ABSTRACT
Objective: Surgery is a cornerstone of treatment for a wide variety of neoplastic, congenital, traumatic, and inflammatory lesions involving the midline anterior skull base and may result in a significant anterior skull base defect requiring reconstruction. This study is a retrospective analysis of the reconstruction techniques and complications seen in a series of 58 consecutive patients with midline anterior skull base pathology treated with craniotomy or a craniofacial approach. The complication rates in this series are compared with other retrospective series and specific techniques that may reduce complications are then discussed. Design: This is a retrospective analysis of 58 consecutive patients who had surgery for a midline anterior skull base lesion between January 1994 and July 2003. Data were collected regarding pathology, surgical approach, reconstruction technique, and complications. Results: Twenty-nine patients underwent surgery for a meningioma (50%). The remainder had frontoethmoidal cancer, mucoceles/invasive nasal polyps, encephalocele, esthesioneuroblastoma, anterior falx dermoid cyst with a nasal sinus tract, or invasive pituitary adenoma. In most patients, a low and narrow two-piece biorbitofrontal craniotomy was used. When possible, the dura was repaired before entering the nasal cavity. Thirteen patients experienced a complication (22%). There was one case of postoperative cerebrospinal fluid (CSF) leak (2%), one case of meningitis (2%), two cases of bone flap infection (3%), and two cases of symptomatic pneumocephalus (3%). There were no deaths, no reoperations for CSF leak, and no patient had a new permanent neurologic deficit other than anosmia. Conclusions: Transcranial approaches for midline anterior skull base lesions can be performed safely with a low incidence of postoperative CSF leak, meningitis, bone flap infection, and symptomatic pneumocephalus. Our results, particularly with regard to CSF leakage, compare favorably with other retrospective series.
Keywords: Anterior skull base, cerebrospinal fluid leak, complications, craniofacial approach, meningioma, skull base surgery
Lesions involving the anterior skull base with intracranial and nasal extension were once considered inoperable and had a correspondingly grave prognosis. In 1963 Ketcham et al1 popularized treating these lesions via a craniofacial approach, combining a craniotomy with a facial incision. This combination approach has been applied to a wide variety of pathology including benign and malignant neoplasms, encephaloceles, mucoceles, and traumatic anterior skull base injury. Since Ketcham's article, numerous reports have described skull base surgical approaches to the midline anterior skull base.2,3,4 With these approaches, the intracranial contents are exposed to a contaminated space; a ready path for cerebrospinal fluid (CSF) drainage into the nasopharynx is created by virtue of the skull base defect. Recently, endonasal approaches have been used as an alternative to cranial and craniofacial approaches for these lesions. The obvious drawback of the endonasal approach is the difficulty in reconstructing skull base and dural defects when the approach is used for intradural pathology. With any approach a substantial part of the surgical plan must address reconstruction of the anterior skull base to avoid postoperative CSF leakage, meningitis, bone flap infection, and pneumocephalus.
A wide variety of techniques have been employed for reconstruction of the anterior skull base with varying degrees of complexity. Ketcham et al1 used a split-thickness skin graft on the dura with no reconstruction of the skull base. Others have used frontal skin flaps5,6 or galea-pericranial flaps.7,8,9 While some authors have stressed that mechanical skull base support is not needed,10 others have emphasized the use of mechanical support in the skull base defect such as rib or iliac autograft8 or a coral plate.11 Other materials in current use include titanium mesh,12,13 vascularized outer-table calvarial flaps,14,15 and hydroxyapatite bone cement. Though it is generally accepted that a pedicled anterior-based pericranial flap is an integral component to reconstruction, there is no consensus as to its configuration or regarding the use of structural supports in the skull base defect.
CSF leakage is often a problem following surgery of the anterior skull base where the dura must be opened. Major series of craniofacial resections where complications were reported in depth have CSF leak rates from 3 to 20% with a mean of between 8% and 10%.16,17,18,19,20,21,22,23,24,25,26,27 In addition, postoperative meningitis occurs in 1 to 10% percent of cases.16,17,18,19,20,21,22,23,24,25,26,27 Infections of the bone flap, epidural, or subdural space all may occur following craniofacial resection and cause significant morbidity and mortality. A review of craniofacial resection for malignancy found that the majority of operative mortality among several large series occurred from meningitis or intracranial abscess.28 Systemic complications such as cardiac events or pulmonary embolism were the next most common cause of operative mortality. Therefore infection is by far the leading cause of operative mortality. Less common but still problematic complications are symptomatic pneumocephalus and postoperative hematoma. In this study we present algorithms for approach selection and reconstruction in midline anterior skull base surgery. We then focus on the prevention of difficult postoperative complications.
PATIENTS AND METHODS
Between January of 1994 and September of 2003, 58 consecutive patients underwent surgery for midline anterior skull base pathology. Medical records, office charts, operative notes, operative photographs, and imaging studies were reviewed retrospectively. Lesion location was classified by the surgical team based on preoperative imaging studies and intraoperative findings. All patients had histologic confirmation of the pathologic lesion. Traumatic CSF fistulas and meningiomas arising from the anterior clinoid or sphenoid wing were excluded.
