 2) the presence of a single positive axon, 3) the presence of positive dendrites, and 4) double labeling with the neuron-specific marker protein gene product (PGP) 9.5 (Figure 2). In sections that were double labeled with β-gal and PGP 9.5, all cells positive for β-gal also expressed PGP 9.5 (Figure 2A–C). In a few β-gal-positive cells where the immunoreactivity extended beyond the soma, we found both β-gal-immunoreactive cilia (arrowheads) and axonal processes (arrows) (Figure 2D–F). A minority of β-gal-positive cells (5 of several hundred positive cells) were located in the basal third of the epithelium, were rounded in shape, and had no identifiable processes. These were tentatively identified as globose basal cells (GBCs, not shown). However, due to the extremely small number of these GBC-like cells found, we did not double label with a GBC-specific marker. We surveyed the β-gal-immunoreactive samples for potential spatial patterns of expression but found no obvious zonal expression pattern (Figure 3). We obtained similar sparse labeling of a few cells within the OE when we stained the samples using the X-gal reaction. No cells were found to be positive using either immunoreactivity or the X-gal reaction in BDNFlacZneo negative control mice, nor were immunoreactive cells present when the primary antibody was omitted (data not shown).
Figure 2.
β-Gal immunoreactivity in the OE. (A–C) Double-label immunohistochemistry with β-gal antibody (green) and PGP 9.5, a marker for mature OSNs (red). (A) β-gal alone. (B) PGP 9.5 alone. (C) Merged image of (A) and (B). (D–F) Images of β-gal-positive cells where immunoreactivity was found throughout the cell. BL: basal lamina. Scale bars: 20 μm. Arrowheads denote cilia, and arrows point to axonal processes.
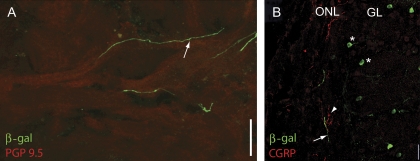
Axons of β-gal-positive OSNs are found in the olfactory nerve
In OSNs expressing the β-gal marker throughout the cytoplasm, we found β-gal-positive basal axonal processes (arrows in Figure 2D,F). Although we were not able to follow a single process from the OE to the OB, we did find processes in nerve bundles underlying the OE (not shown) and in the olfactory nerve (Figure 4). The putative OSN axons traversed the olfactory nerve following the sinuous trajectories of PGP 9.5–positive OSN axon bundles (Figure 4A). However, unlike axons from OSNs expressing a particular olfactory receptor, β-gal-positive fibers did not coalesce in a single glomerulus. Instead, we found these fibers scattered throughout the olfactory nerve layer (ONL) overlaying the glomerular layer (Figure 4B). The β-gal-positive fibers did not express calcitonin gene-related peptide (CGRP), a marker for trigeminal fibers (Figure 4B). We did not see evidence for axonal branching within any given glomerulus.
Figure 4.
β-gal-positive fibers in the olfactory nerve. (A) β-gal-positive fibers (green) traverse the olfactory nerve layer following the route of PGP 9.5–positive fibers (red). (B) Image of the OB nerve layer and the glomerular layer. CGRP in red; β-gal in green. Notice the lack of overlap of the CGRP and β-gal immunoreactivity. Asterisks mark β-gal-positive PG cells surrounding the glomeruli. Scale bar 20 μm.
β-Gal expression in the OB
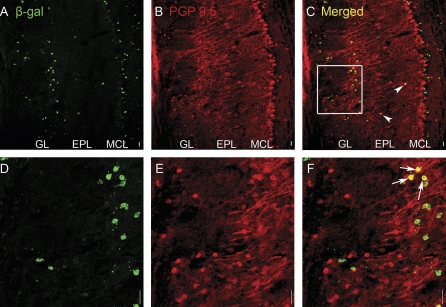
We detected β-gal immunoreactivity in a subset of OB cells. In order to identify cell types expressing β-gal, we used cell morphology, location of the cell soma, and double labeling for cell-specific markers. We identified mitral cells in PGP 9.5–labeled sections due to the location of their somata in the mitral cell layer, their large cell bodies, and PGP 9.5–immunoreactive primary dendrites (Figure 5B). Although we did not find a single mitral cell immunoreactive for β-gal, we did find some cells with the β-gal label in the internal part of the mitral cell layer. However, due to the small size of their somata and the lack of PGP 9.5 immunoreactivity, these cells are presumably interneurons. Approximately 20% of β-gal-positive juxtaglomerular cells were also immunoreactive to PGP 9.5 (Figure 5F, arrows). These cells typically bordered the external plexiform layer (EPL) and are likely external tufted cells. Finally, a few somata, presumably from middle tufted cells, were double labeled in the EPL (Figure 5C, arrowheads).
