ABSTRACT
Objective: To evaluate the use of the temporalis myofascial flap in primary cranial base reconstruction following surgical tumor ablation and to explain technical issues, potential complications, and donor site consequences along with their management. Design: Retrospective case series. Setting: Tertiary referral center. Participants: Forty-one consecutive patients receiving primary temporalis myofascial flap reconstructions following cranial base tumor resections in a 4-year period. Main Outcome Measures: Flap survival, postoperative complications, and donor site morbidity. Results: Patients included 37 males and 4 females ranging in age from 10 to 65 years. Two patients received preoperative and 18 postoperative radiation therapy. Patient follow-up ranged from 4 to 39 months. The whole temporalis muscle was used in 26 patients (63.4%) and only part of a coronally split muscle was used in 15 patients (36.6%). Nine patients had primary donor site reconstruction using a Medpor® (Porex Surgical, Inc., Newnan, GA) temporal fossa implant; these had excellent aesthetic results. There were no cases of complete flap loss. Partial flap dehiscence was seen in six patients (14.6%); only two required surgical débridement. None of the patients developed cerebrospinal leaks or meningitis. One patient was left with complete paralysis of the temporal branch of the facial nerve. Three patients (all had received postoperative irradiation) developed permanent trismus. Conclusions: The temporalis myofascial flap was found to be an excellent reconstructive alternative for a wide variety of skull base defects following tumor ablation. It is a very reliable, versatile flap that is usually available in the operative field with relatively low donor site aesthetic and functional morbidity.
Keywords: Temporalis muscle flap, cranial base, reconstruction
The temporalis flap is one of the first reported muscle flaps described by Lentz1 in 1895, who used it after resection of the condylar neck for temporomandibular joint ankylosis. In 1898, Golovine2 used the flap to reconstruct an orbital exenteration defect. Subsequently, this flap was used in reconstructions involving several head and neck regions including the mastoid,3 maxilla,4 periorbital region,5 oral cavity,6 oropharynx,7 and the cranial base.8,9,10
The pedicled temporalis muscle flap is a reliable and highly versatile flap that receives blood supply from the anterior and posterior deep temporal arteries, which arise from the internal maxillary system deep to the zygomatic arch. Accessory blood supply is derived from the middle temporal artery, originating from the superficial temporal artery and entering the muscle laterally. This rich blood supply allows the temporalis muscle to be transferred in its entirety or in part, depending on the reconstructive challenge.11
The purpose of this work was to examine our series of temporalis myofascial flaps used for reconstruction of cranial base defects following tumor resection. In this work we studied the complications associated with use of the muscle. We also attempted to establish the frequency and extent of the principal donor site defect (temporal hollowing), and recommend techniques for the prevention and management of this contour deformity.
PATIENTS AND METHODS
This study was based on data of 41 consecutive patients who had primary temporalis myofascial flap reconstructions following resection of cranial base tumors at the department of Otolaryngology—Head and Neck Surgery, Alexandria University, in the period between August 2002 and July 2006.
Flap Exposure, Elevation, and Inset
Access to the temporalis muscle was achieved via a bicoronal or hemicoronal incision, which in most cases was the same incision used for the cranial base ablative surgical procedure. The scalp flap was elevated down to the supraorbital ridge in a subgaleal plane.
An oblique incision was then made extending from the upper margin of the lateral orbital rim to just anterior to the temporomandibular joint at the zygomatic arch. A plane of dissection was developed beneath the superficial temporal fat pad following the deep layer of deep temporal fascia to the level of the zygoma, and the periosteum was elevated off the entire length of the zygomatic arch. The superficial temporal fat pad was elevated intact, thus protecting the temporal branch of the facial nerve. This maneuver may also help to prevent postoperative temporal hollowing.
The inferior border of the zygoma was identified and the masseter muscle origin divided. The zygomatic arch was now free of attachments. Anterior and posterior osteotomies were made in the zygomatic arch after predrilling holes for subsequent fixation. Removal of the zygomatic arch facilitates elevation of the temporalis muscle flap, provides extra length, facilitates coronoid resection, and reduces trauma to the flap during rotation.