RESULTS
Operative and clinical records were available in all patients. The mean follow-up was 25.8 months (range, 1 to 95 months; median, 19 months). No patients were lost to follow-up. The pathology of the lesions is demonstrated in Tables 1 and 2. Meningioma was the most common pathologic entity, accounting for ~50% of all lesions. Though the vast majority of meningiomas considered in this series arose in the true midline, three arose from the medial orbital roof. Malignant lesions (esthesioneuroblastoma and frontoethmoidal cancers) accounted for 28% of our series, non-neoplastic lesions for 17%, dermoid cysts for 3%, and invasive pituitary adenoma for ~2%.
Table 1.
Lesion Pathology
| Meningioma | 29 |
| Frontoethmoidal cancer | 12 |
| Esthesioneuroblastoma | 4 |
| Encephalocele | 4 |
| Nasofrontal mucocele | 6 |
| Dermoid | 2 |
| Invasive pituitary adenoma | 1 |
Table 2.
Frontoethmoidal Cancer Pathology
| Adenocarcinoma | 1 |
| Adenoid cystic carcinoma | 3 |
| Fibrosarcoma | 2 |
| Neuroendocrine carcinoma | 1 |
| Sinonasal undifferentiated carcinoma | 3 |
| Squamous cell carcinoma | 2 |
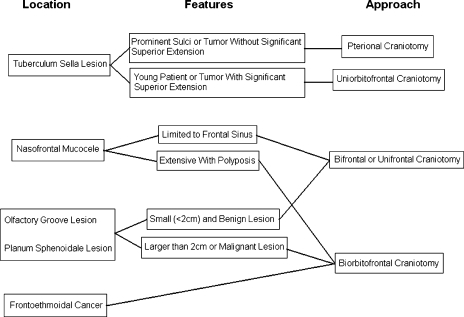
The choice of approach and type of craniotomy depended on the location and anatomy of the individual lesion. The approaches used are listed in Table 3 and an algorithm for their selection is presented in Fig. 1. The biorbitofrontal approach was the most commonly employed and is the most versatile approach. Bifrontal and unifrontal craniotomies were employed without an orbital osteotomy in selected cases involving small tumors when exposure without an orbital osteotomy was excellent. Pterional craniotomies were used only for tuberculum sella meningiomas. A facial incision was used only once; this patient had two separate radiation-induced tumors, an olfactory groove meningioma and a nasal sarcoma. The nasal incision was used for the nasal cavity sarcoma.
Table 3.
Approaches Utilized
| Biorbitofrontal | 34 |
| Bifrontal | 8 |
| Pterional | 6 |
| Uniorbitofrontal | 5 |
| Unifrontal | 5 |
Figure 1.
Algorithm for selection of surgical approach.
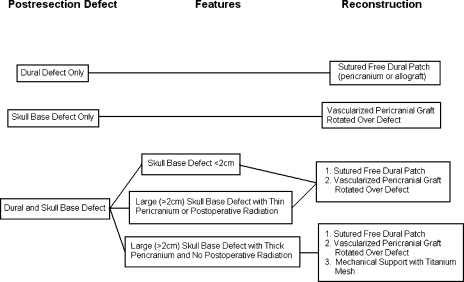
The reconstruction methods used were varied, but fall into several major categories as shown in Table 4. Our reconstruction technique evolved somewhat over the course of the series; our current reconstruction algorithm is depicted in Fig. 2. A vascularized pericranial flap rotated over the skull base defect and sutured to the dura posterior to the primary dural repair was the foundation of our reconstructions and was used in 90% of cases. It was not used only when it was unavailable due to previous surgery. We used the vascularized pericranial flap alone in 31% of cases when the dura could be closed primarily or when the dura was not involved. This was usually the case with encephaloceles and mucoceles. More often, a substantial dural defect was created and dural closure without excessive tension necessitated a dural patch. We used a variety of materials to patch the dura over the course of the series. In general, we used a free pericranial graft from the parietal area when available. If free pericranium was not available, we used autologous temporal fascia, AlloDerm (LifeCell Corp., Branchburg, NJ), or cadaveric fascia lata with success. When the skull base defect was greater than 2 cm wide, we performed a mechanical skull base reconstruction with vascularized outer-table bone or titanium mesh in addition to the vascularized pericranial flap. In three patients a vascularized pericranial flap was not available due to previous surgery or radiation. The reconstructions for these patients were performed using a free tissue flap, inner-table bone graft, and a dural patch, respectively.
Table 4.
Reconstruction Methods
| Vascularized pericranium only | 18 |
| Free dural patch and vascularized pericranium | 18 |
| Vascularized outer-table bone graft | 7 |
| Titanium mesh and vascularized pericranium | 9 |
| Other | 6 |
Figure 2.
Algorithm for reconstruction technique.
Complications occurred in 13 patients in our series (22%) and are presented in Table 5. Two patients had more than one complication. Pneumocephalus occurred in two patients. In both of these cases, lumbar spinal drainage was used intraoperatively and continued postoperatively. One patient who experienced a postoperative seizure also had symptomatic pneumocephalus while another two patients had a postoperative seizure without pneumocephalus. A postoperative subdural hematoma occurred in one patient and required burr-hole drainage. This patient also developed meningitis and resulting hydrocephalus which required a ventriculoperitoneal shunt. A postoperative middle cerebral artery stroke occurred in one patient, who made a full recovery. Two patients had complications related to the nasal skin: one patient who had had a previous craniofacial resection with a nasal skin incision followed by radiation developed dehiscence of the facial incision; another patient had carcinoma infiltrating into the skin of the nose and medial canthus and developed a defect following radiation treatment. There were no deaths, one transient CSF leak treated with lumbar drainage, and no new permanent neurologic deficits other than anosmia. No patients underwent readmission for CSF leak. There were no reoperations for CSF leak. There were no deaths.