Figure 5.
Double-label immunohistochemistry (IHC) for PGP 9.5 (red) and β-gal (green) in the OB of BDNFlacZneo heterozygous mice. (A, D) β-gal IHC (green). (B, E) PGP 9.5 IHC (red). (C, F) Combined β-gal/PGP 9.5 images. (A) to (C) are lower magnification than (D) to (F). Arrows indicate PGP 9.5+/β-gal+ juxtaglomerular cells. Arrowheads indicate PGP 9.5+/β-gal+ cells in the EPL. Square in (C) indicates region shown in (D) to (F). Scale bar: 20 μm.
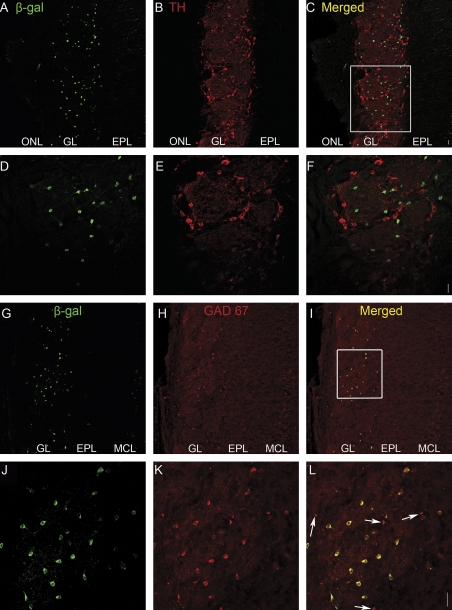
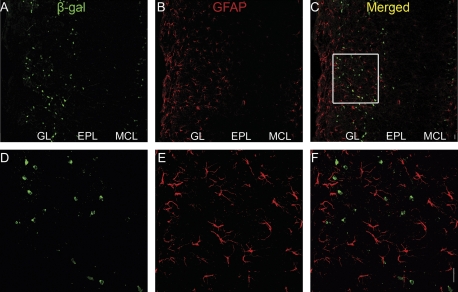
Due to the heterogeneous nature of juxtaglomerular cells, we sought to determine which type of juxtaglomerular cell expresses the β-gal marker. We used immunocytochemical markers known to label subsets of PG cells (Parrish-Aungst et al. 2007). We never found β-gal-positive cells that co-labeled with tyrosine hydroxylase, a marker for dopaminergic neurons (Figure 6A–F), or glial fibrillary acidic protein (GFAP), a marker for astrocytes (Figure 7). In contrast, we found a large population of β-gal-positive cells that did co-label with γ-aminobutyric acid decarboxylase 67 (GAD67), a marker of a subset of γ-aminobutyric acidergic (GABAergic) PG cells (Figure 6G–L). Note that not all the PG cells were immunoreactive for GAD67.
Figure 6.
Double-label immunohistochemistry (IHC) for β-gal in green and either tyrosine hydroxylase (TH, A through F) or GAD67 (G through L) in red in the OB of BDNFlacZneo heterozygous mice. TH and GAD67 are markers for 2 different populations of PG cells. (A, D) β-gal IHC (green). (B, E) Corresponding TH IHC (red). (C, F) Combined β-gal/TH images. Square in (C) indicates region shown in (D) to (F). (G, J) β-gal IHC (green). (H, K) Corresponding GAD67 IHC (red). (I, L) Combined β-gal/GAD67 images. Arrows indicate β-gal-negative/GAD67-positive cells. Square in (C) indicates region shown in (D) to (F). Scale bar: 20 μm.
Figure 7.
Immunohistochemistry (IHC) for GFAP and β-gal in the OB of BDNFlacZneo heterozygous mice. (A, D) β-gal IHC (green). (B, E) GFAP IHC (red). (C, F) Combined β-gal/GFAP images. Square in (C) indicates region shown in (D) to (F). Scale bar: 20 μm.
The number of β-gal-positive juxtaglomerular cells varied widely between glomeruli. In order to survey patterns of PG cell–specific β-gal expression, we performed a colorimetric detection of β-gal expression in whole-mount OBs. A visual inspection of these bulbs revealed loose clusters of β-gal-positive juxtaglomerular cells all along the surface of the OB with a dense ring of cells at the posterior end of the dorsal OB (Figure 8A). These structures, which could be necklace glomeruli, were also visible in the posterior ventral OB (Figure 8B,C). Further work is necessary to define the identity of these structures.