Following removal of the zygomatic arch, the entire temporalis muscle was then elevated from the temporal bone using a cutting diathermy at a low power setting. The muscle was then rotated to the site of the defect. The edges of the muscle were sutured with heavy nonabsorbable sutures to drill holes made in the cut edge of the bony cranial base defect.
According to the nature of the defect, the following steps were taken. (1) The entire temporalis muscle was used in the reconstruction or the muscle was carefully split in the coronal plane (along the same direction of its blood supply), thus dividing the muscle into anterior and posterior portions (Fig. 1). The portion of muscle that was not used for reconstruction was sutured back to the anterior part of the temporal fossa to help decrease postoperative temporal fossa hollowing. (2) The coronoid process was excised in some cases to increase the arc of muscle rotation, especially when reconstructing midline cranial base defects such as the clivus (Fig. 2). (3) The temporoparietal fascial layer was elevated together with a pericranial sheet extending to the midline as a single flap supplied by the superficial temporal vessels. This was used along with the temporalis muscle flap to reconstruct defects involving the orbital floor and cribriform region (Fig. 3).
Figure 1.
A vertically split temporalis flap used to reconstruct a surgical defect following ablation of a recurrent nasopharyngeal carcinoma through an infratemporal fossa approach. (A) Anterior (Ant) and posterior (Post) halves of the split muscle. Arrowheads point to the site of the defect. Asterisks delineate the craniotomy. (B) Rotation of the anterior half of the muscle to the surgical defect (white arrow). Asterisk points to the remaining posterior half of the muscle that is used to fill the anterior part of the temporal fossa hollow.
Figure 2.
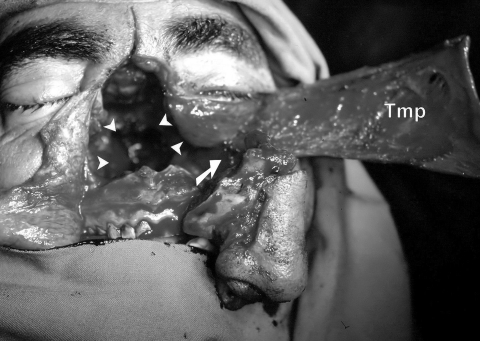
The temporalis flap used for clival defect reconstruction following surgical ablation of a clival chordoma using a medially extended facial translocation approach. Note that excision of the coronoid process is essential to safely achieve such a wide angle of muscle rotation. Arrowheads point to the site of the clival defect. Arrow points to the site of surgically created window behind the maxilla for passage of the flap. Tmp, temporalis flap.
Figure 3.
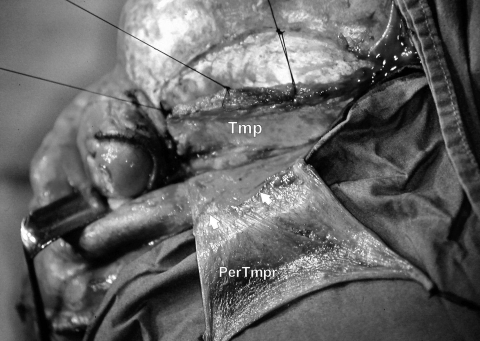
The use of a pericranial-temporoparietal fascial flap (PerTmpr) together with a temporalis muscle flap (Tmp). Flaps were developed at an early stage of the operation that involved a cranio-orbital resection for an extensive malignant fibrous histiocytoma of the orbit. Small arrows point to the line of demarcation between the pericranium and the temporoparietal fascia.
Donor Site Reconstruction and Closure
Whenever available, we used a Medpor® Flexblock™ temporal fossa implant (Porex Surgical, Inc., Newnan, GA) made of a porous high-density polyethylene (PHDPE) of appropriate size to reconstruct the temporal fossa after complete mobilization of the muscle. The implant usually required some final reshaping and adjustments of its size to adequately fill the temporal fossa hollow. The implant was then screw-fixed using 5-mm self-tapping titanium screws (Fig. 4). The zygoma was replaced and secured using titanium miniplates and screws and the coronal incision was then closed with or without a lateral canthopexy to the temporal aponeurosis. Active surgical drains were placed posterior to the coronal incision for adequate postoperative drainage.