Table 5.
Complications
| Number | Percent | |
|---|---|---|
| Patients experiencing a complication | 13 | 22% |
| Cerebrospinal fluid leak | 1 | 2% |
| Meningitis | 1 | 2% |
| Symptomatic pneumocephalus | 2 | 3% |
| Bone flap infection | 2 | 3% |
| Seizure | 2 | 3% |
| Subdural hematoma | 1 | 2% |
| Pneumonia | 1 | 2% |
| Pulmonary embolism | 2 | 3% |
| Cerebrovascular accident | 1 | 2% |
| Facial incision dehiscence | 2 | 3% |
| Death | 0 | 0% |
| Permanent deficit other than anosmia | 0 | 0% |
We also analyzed the complication rate in the subgroup of 29 patients who underwent meningioma resection. The results are summarized in Table 6. Four of these patients experienced a complication, yielding an overall complication rate of 14%. There were no CSF leaks, deaths, or permanent deficits other than anosmia.
Table 6.
Complications in Meningioma Subgroup
| Number | Percent | |
|---|---|---|
| Patients experiencing a complication | 4 | 14% |
| Cerebrospinal fluid leak | 0 | 0% |
| Meningitis | 1 | 3% |
| Symptomatic pneumocephalus | 1 | 3% |
| Seizure | 2 | 6% |
| Subdural hematoma | 1 | 3% |
| Pulmonary embolism | 1 | 3% |
| Death | 0 | 0% |
| Permanent deficit other than anosmia | 0 | 0% |
DISCUSSION
Skull base surgery techniques have improved the treatment of anterior skull base pathology by cranial and craniofacial approaches. Recent surgical series have reported CSF leak rates of 3 to 20% and operative mortality of 2 to 10%,16,17,18,19,20,21,22,23,24,25,26,27 with bacterial meningitis as a major source of mortality. In this series, there was only one transient CSF leak. There were two cases of bone flap infection and two cases of symptomatic pneumocephalus. There was no permanent neurologic morbidity and no operative mortality. We believe that most complications in anterior skull base surgery are related to bacterial contamination at the time of surgery or inadequate dural and skull base reconstruction. Infectious complications likely occur from one or a combination of these factors. Therefore, our surgical technique has been adapted to minimize intraoperative bacterial contamination of the intracranial space as well as provide a sound, watertight skull base reconstruction.
Exposure
Meningiomas of the tuberculum sella and medial orbital roof were usually approached through a unilateral frontal approach. Either a pterional craniotomy or an orbitofrontal craniotomy was used. Our preference was to use a pterional craniotomy unless the tumor had significant superior extension between the frontal lobes, or there was significant peritumoral edema. In this circumstance, an orbitofrontal craniotomy was used to provide the best exposure with the least brain retraction. The craniotomy was fashioned to avoid the lateral extension of the frontal sinus if possible, but the exposure was not compromised to avoid the frontal sinus. We usually estimate the location of the frontal sinus based on preoperative imaging. On occasion we have used transillumination of the medial orbital roof by a focused light source to mark the extent of the frontal sinus. If the lateral aspect of the frontal sinus was entered it was sealed by rotating a small anterior-based pericranial flap over it. The pericranial flap was sutured to the dura, tight against the inner table of the skull, under the sinus opening. In such instances, the frontal sinus was not stripped of mucosa or packed with anything, other than perhaps a small piece of Gelfoam (Pharmacia UpJohn, Bridgewater, NJ). During closure, the bone flap was replaced snugly against the small pericranial flap rotated over the frontal sinus opening, gently compressing against the frontal sinus cavity.
In the majority of patients, a midline biorbitofrontal craniotomy was used. Our technique evolved slightly over the years of this study, but was generally performed in the following manner. After a bicoronal skin incision, the frontal scalp flap was reflected anteriorly in the subgaleal plane. The pericranium was then dissected off the skull and pedicled anteriorly preserving its blood supply as much as possible (Fig. 3). A bifrontal craniotomy bone flap was then removed after predrilling holes for miniplate fixation. A biorbital or a biorbitonasal bar was then removed. If the lesion extended into the anterior inferior nasal cavity, then the superior portion of the nasal bones was removed along with the biorbital bar (Fig. 4). The lateral osteotomies of the orbital bar were usually made just medial to the supraorbital notch. The lateral orbital rims were not included in the biorbital bar osteotomy. Any frontal sinus mucosa present in the craniotomy bone flap or the biorbital bar was removed. The frontal sinus surfaces on the bone flaps were drilled with a diamond drill bit to remove any mucosal remnants. The frontal sinus mucosa on the medial orbital roof and just above the nasofrontal ducts was removed and the underlying bone drilled with a diamond burr. The nasofrontal ducts were then plugged with Gelfoam.
Figure 3.