Discussion
The role of BDNF in the main olfactory system remains controversial primarily due to the well-known reactivity problems of the BDNF antibodies (Bennett et al. 1999; Yee et al. 2003) and the complex mRNA splicing and posttranslational processing of the BDNF molecule itself. In this study, we characterize BDNF gene expression by using the highly sensitive method of detecting lacZ in a heterozygous BDNF-lacZ mouse in which one allele directs the expression of β-gal under the control of the BDNF promoter. In these mice, we find evidence for BDNF expression in a subset of GABAergic PG and external tufted cells in the OB. These results are consistent with proposed modulation by BDNF of activity-dependent competition in axonal branching among OSNs within the glomeruli (Cao et al. 2007). Consistent with this premise, mice deficient in the low-affinity BDNF receptor p75(NTR) display aberrant axonal branching resulting in the formation of extraneous glomeruli (Tisay et al. 2000).
Contrary to previous reports of BDNF antibody immunoreactivity, we do not find expression of β-gal in the majority of OSNs or in HBCs (Buckland and Cunningham 1999; Takami et al. 2005). Rather we find β-gal expressed in a small subset of mature OSNs distributed throughout the epithelium. These results do not support a proposed role for BDNF in the differentiation and generation of new OSNs which is based on the previous report of widespread expression of BDNF in HBCs (Buckland and Cunningham 1999). Instead, our results are consistent with the reports that the number of OSNs does not change in mice deficient for BDNF and that BDNF is not expressed in the OE in neonatal mice (Nef et al. 2001).
Although we found BDNF expression in OSNs to be far from comprehensive, the broad distribution of β-gal-positive axons along the entire surface of the OB indicates that BDNF expression was not limited to single OSN type expressing a single odorant receptor. As any given bulb, after animal sacrifice, can represent only a single snapshot in the time of BDNF expression, we cannot ignore the possibility that BDNF is transiently expressed during a stage of OSN maturation. As such, BDNF may in fact enjoy a broader expression in OSNs over the lifetime of an animal. Indeed, it is curious that β-gal-labeled axons that originate in OSNs expressing the mature neuron marker PGP 9.5 approach the glomeruli but do not branch within them. One interpretation of this finding would be that shortly after maturation, an OSN undergoes a surge in BDNF expression when the axon approaches the target glomerulus. An abrupt and transient onset of BDNF expression would be consistent with the fact that in some OSNs, lacZ expression is confined to the cell body, whereas in others it spreads to the axon reaching the OB (Figure 2). Although speculative, considering the current available data, this potential explanation for BDNF expression in a subset of OSNs warrants future investigation.
Funding
National Institutes of Health to D.R., E.S., and A.C.
Acknowledgments
We would like to thank Drs Tom Finger and Cindy Yee for providing a β-gal antibody generated in guinea pig.
References
- Bennett JL, Zeiler SR, Jones KR. Patterned expression of BDNF and NT-3 in the retina and anterior segment of the developing mammalian eye. Invest Ophthalmol Vis Sci. 1999;40:2996–3005. [PubMed] [Google Scholar]
- Buckland ME, Cunningham AM. Alterations in expression of the neurotrophic factors glial cell line-derived neurotrophic factor, ciliary neurotrophic factor and brain-derived neurotrophic factor, in the target-deprived olfactory neuroepithelium. Neuroscience. 1999;90:333–347. doi: 10.1016/s0306-4522(98)00270-x. [DOI] [PubMed] [Google Scholar]