Figure 4.
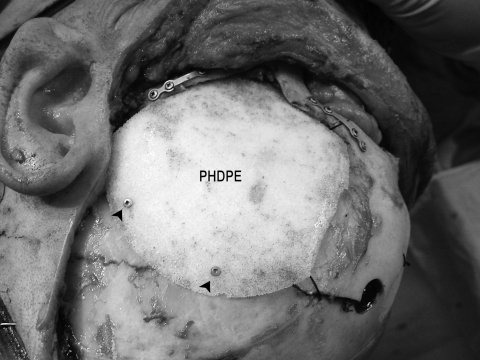
Medpor® temporal fossa implant made of porous high density polyethylene (PHDPE) used for temporal fossa reconstruction following complete transfer of the temporalis muscle. Titanium screws are used for implant fixation (arrowheads). Note that the zygomatic arch has been fixed in place by titanium miniplates and screws.
RESULTS
During the study period, 41 patients underwent primary temporalis flap reconstruction of cranial base defects following oncologic resection of a wide variety of cranial base tumors. There were 37 males (90.2%) and 4 females (9.8%). Patients ranged in age from 10 to 65 years, with a mean age of 31.6 years. Only 2 patients (both had nasopharyngeal carcinomas) received preoperative irradiation, while 18 patients received postoperative radiotherapy. There were no perioperative mortalities in this case series and none of the patients developed postoperative cerebrospinal leaks or meningitis. The average follow-up period was 11.3 months (range, 4 to 39 months).
Cranial Base Defects
The nature of the cranial base procedures performed and their histopathological diagnosis are outlined in Table 1. The location of the surgically created cranial base defects classified according to a previously published practical classification system described by Irish et al12 is shown in Table 2. This classification integrates anatomic boundaries with tumor growth patterns in various regions. It classifies the skull base into regions I, II, and III. Region I includes tumors arising anteriorly and those extending down the clivus to the foramen magnum. Region II tumors arise laterally and involve primarily the infratemporal and pterygopalatine fossae, with extension into the middle cranial fossa through various foramina. Region III lesions arise posteriorly, within or around the temporal bone, and extend intracranially into the middle or posterior cranial fossa.
Table 1.
Nature of Cranial Base Ablative Procedures and Tumor Histopathology
| Type of Surgical Resection | Tumor Pathology (Location) | n |
|---|---|---|
| MFH, malignant fibrous histiocytoma; MM, medial maxillectomy; PPF, pterygopalatine fossa. | ||
| Anterior craniofacial resection + orbitectomy | MFH (ethmoido-orbital) | 1 |
| Cranio-orbital resection (orbitectomy) | Malignant tumor (orbit) | 8 |
| Facial translocation | ||
| Classical | Angiofibroma | 1 |
| Medially extended | Chordoma (clivus) | 4 |
| Giant cell tumor (clivus) | 1 | |
| Complete rhinotomy + bilateral MM | Chordoma (clivus) | 3 |
| Preauricular infratemporal fossa approach | Angiofibroma | 13 |
| + nasopharyngectomy | Angiosarcoma (nasopharynx + PPF) | 1 |
| Nasopharyngeal carcinoma | 2 | |
| Temporal bone resection | ||
| Lateral | Carcinoma (temporal bone) | 6 |
| Subtotal | Carcinoma (temporal bone) | 1 |
Table 2.