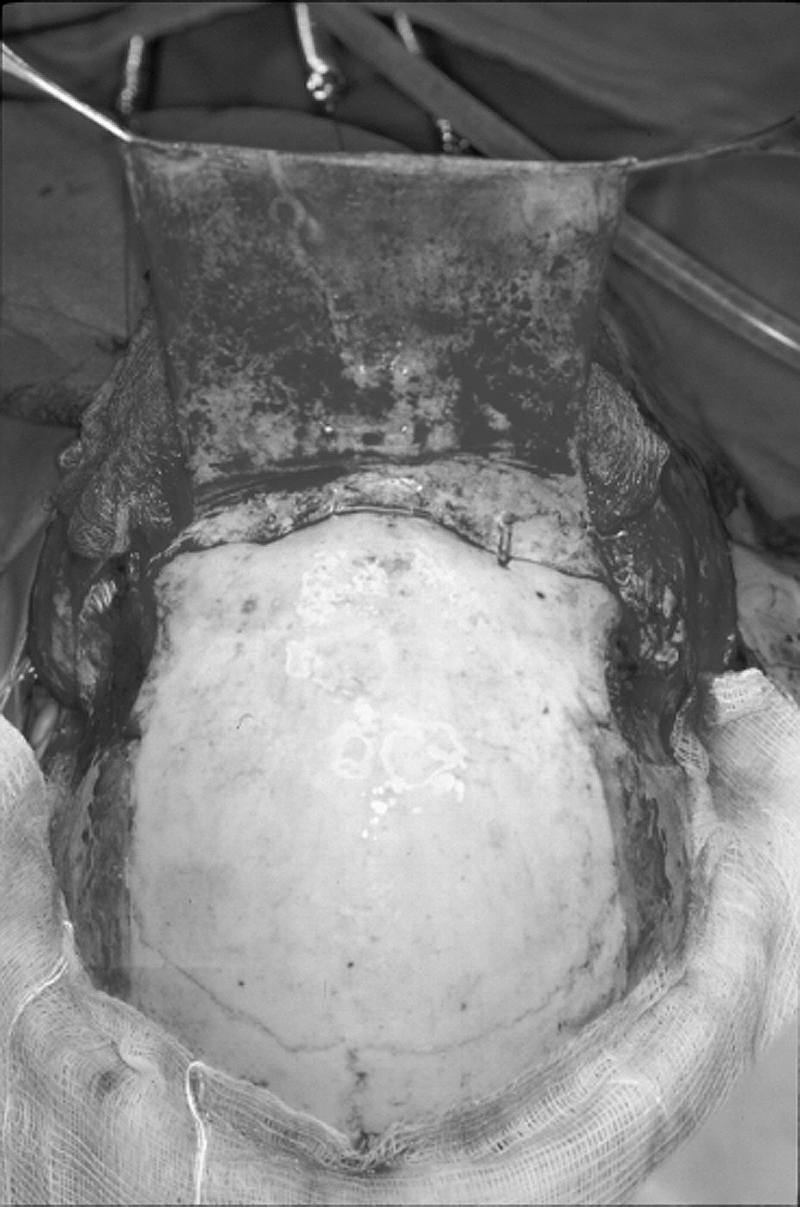
An anterior-based pedicled pericranial flap.
Figure 4.
A two-piece biorbitofrontal craniotomy incorporating the nasal bones for lesions with significant nasal extension. The pericranial flap must be placed above the orbitonasal bar during reconstruction.
Resection and Dural Repair
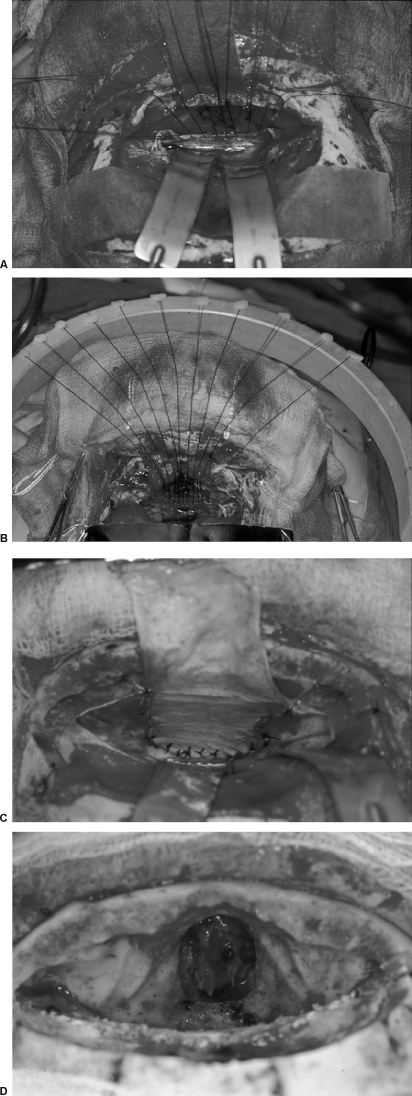
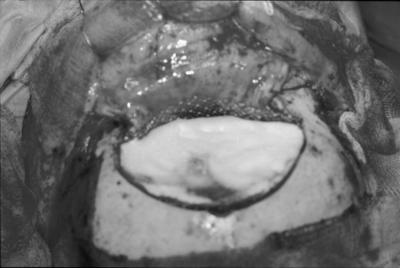
Anterior skull base lesions without nasoethmoid extension, such as most olfactory groove meningiomas, were then removed at this point. The dura of origin and any abnormal underlying bone were removed with the drill. The dural defect created was then repaired with a free pericranial graft, usually harvested from behind the coronal suture over the parietal bones. The dural closure was performed with interrupted sutures along the deep posterior dural margin as shown in Fig. 5. The sutures were placed sequentially along the deep border of the dural defect and then through the corresponding edge of the dural graft. The dural graft was then advanced into the location of the dural defect along the sutures, as if lowered down the strings of a parachute. The “parachute technique” results in a very secure suturing of the deep dural margin. The lateral dural margins and superficial margin were then sewn with running sutures. The vascularized pericranial graft was then rotated under the orbitonasal bar if possible and sutured to the dura in an extradural fashion, deep to the posterior margin or the primary dural repair. This technique provides a second tissue layer in addition to the repaired dura in between the brain and frontal sinus ostea.