- Cao L, Dhilla A, Mukai J, Blazeski R, Lodovichi C, Mason CA, Gogos JA. Genetic modulation of BDNF signaling affects the outcome of axonal competition in vivo. Curr Biol. 2007;17:911–921. doi: 10.1016/j.cub.2007.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner JM, Lauterborn JC, Yan Q, Gall CM, Varon S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J Neurosci. 1997;17:2295–2313. doi: 10.1523/JNEUROSCI.17-07-02295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckner ML, Frisen J, Verge VM, Hokfelt T, Risling M. Localization of neurotrophin receptors in olfactory epithelium and bulb. Neuroreport. 1993;5:301–304. doi: 10.1097/00001756-199312000-00030. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Wetmore C, Olson L, Persson H. Identification of cells in rat brain and peripheral tissues expressing mRNA for members of the nerve growth factor family. Neuron. 1990;5:511–526. doi: 10.1016/0896-6273(90)90090-3. [DOI] [PubMed] [Google Scholar]
- Graziadei PP, Metcalf JF. Autoradiographic and ultrastructural observations on the frog's olfactory mucosa. Z Zellforsch Mikrosk Anat. 1971;116:305–318. doi: 10.1007/BF00330630. [DOI] [PubMed] [Google Scholar]
- Guthrie KM, Gall CM. Differential expression of mRNAs for the NGF family of neurotrophic factors in the adult rat central olfactory system. J Comp Neurol. 1991;313:95–102. doi: 10.1002/cne.903130107. [DOI] [PubMed] [Google Scholar]
- Hofer M, Pagliusi SR, Hohn A, Leibrock J, Barde YA. Regional distribution of brain-derived neurotrophic factor mRNA in the adult mouse brain. EMBO J. 1990;9:2459–2464. doi: 10.1002/j.1460-2075.1990.tb07423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horch HW. Local effects of BDNF on dendritic growth. Rev Neurosci. 2004;15:117–129. doi: 10.1515/revneuro.2004.15.2.117. [DOI] [PubMed] [Google Scholar]
- Huard JM, Youngentob SL, Goldstein BJ, Luskin MB, Schwob JE. Adult olfactory epithelium contains multipotent progenitors that give rise to neurons and non-neural cells. J Comp Neurol. 1998;400:469–486. [PubMed] [Google Scholar]
- Kawamoto Y, Nakamura S, Oka N, Akiguchi I, Kimura J. Immunohistochemical localization of brain-derived neurotrophic factor in adult rat brain. Neuroscience. 1996;74:1209–1226. doi: 10.1016/0306-4522(96)00245-x. [DOI] [PubMed] [Google Scholar]
- Lewin GR, Barde YA. Physiology of the neurotrophins. Annu Rev Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- Lindsay RM. Role of neurotrophins and trk receptors in the development and maintenance of sensory neurons: an overview. Philos Trans R Soc Lond B Biol Sci. 1996;351:365–373. doi: 10.1098/rstb.1996.0030. [DOI] [PubMed] [Google Scholar]
- McLean JH, rby-King A, Bonnell WS. Neonatal olfactory sensory deprivation decreases BDNF in the olfactory bulb of the rat. Brain Res Dev Brain Res. 2001;128:17–24. doi: 10.1016/s0165-3806(01)00144-4. [DOI] [PubMed] [Google Scholar]
- Nef S, Lush ME, Shipman TE, Parada LF. Neurotrophins are not required for normal embryonic development of olfactory neurons. Dev Biol. 2001;234:80–92. doi: 10.1006/dbio.2001.0240. [DOI] [PubMed] [Google Scholar]
- Parrish-Aungst S, Shipley MT, Erdelyi F, Szabo G, Puche AC. Quantitative analysis of neuronal diversity in the mouse olfactory bulb. J Comp Neurol. 2007;501:825–836. doi: 10.1002/cne.21205. [DOI] [PubMed] [Google Scholar]
- Phillips HS, Hains JM, Laramee GR, Rosenthal A, Winslow JW. Widespread expression of BDNF but not NT3 by target areas of basal forebrain cholinergic neurons. Science. 1990;250:290–294. doi: 10.1126/science.1688328. [DOI] [PubMed] [Google Scholar]
- Takami S, Hasegawa R, Nishiyama F. The roles of brain-derived neurotrophic factor in the development of nasal chemoreceptor neurons. Chem Senses. 2005;30(Suppl 1):i121–i122. doi: 10.1093/chemse/bjh144. [DOI] [PubMed] [Google Scholar]
- Tisay KT, Bartlett PF, Key B. Primary olfactory axons form ectopic glomeruli in mice lacking p75NTR. J Comp Neurol. 2000;428:656–670. doi: 10.1002/1096-9861(20001225)428:4<656::aid-cne6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Weiler E, Farbman AI. Proliferation in the rat olfactory epithelium: age-dependent changes. J Neurosci. 1997;17:3610–3622. doi: 10.1523/JNEUROSCI.17-10-03610.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee CL, Jones KR, Finger TE. Brain-derived neurotrophic factor is present in adult mouse taste cells with synapses. J Comp Neurol. 2003;459:15–24. doi: 10.1002/cne.10589. [DOI] [PubMed] [Google Scholar]