Classification of Skull Base Defects According to Location
| Cranial Base Region | n |
|---|---|
| Region I | |
| Anterior cranial base | 9 |
| Clivus | 8 |
| Region II | |
| Pterygopalatine fossa/infratemporal fossa | 13 |
| Region III | |
| Temporal bone | 7 |
| Middle cranial fossa | 4 |
The dura was breached during resection in only 3 out of the 41 cases performed (7.3%). In 2 cases (both clival chordoma resections), primary watertight dural repair was achieved. In the remaining case (an orbitectomy for an adenoid cystic carcinoma arising from the lacrimal gland), a 2-cm2 dural defect was repaired using a temporalis fascia graft. The temporalis muscle flap was used to reinforce the reconstruction. In all other cases the resection was performed extradurally.
Temporalis Reconstruction Details
The temporalis muscle was accessed via the same coronal incision utilized for the surgical resection procedure in 33 cases (80.5%). In the remaining 8 cases (19.5%), an ipsilateral hemicoronal incision was needed for temporalis muscle elevation.
In this series, the whole temporalis muscle was used for cranial base defect reconstruction in 26 patients (63.4%), while in 15 patients (36.6%) only part of a coronally split temporalis muscle was used. The anterior portion of the split muscle was used for reconstruction in 9 cases (2 with orbital roof defects and 7 with infratemporal fossa/nasopharyngeal defects). In these patients, the posterior portion of the muscle was reattached to the lateral orbital rim and temporal line to help decrease postoperative temporal fossa hollowing. In the remaining 6 patients (all had lateral temporal bone resections), the posterior portion of the coronally split muscle was used for reconstruction, while the anterior one third of the muscle was not elevated and was left undisturbed in the temporal fossa. Nine patients received primary reconstruction of the temporal fossa following complete transfer of the temporalis muscle using a Medpor® temporal fossa implant.
The coronoid process was resected to increase the arc of rotation in all cases where the muscle was used to reconstruct midline cranial base defects (eight cases). In seven patients who had cranio-orbital resection for extensive malignant orbital tumors, the temporalis muscle was used together with a separate flap of temporoparietal fascia and pericranium based on the superficial temporal artery to provide for a more stable reconstruction of the anterior cranial base.
Temporalis Flap Complications
Table 3 lists postoperative complications related to the use of the temporalis myofascial flap in our patient series. There was no instance of complete flap necrosis. Partial dehiscence of the flap was seen in six patients (14.6%). Only two of them required surgical débridement due to partial necrosis of the distal end of the flap.
Table 3.
Postoperative Complications of Temporalis Flap Reconstruction
| Complication | n |
|---|---|
| CN VII, facial nerve. | |
| Seroma | 7 |
| Hematoma | 2 |
| Infection | 1 |
| Partial flap dehiscence | |
| Mild | 4 |
| Severe | 2 |
| Injury to temporal branch of CN VII | |
| Temporary paresis | 4 |
| Complete paralysis | 1 |
| Trismus | |
| Temporary | 12 |
| Permanent | 3 |
Four patients developed temporary paresis of the temporal branch of the facial nerve that recovered completely after periods ranging from 1 to 5 months. Only one patient was left with complete paralysis.
Fifteen patients (36.6%) developed some degree of jaw movement limitation after the operation. Postoperative physiotherapy was successful in 12 of them (80%) in restoring full range of jaw movement in a period of 6 to 9 weeks. In 3 patients (all had received postoperative irradiation) the development of trismus was considered to be permanent.
The incidence of seroma developing after removal of the surgical drain was high among the group of patients who had Medpor® reconstructions (4/9) compared with those who did not (3/32). One patient developed infection over the temporal fossa implant that required reexploration with curettage of infected granulations and local antibiotic irrigation, followed by intravenous antibiotic therapy for 10 days to control the infection.
Severe hollowing of the temporal fossa was seen in 17 patients (those who had complete temporalis muscle transfer without primary donor site reconstruction). Temporal aesthetic appearance was excellent in all patients receiving Medpor® donor site reconstructions except one who had an oversized implant that gave a slightly visible edge. All patients who had transfer of the anterior portion of the split temporalis muscle developed some degree of temporal fossa hollowing that became more noticeable with time, probably due to some atrophy of the remaining part of the muscle. However, patients who had transfer of the posterior portion of the muscle for reconstruction of lateral temporal bone defects had a relatively better aesthetic appearance.