Figure 5.
(A) Sutures have been placed in the deep margin of the dural defect. (B) The parachute technique for suturing the dural graft. After placement of interrupted sutures along the deep margin of the dural defect the graft is slid into place. (C) Appearance of the deep dural defect repair. (D) View of typical anterior skull base defect following dural repair.
In cases where the anterior skull base lesion extended into the nasal cavity, our preference was to separate the lesion from the brain and close the dura in watertight fashion as described above. This was performed prior to entering into the ethmoid air cells or nasal cavity proper to minimize contamination of the intradural space with bacteria from the nasal cavity. In lesions with minimal intracranial extension, there usually was ample room to repair the dura with a pericranial patch graft without first debulking the lesion. Primary dural closure without a free pericranial tissue graft was occasionally possible in cases of encephalocele. In cases with a large amount of intracranial extension, it was necessary to debulk the intracranial aspect of the lesion to provide enough room to repair the dura. This eliminated the possibility of an en bloc resection, but in large lesions with frontal lobe extension an en bloc resection is difficult in any event. Once the dura is closed and the intracranial compartment sealed, the nasal and ethmoid air spaces are entered and resection of the nasal extent of the lesion was performed.
Reconstruction
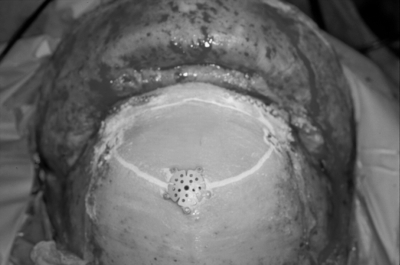
For small skull base defects (2 cm wide or less side to side) vascularized pericranium alone was used for skull base reconstruction without mechanical support. The vascularized pericranial flap was reflected under the orbital bar if possible and placed to cover the defect. The flap was then sutured to the dura posterior to the dural repair suture line. For larger defects, a mechanical construct was used to provide structural support to the skull base. Early in this series, mechanical reconstruction was performed with the use of a vascularized outer-table bone graft. Vascularized outer-table bone was harvested as previously described.9 The pericranial-calvarial flap was rotated under the orbital bar if possible and the bone fitted to the defect (Fig. 6). The pericranium was then tacked to the dura posteriorly. Titanium mesh reconstruction was used later in the series and was performed by first rotating the pericranial flap under the orbital bar and tacking it to the posterior dura. The titanium mesh was then fitted to the defect and placed on top of the pericranium but under the dural repair (Fig. 7). It was held in place by one screw in a tab of titanium mesh on either side. Thin titanium mesh with dynamic qualities works best because it can be molded easily and the location of screw fixation adjusted as needed. After replacement of the bone flap and fixation with miniplates, hydroxyapatite bone cement is used to fill the burr-hole and craniotome defects. The bone cement is also feathered around the miniplates and screws to guarantee that a prominence is never seen or felt through the scalp.
Figure 6.
The pericranium has been rotated beneath the orbitonasal bar to cover the skull base defect. Following miniplate fixation of the craniotomy flaps, the craniotome and burr-hole defects are filled with hydroxyapatite bone cement.
Figure 7.
Titanium mesh in place above pedicled pericranial flap prior to bone flap replacement. The frontal dura is covered with Gelfoam (Pharmacia UpJohn, Bridgewater, NJ).
Complication Avoidance
Complication avoidance must begin with operative planning and the selection of a surgical approach that provides both excellent exposure of the lesion and the ability to perform a meticulous reconstruction. In our opinion, most anterior skull base lesions in the region of the tuberculum sella, such as a tuberculum sella meningioma, can be approached with a simple pterional approach. This approach preserves olfaction, minimizes the chance of opening into the frontal sinus, and does not require significant brain retraction for small lesions, particularly in elderly patients. The unilateral orbital-frontal approach is appropriate for tuberculum sella meningiomas with significant superior extension. This is particularly true in young patients without any degree of brain atrophy. The midline biorbitofrontal approach is most appropriate for lesions of the olfactory groove, planum sphenoidale with impaired olfaction, and frontoethmoidal cancers.
The two-piece biorbitofrontal craniotomy with variable inclusion of the nasal bones provides excellent exposure of the anterior skull base. This bone flap does not need to be very wide, nor very tall. The lateral extent of the bifrontal craniotomy need only provide access to the lateral aspects of the frontal sinus, so that they can be cleaned free of mucosal remnants and cranialized. We never include a pterional craniotomy with the midline biorbital-frontal craniotomy. Once the bifrontal craniotomy flap is removed, and the dura elevated off the orbital rims and roof bilaterally, the biorbital bar osteotomy cuts are easy to perform while protecting the dura and orbital tissues. The biorbital bar does not have to be very wide. The lateral orbital rims do not need to be included in the osteotomy if the lesion is anterior to the optic chiasm and internal carotid arteries. If the orbital bar osteotomies are made medial to the supraorbital nerves, and beveled toward the midline, the biorbital bar is easy to remove and yet still provides excellent low exposure. If a postoperative bone flap infection were to occur, we believe that in most cases only the bifrontal craniotomy would need to be removed. The relatively thin bone of the orbital bar might revascularize and allow the infection to be cured without its removal. An additional advantage of this bone flap is that inclusion of a portion of the nasal bones with the biorbital bar osteotomy provides excellent exposure of the nasal cavity. This eliminates the need for a facial incision, even if the lesion extends to the nares.