DISCUSSION
Reconstruction of the cranial base remains one of the most challenging areas facing reconstructive surgeons worldwide. The extent of resection as well as the proximity to vital neurovascular structures can make most conventional reconstructive options less than ideal. In addition, life-threatening complications may develop if the reconstruction fails, thus delaying the initiation of adjuvant therapy. There are several reconstructive goals, the most important being adequate separation of the intracranial contents from the bacteria-laden sinuses and oropharyngeal cavities. Other objectives for successful reconstruction include a watertight dural repair, elimination of dead space, and restoration of facial function and appearance. With each objective, multiple options exist.13 Each case should be individualized to provide a functional reconstruction with the lowest possible morbidity. Local flaps including the pericranial, galeal, and scalp flaps are sometimes adequate to achieve most of these reconstructive objectives. Pedicled flaps including the latissimus dorsi, pectoralis major, and trapezieus flaps are seldomly used in skull base reconstruction as their reach is limited by the vascular attachment below the clavicle. Microvascular free flaps have undoubtedly revolutionized skull base reconstruction and offer many advantages, especially in large defects requiring a significant amount of tissue bulk. They have been widely accepted as the best method to repair skull base defects.10 However, these flaps may be associated with significant donor site morbidity and increased operative time. Moreover, they require a surgical team with specific microvascular expertise.
The temporalis muscle flap is a versatile and reliable flap that has been used extensively for orbital reconstruction and facial reanimation. It is most useful for reconstructing defects in which the ideal flap is a flexible, tailored muscle with moderate thickness.14 The length of the temporalis muscle in cadavers ranges between 12 and 16 cm as measured by Bradley and Brockbank.6 It is ~0.5 to 1.0 cm in thickness.8 The flap maintains the majority of its mass because it is usually not denervated during transfer.10,14 Its pedicle can tolerate ~130 degrees of rotation.6 The flap may also be extended to the midline by including the temporoparietal fascia.15
The temporalis muscle flap is accessible through a coronal incision that is hidden in the hair-bearing scalp and preauricular crease. Another advantage in cranial base reconstruction is that the temporalis muscle usually lies in the same operative field as the defect, potentially reducing operative time and motion-induced viability issues during the recovery period.16 The deep temporal fascia lines the flap and obviates the need for skin grafting. Furthermore, its adequate length and flexible arc of rotation make it suitable to cover defects in the anterior and lateral cranial base, the midline cranial base, and the temporal bone.17
In this study, we used the temporalis myofascial flap in 41 patients to reconstruct a variety of different cranial base defects. Except for 8 patients (19.5%), the flap was always available in the surgical field through the same scalp incision used in the ablative surgery, thus reducing the operative time.
Total necrosis of the temporalis flap is a very infrequent complication. A previous review of 11 published case series in the English-language literature between 1987 and 2000 on the use of this flap has shown a very low flap failure rate (1.6%).18 Clauser and colleagues19 also reported total necrosis of the temporalis muscle flap in 1.6% and partial flap dehiscence in 13.4%. In our study, we had no instance of total necrosis of the flap; however, partial necrosis was seen in 14.6% of patients. In all these patients, the flap was used to cover clival defects. We believe that the extensive dissection required to mobilize the flap that included resection of the coronoid process and the passage through a window behind the maxilla might have partially compromised the flap's blood supply. Partial necrosis was mainly seen in the form of dehiscence at the distal end of the flap.