In an attempt to minimize contamination of the intradural space with bacteria from the nasal cavity, we complete the intradural portion of the procedure and meticulously seal the dura prior to entering the nasal cavity. The frontal sinus must be entered as part of the initial exposure, but this space is considered usually sterile. The one case in this series complicated by postoperative bacterial meningitis was performed before we began closing the dura prior to entering the nasal cavity. It was this case that led us to this technique. The dural defect created by removing the cranial component of a lesion is repaired primarily, usually with a free pericranial graft taken from the parietal area. When a pericranial graft is not available, we have had success with AlloDerm (LifeCell Corp., Branchburg, NJ) or cadaveric fascia lata. Meticulous suturing of this graft, as described earlier, cannot be overemphasized. The parachute suture technique as described above allows a watertight closure along the deepest and most difficult-to-suture border of the dural graft.
Closing the dura prior to entering the nasal cavity will occasionally hinder the ability to obtain an en bloc resection of a malignant lesion, because tumors with significant intracranial extension will have to be debulked to allow access to perform the dural closure. In our experience, however, it is rarely possible in any circumstance to perform a strictly en bloc resection of tumors with significant frontal lobe extension.
In this series we utilized mechanical reconstruction for large (> 2 cm) skull base defects. This is not done to prevent brain herniation, since this rarely, if ever, occurs. We believe that mechanical reconstruction may decrease pulsations and downward displacement of the primary dural repair and thus may take tension off of the dural suture line. Early in the series we employed the use of a vascularized outer-table bone graft together with the pericranial graft as described by Kantrowitz.29 Later in the series we switched to titanium mesh because it is easier and faster. Thin dynamic titanium mesh (Stryker, Leibinger, Kalamazoo, MI) is well suited to this purpose because it can easily be molded to the contours of the medial orbital roofs once in place, and the securing tabs can be expanded as needed to reach good bone for screw fixation. The titanium mesh is placed below the primary dural repair but above the anterior-based pericranial flap, so that the mesh is not exposed to the nasal cavity. We have since discontinued the use of titanium mesh in patients with thin pericranium or nasoethmoidal cancers necessitating postoperative radiation therapy. There were five patients in this series with titanium mesh reconstructions that underwent postoperative radiation and two of them developed visible mesh on follow-up nasal endoscopy. Significant nasal crusting over the mesh developed and both patients require periodic office-based débridement of the crusting. Currently we use titanium mesh reconstruction only in patients with thick pericranium and large skull base defects who will not require postoperative radiation.
Ideally, the anterior-based vascularized pericranial graft is placed below both the orbital bar osteotomy and the bifrontal bone flap. This allows complete separation of the bone flaps from the nasal cavity and may decrease the incidence of bone flap infection. However, if the nasal bones are removed with the orbital bar, it is difficult, if not impossible, to reflect the pericranium under it. Thus in selected cases the pericranium was placed above the orbital bar, and then rotated to cover the anterior skull base. This vascularized tissue flap must not be compressed too tightly between the two bone flaps or severe swelling of the graft can occur, requiring revision surgery.30
The use of spinal drainage varied throughout the series. Early in the series, spinal drainage was used frequently during surgery and continued in the postoperative period. However, we believe that this produced symptomatic pneumocephalus in two cases and produced a decreased level of consciousness in another patient. Lumbar spinal fluid drainage is not risk-free and has been associated with a 59% incidence of minor complications (headache, nausea) and a 12.5% incidence of major complications including vocal cord paralysis and posterior cerebral artery infarction from transtentorial herniation.31 Therefore, later in the series we stopped using prophylactic spinal drainage and reserve it only for postoperative CSF leak.
In patients with a relatively thick scalp (usually men), excellent forehead cosmesis is achieved with miniplate fixation of the bone flaps alone. In patients with a thin scalp or in whom radiation will be used postoperatively, the margins of the craniotomy bone flaps can become visible on the forehead over time. We now fill the craniotomy margins in these patients with hydroxyapatite bone cement (Stryker Leibinger, Kalamazoo, MI) to guarantee an optimal cosmetic result.
SUMMARY
Resection of anterior midline skull base pathology can be performed with a very low incidence of postoperative CSF leak, meningitis, pneumocephalus, and bone flap infection. In this series no patient required readmission or reoperation for CSF leak. There has been considerable recent interest in approaching anterior skull base lesions endonasally using advanced endoscopic instrumentation. For intradural lesions such as meningiomas, endonasal approaches do not allow for a sutured watertight dural repair or use of the vascularized pericranium in reconstruction. Instead, reconstructions usually involve the use of multilayer onlay materials and would intuitively be more prone to CSF leak and other complications.