The main complication with the use of the temporalis muscle flap is a deficit in function of the frontal (temporal) branch of the facial nerve. Such a deficit can be transient or permanent. Injury to the temporal branch of the facial nerve can be avoided by elevating the temporal fat pad as described earlier. This protects the nerve within the temporoparietal fascia. The nerve also can be injured as a result of retraction during the elevation of the flap and removal of the zygomatic arch.14 In the largest published case series of temporalis flaps (N = 182), Clauser and associates19 reported temporal branch paresis in 19.2% and paralysis in 2.7%. In our series, one case (2.4%) of permanent damage to the temporal branch of facial nerve occurred and four patients (9.8%) developed temporary paresis. We have observed that in all these patients, a hemicoronal incision was used in elevation of the temporalis flap. The relatively narrow exposure provided by the hemicoronal incision might have resulted in the use of excessive retraction on the temporal fat pad during elevation of the flap, which could have led to the nerve injury. We thus recommend extra care while using retractors on the temporal fat pad during flap elevation, especially when a hemicoronal incision is being used. Knowledge of the surgical anatomy, meticulous dissection, and delicate handling of tissues can minimize the risk of a facial nerve deficit. However, the patient should be always warned preoperatively about such an inherent risk.
During the healing phase, temporary difficulties in chewing and jaw opening can occur after temporalis muscle transfer. These are attributed to the development of local edema and swelling of the muscle with subsequent fibrosis. However, in most cases, intensive postoperative physiotherapy can restore full range of motion in ~2 to 3 months.20 Limitation in jaw movement was seen in 36.5% of our patients. The condition was reversed in 80% of them by using jaw stretching exercises. In a series of 26 patients reported by Colmenero and others,21 restricted mouth opening occurred in 11.5%. The relatively higher incidence in our series might have been due to the extensive nature of the surgical procedures employed. We also believe that the use of postoperative irradiation is a major factor in delaying rehabilitation or causing permanent trismus in those cases, as all patients who developed permanent trismus had received postoperative radiotherapy.
Temporal hollowing is the most commonly cited aesthetic donor site morbidity associated with the temporalis muscle flap.14 Different measures have been described to eliminate temporal hollowing. Cordeiro and Wolfe17 suggested rotating the temporal fat pad into the anterior temporal region and harvesting a large pericranial flap from the frontal and contralateral parietal regions and folding them into the defect. Other authors have reported the use of rib cartilage, bone grafts, rolled dermis, alloplastic materials, or even free flaps to fill unacceptable temporal defects.22,23,24
In our experience, replacing the zygomatic arch prevents major depression in the non–hair-bearing region of the donor site, while preserving the normal position of the temporal fat pad and anterior portion of the temporalis muscle nearly eliminates any additional hollowing. Nevertheless, if the entire muscle is used for reconstruction, or in cases in which the remaining portion of the muscle appears inadequate to restore the original contour, immediate donor site reconstruction is often needed. Such reconstruction is usually best accomplished by using alloplastic materials. A prefabricated block made from PHDPE is specifically marketed for reconstruction of the temporal fossa after transfer of all or part of the temporalis muscle. The firm nature of the implant material easily allows carving with a sharp instrument without collapsing the pore structure. Thus, the block can be further modified intraoperatively to fit the resulting defect more precisely.
In this study, significant postoperative hollowing of the temporal fossa occurred in all patients in whom the whole temporalis muscle was transferred without primary donor site reconstruction. However, the deformity was not considered by any of these patients severe enough to warrant secondary reconstruction. Most came from rural areas and thus camouflaged the deformity with their traditional costume. On the other hand, aesthetic appearance was almost always perfectly symmetrical in those patients that had immediate intraoperative donor site reconstruction using a Medpor® Flexblock™ temporal fossa implant. However, the incidence of postoperative seroma among this group of patients was high (55.5%). This observation might be preventable by delaying removal of the surgical drains after using such implants. Most defects require the full length of the temporalis muscle for reconstruction but some may not need the full width. None of the 15 patients who had a split partial temporalis flap for reconstruction developed a significant temporal fossa hollowing, especially when the posterior part of the muscle was used for reconstruction, leaving the anterior part undisturbed in the temporal fossa.