The subgroup of 29 meningioma patients in this microsurgical series had no postoperative CSF leakage, deaths, or permanent deficits other than anosmia and one case of meningitis. There have been only a few small series reporting removal of midline skull base meningiomas through an endoscopic endonasal approach. Laufer et al32 reported 10 cases of suprasellar lesions resected through an endoscopic endonasal approach, of which 5 were meningiomas of the tuberculum sella or planum sphenoidale. One of the meningioma patients in the series had CSF leak and long-term diabetes insipidus. Several other reports only include one or two patients with midline skull base meningiomas.33,34,35 Several authors have also reported extended trans-sphenoidal resection of meningiomas and craniopharyngiomas using microscopic visualization but with significant rates of reoperation, CSF leak, and subtotal resection.36,37
Though the decreased invasiveness of endonasal surgery is favorable, our results demonstrate that craniotomy for midline anterior skull base lesions can be employed with a very small incidence of major complications and reoperation. Endoscopic endonasal procedures addressing these lesions must demonstrate similar safety, particularly with regard to infectious complications, if they are to become a reasonable alternative.
REFERENCES
- Ketcham A S, Wilkins R H, Buren J M Van, et al. A combined intracranial facial approach to the paranasal sinuses. Am J Surg. 1963;106:698–703. doi: 10.1016/0002-9610(63)90387-8. [DOI] [PubMed] [Google Scholar]
- Raveh J, Turk J B, Ladrach K, et al. Extended anterior subcranial approach for skull base tumors: long-term results. J Neurosurg. 1995;82:1002–1010. doi: 10.3171/jns.1995.82.6.1002. [DOI] [PubMed] [Google Scholar]
- Raveh J, Laedrach K, Speiser M, et al. The subcranial approach for fronto-orbital and anteroposterior skull base tumors. Arch Otolaryngol Head Neck Surg. 1993;119:385–393. doi: 10.1001/archotol.1993.01880160029006. [DOI] [PubMed] [Google Scholar]
- Sekhar L N, Janecka I P, Jones N F. Subtemporal-infratemporal and basal subfrontal approach to extensive cranial base tumors. Acta Neurochir (Wien) 1988;92:83–92. doi: 10.1007/BF01401977. [DOI] [PubMed] [Google Scholar]
- Terz J J, Young H G, Lawrence W., Jr Combined craniofacial resection for locally advanced carcinoma of the head and neck. Am J Surg. 1980;140:618–624. doi: 10.1016/0002-9610(80)90043-4. [DOI] [PubMed] [Google Scholar]
- Westbury G, Wilson J S, Richardson A. Combined craniofacial resection for malignant disease. Am J Surg. 1975;130:463–469. doi: 10.1016/0002-9610(75)90485-7. [DOI] [PubMed] [Google Scholar]
- Jackson I T, Adham M N, Marsh W R. Use of the galeal frontalis myocutaneous flap in craniofacial surgery. Plast Reconstr Surg. 1986;77:905–910. doi: 10.1097/00006534-198606000-00005. [DOI] [PubMed] [Google Scholar]
- Schaefer S D, Close L G, Mickey B E. Axial subcutaneous scalp flaps in the reconstruction of the anterior cranial fossa. Arch Otolaryngol Head Neck Surg. 1986;112:745–749. doi: 10.1001/archotol.1986.03780070057012. [DOI] [PubMed] [Google Scholar]
- Schramm V L, Myers E N, Maroon J C. Anterior skull base surgery for benign and malignant disease. Laryngoscope. 1979;89:1077–1091. [PubMed] [Google Scholar]
- Cantu G, Solero C L, Pizzi N, et al. Skull base reconstruction after anterior craniofacial resection. J Craniomaxillofac Surg. 1999;27:228–234. doi: 10.1016/s1010-5182(99)80034-1. [DOI] [PubMed] [Google Scholar]
- Roux F X, Brasnu D, Menard M, et al. Combined approach to malignant tumors of the ethmoid and other paranasal sinuses: principles and results [in French] Ann Otolaryngol Chir Cervicofac. 1991;108:292–297. [PubMed] [Google Scholar]
- Badie B, Preston J K, Hartig G K. Use of titanium mesh for reconstruction of large anterior cranial base defects. J Neurosurg. 2000;93:711–714. doi: 10.3171/jns.2000.93.4.0711. [DOI] [PubMed] [Google Scholar]
- Sinha U K, Johnson T E, Crockett D, et al. Three-layer reconstruction for large defects of the anterior skull base. Laryngoscope. 2002;112:424–427. doi: 10.1097/00005537-200203000-00003. [DOI] [PubMed] [Google Scholar]
- McCarthy J G, Zide B M. The spectrum of calvarial bone grafting: introduction of the vascularized calvarial bone flap. Plast Reconstr Surg. 1984;74:10–18. doi: 10.1097/00006534-198407000-00002. [DOI] [PubMed] [Google Scholar]
- Psillakis J M, Grotting J C, Casanova R, et al. Vascularized outer-table calvarial bone flaps. Plast Reconstr Surg. 1986;78:309–319. doi: 10.1097/00006534-198609000-00005. [DOI] [PubMed] [Google Scholar]
- Catalano P J, Hecht C S, Biller H F, et al. Craniofacial resection: an analysis of 73 cases. Arch Otolaryngol Head Neck Surg. 1994;120:1203–1208. doi: 10.1001/archotol.1994.01880350017004. [DOI] [PubMed] [Google Scholar]