Contraindications to the use of the temporalis muscle flap include previous damage to the muscle or reduction in its blood supply through interference with the ipsilateral internal maxillary or external carotid arteries. Such interference can occur after radical excision of tumors or radiation of the muscle for treatment of neoplastic lesions in adjacent areas. However, in cases in which there has been previous radiation therapy to the temporal region, the radiated muscle generally may retain adequate blood supply and can still be used for defect reconstruction, provided that special care is taken during elevation and transposition of the muscle.25 Neither patient who had received preoperative irradiation in this series had a postoperative healing problem. It should be noted, however, that in such instances healing of the flap may be delayed20; therefore, radiotherapy to the temporal region should always be considered a risk factor that could compromise flap healing. On the other hand, when the temporalis muscle is subjected to radiotherapy after complete flap healing, relative loss of muscle volume may occur, but with no significant effect on flap survival.26,27
SUMMARY
On the basis of our experience and on review of the experience of others, it is evident that the temporalis flap is a useful, reliable, and versatile option for reconstruction of a wide variety of cranial base defects. In most instances the muscle provides abundant well-vascularized tissue, with minimal to no functional morbidity or aesthetic deformity at the donor site. Since we began using the Medpor® Flexblock™ temporal fossa implant for donor site reconstruction, the temporal hollowness resulting from the use of temporalis muscle has generally been negligible.
REFERENCES
- Lentz J. Résection du col du condyle avec interposition d'un lambeau temporal entre les surfaces de résection. Guérison. Assoc Franç de Chirur (Paris) 1895;9:113–117. [Google Scholar]
- Golovine S S. Procédé de cloture plastique de l'orbite après l'exentération. Arch Ophthalmol. 1898;18:679–680. [Google Scholar]
- Rambo J H. Musculoplasty: a new operation for suppurative middle ear deafness. Trans Am Acad Ophthalmol Otolaryngol. 1958;62:166–177. [PubMed] [Google Scholar]
- Bakamjian V. A technique for primary reconstruction of the palate after radical maxillectomy for cancer. Plast Reconstr Surg. 1963;31:103–117. doi: 10.1097/00006534-196302000-00001. [DOI] [PubMed] [Google Scholar]
- Cramer L M. Surgical management of recurrent periorbital tumors. Plast Reconstr Surg Transplant Bull. 1962;29:14–21. doi: 10.1097/00006534-196201000-00003. [DOI] [PubMed] [Google Scholar]
- Bradley P, Brockbank J. The temporalis muscle flap in oral reconstruction: a cadaveric, animal and clinical study. J Maxillofac Surg. 1981;9:139–145. doi: 10.1016/s0301-0503(81)80034-3. [DOI] [PubMed] [Google Scholar]
- Koranda F C, McMahon M F, Jernstrom V R. The temporalis muscle flap for intraoral reconstruction. Arch Otolaryngol Head Neck Surg. 1987;113:740–743. doi: 10.1001/archotol.1987.01860070054015. [DOI] [PubMed] [Google Scholar]
- Shagets F W, Panje W R, Shore J W. Use of temporalis muscle flaps in complicated defects of the head and face. Arch Otolaryngol Head Neck Surg. 1986;112:60–65. doi: 10.1001/archotol.1986.03780010062011. [DOI] [PubMed] [Google Scholar]
- Maegawa J, Saijo M, Ogino H, Yamamoto I. Reverse U-shaped split temporalis musculofascial flap in cranial base reconstruction. Ann Plast Surg. 1999;42:644–650. doi: 10.1097/00000637-199906000-00011. [DOI] [PubMed] [Google Scholar]
- Chang D W, Langstein H N, Gupta A, et al. Reconstructive management of cranial base defects after tumor ablation. Plast Reconstr Surg. 2001;107:1346–1355. doi: 10.1097/00006534-200105000-00003. [DOI] [PubMed] [Google Scholar]
- Cheney M L. In: Urken ML, Cheney ML, Sullivan MJ, Biller HF, editor. Atlas of Regional and Free Flaps for Head and Neck Reconstruction. New York: Raven Press; 1995. Temporalis. pp. 65–76.
- Irish J C, Gullane P J, Gentili F, et al. Tumors of the skull base: outcome and survival analysis of 77 cases. Head Neck. 1994;16:3–10. doi: 10.1002/hed.2880160103. [DOI] [PubMed] [Google Scholar]
- Bull W J, Vandevender D, Cimino V G. Reconstruction of defects of the cranial base. Tech Neurosurg. 2003;9:106–112. [Google Scholar]
- Hanasono M M, Utley D S, Goode R L. The temporalis muscle flap for reconstruction after head and neck oncologic surgery. Laryngoscope. 2001;111:1719–1725. doi: 10.1097/00005537-200110000-00009. [DOI] [PubMed] [Google Scholar]
- Bakamjian V Y, Souther S G. Use of the temporal muscle flap for reconstruction after orbito-maxillary resections for cancer. Plast Reconstr Surg. 1975;56:171–177. doi: 10.1097/00006534-197508000-00009. [DOI] [PubMed] [Google Scholar]
- Holmes A D, Marshall K A. Uses of the temporalis muscle flap in blanking out orbits. Plast Reconstr Surg. 1979;63:336–342. doi: 10.1097/00006534-197903000-00007. [DOI] [PubMed] [Google Scholar]
- Cordeiro P G, Wolfe S A. The temporalis muscle flap revisited on its centennial: advantages, newer uses, and disadvantages. Plast Reconstr Surg. 1996;98:980–987. doi: 10.1097/00006534-199611000-00008. [DOI] [PubMed] [Google Scholar]
- Abubaker A O, Abouzgia M B. The temporalis muscle flap in reconstruction of intraoral defects: an appraisal of the technique. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:24–30. doi: 10.1067/moe.2002.126077. [DOI] [PubMed] [Google Scholar]
- Clauser L, Curioni C, Spanio S. The use of the temporalis muscle flap in facial and craniofacial reconstructive surgery: a review of 182 cases. J Craniomaxillofac Surg. 1995;23:203–214. doi: 10.1016/s1010-5182(05)80209-4. [DOI] [PubMed] [Google Scholar]
- Wolff K D, Dienemann D, Hoffmeister B. Intraoral defect coverage with muscle flaps. J Oral Maxillofac Surg. 1995;53:680–686. doi: 10.1016/0278-2391(95)90170-1. [DOI] [PubMed] [Google Scholar]
- Colmenero C, Martorell V, Colmenero B, Sierra I. Temporalis myofascial flap for maxillofacial reconstruction. J Oral Maxillofac Surg. 1991;49:1067–1073. doi: 10.1016/0278-2391(91)90141-8. [DOI] [PubMed] [Google Scholar]
- Falconer D T, Phillips J G. Reconstruction of the defect at the donor site of the temporalis muscle flap. Br J Oral Maxillofac Surg. 1991;29:16–18. doi: 10.1016/0266-4356(91)90167-4. [DOI] [PubMed] [Google Scholar]
- Cheung L K, Samman N, Tideman H. The use of mouldable acrylic for restoration of the temporalis flap donor site. J Craniomaxillofac Surg. 1994;22:335–341. doi: 10.1016/s1010-5182(05)80114-3. [DOI] [PubMed] [Google Scholar]
- de Visscher J GAM, der Wal K GH van. Temporalis muscle flap revisited on its centennial: advantages, newer uses, and disadvantages [letter] Plast Reconstr Surg. 1997;100:1936–1938. doi: 10.1097/00006534-199712000-00062. [DOI] [PubMed] [Google Scholar]
- Demas P N, Sotereanos G C. Transmaxillary temporalis transfer for reconstruction of a large palatal defect: report of a case. J Oral Maxillofac Surg. 1989;47:197–202. doi: 10.1016/s0278-2391(89)80119-3. [DOI] [PubMed] [Google Scholar]
- Huttenbrink K B. Temporalis muscle flap: an alternative in oropharyngeal reconstruction. Laryngoscope. 1986;96:1034–1038. [PubMed] [Google Scholar]
- Alonso del Hoyo J, Fernandez Sanroman J, Gil-Diez J L, Diaz Gonzalez F J. The temporalis muscle flap: an evaluation and review of 38 cases. J Oral Maxillofac Surg. 1994;52:143–147. doi: 10.1016/0278-2391(94)90396-4. [DOI] [PubMed] [Google Scholar]