- Dias F L, Sa G M, Kligerman J, et al. Prognostic factors and outcome in craniofacial surgery for malignant cutaneous tumors involving the anterior skull base. Arch Otolaryngol Head Neck Surg. 1997;123:738–742. doi: 10.1001/archotol.1997.01900070082013. [DOI] [PubMed] [Google Scholar]
- Janecka I P, Sen C, Sekhar L N, et al. Cranial base surgery: results in 183 patients. Otolaryngol Head Neck Surg. 1994;110:539–546. doi: 10.1177/019459989411000611. [DOI] [PubMed] [Google Scholar]
- Ketcham A S, Buren J M Van. Tumors of the paranasal sinuses: a therapeutic challenge. Am J Surg. 1985;150:406–413. doi: 10.1016/0002-9610(85)90145-x. [DOI] [PubMed] [Google Scholar]
- Dos Santos L R, Cernea C R, Brandao L G, et al. Results and prognostic factors in skull base surgery. Am J Surg. 1994;168:481–484. doi: 10.1016/s0002-9610(05)80106-0. [DOI] [PubMed] [Google Scholar]
- Tuyl R Van, Gussack G S. Prognostic factors in craniofacial surgery. Laryngoscope. 1991;101:240–244. doi: 10.1288/00005537-199103000-00004. [DOI] [PubMed] [Google Scholar]
- Ross D A, Marentette L J, Moore C E, et al. Craniofacial resection: decreased complication rate with a modified subcranial approach. Skull Base Surg. 1999;9:95–100. doi: 10.1055/s-2008-1058155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman B J, Traynelis V C, McCulloch T M, et al. Midline anterior craniofacial approach for malignancy: results of en bloc versus piecemeal resections. Skull Base Surg. 1999;9:41–46. doi: 10.1055/s-2008-1058171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantu G, Riccio S, Bimbi G, et al. Craniofacial resection for malignant tumours involving the anterior skull base. Eur Arch Otorhinolaryngol. 2006;263:647–652. doi: 10.1007/s00405-006-0032-z. [DOI] [PubMed] [Google Scholar]
- Dias F L, Sa G M, Klingerman J, et al. Complications of anterior craniofacial resection. Head Neck. 1999;21:12–20. doi: 10.1002/(sici)1097-0347(199901)21:1<12::aid-hed2>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Donald P J. Complications in skull base surgery for malignancy. Laryngoscope. 1999;109:1959–1966. doi: 10.1097/00005537-199912000-00012. [DOI] [PubMed] [Google Scholar]
- Ganly I, Patel S G, Singh B, et al. Complications of craniofacial resection for malignant tumors of the skull base: report of an International Collaborative Study. Head Neck. 2005;27:575–584. doi: 10.1002/hed.20166. [DOI] [PubMed] [Google Scholar]
- Boyle J O, Shah K C, Shah J P. Craniofacial resection for malignant neoplasms of the skull base: an overview. J Surg Oncol. 1998;69:275–284. doi: 10.1002/(sici)1096-9098(199812)69:4<275::aid-jso13>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Kantrowitz A B, Hall C, Moser F, et al. Split-calvaria osteoplastic rotational flap for anterior fossa floor repair after tumor excision: technical note. J Neurosurg. 1993;79:782–786. doi: 10.3171/jns.1993.79.5.0782. [DOI] [PubMed] [Google Scholar]
- Jensen R, McCutcheon I E, DeMonte F. Postoperative swelling of pericranial pedicle graft producing intracranial mass effect: report of two cases. J Neurosurg. 1999;91:124–127. doi: 10.3171/jns.1999.91.1.0124. [DOI] [PubMed] [Google Scholar]
- Roland P S, Marple B F, Meyerhoff W L, et al. Complications of lumbar spinal fluid drainage. Otolaryngol Head Neck Surg. 1992;107:564–569. doi: 10.1177/019459989210700409. [DOI] [PubMed] [Google Scholar]
- Laufer I, Anand V K, Schwartz T H. Endoscopic, endonasal extended transsphenoidal, transplanum transtuberculum approach for resection of suprasellar lesions. J Neurosurg. 2007;106:400–406. doi: 10.3171/jns.2007.106.3.400. [DOI] [PubMed] [Google Scholar]
- de Divitiis E, Cavallo L M, Cappabianca P, et al. Extended endoscopic endonasal transsphenoidal approach for the removal of suprasellar tumors: part 2. Neurosurgery. 2007;60:46–59. doi: 10.1227/01.NEU.0000249211.89096.25. [DOI] [PubMed] [Google Scholar]
- Jho H D. Endoscopic endonasal approach to the optic nerve: a technical note. Minim Invasive Neurosurg. 2001;44:190–193. doi: 10.1055/s-2001-19927. [DOI] [PubMed] [Google Scholar]
- Prevedello D M, Thomas A, Gardner P, et al. Endoscopic endonasal resection of a synchronous pituitary adenoma and a tuberculum sellae meningioma: technical case report. Neurosurgery. 2007;60(suppl 2):E401. doi: 10.1227/01.NEU.0000255359.94571.91. [DOI] [PubMed] [Google Scholar]
- Dusick J R, Esposito F, Kelly D F, et al. The extended direct endonasal transsphenoidal approach for nonadenomatous suprasellar tumors. J Neurosurg. 2005;102:832–841. doi: 10.3171/jns.2005.102.5.0832. [DOI] [PubMed] [Google Scholar]
- Cook S W, Smith Z, Kelly D F. Endonasal transsphenoidal removal of tuberculum sellae meningiomas: technical note. Neurosurgery. 2004;55:239–244. discussion 244–246. doi: 10.1227/01.neu.0000126952.51782.4d. [DOI] [PubMed] [Google Scholar]