Abstract
Cpefat mice carry a mutation in the carboxypeptidase E/H gene which encodes an exopeptidase that removes C-terminal basic residues from endoproteolytically cleaved hormone intermediates. These mice have endocrine disorders including obesity, infertility, and hyperproinsulinemia–diabetes syndrome, but the etiology remains an enigma. Because studies have identified membrane carboxypeptidase E as a sorting receptor for targeting prohormones to the regulated secretory pathway for processing and secretion, the intracellular routing and secretion of pro-opiomelanocortin/adrenocorticotropin and growth hormone from anterior pituitary cells were investigated in Cpefat mice. In Cpefat mice, pro-opiomelanocortin was accumulated 24-fold above normal animals in the pituitary and it was poorly processed to adrenocorticotropin. Furthermore, pro-opiomelanocortin was secreted constitutively at high levels, showing no response to stimulation by corticotropin-releasing hormone. Similarly, growth hormone release was constitutive and did not respond to high K+ stimulation. Both pro-opiomelanocortin and growth hormone levels were elevated in the circulation of Cpefat mice versus normal mice. These data provide evidence that the lack of carboxypeptidase E, the sorting receptor, results in the intracellular misrouting and secretion of pro-opiomelanocortin and growth hormone via the constitutive pathway in the pituitary of Cpefat mice.
Keywords: prohormone sorting, neuropeptides, pro-opiomelanocortin
Peptide hormones and neuropeptides are synthesized as larger percursors (1). These precursors (prohormones) are transported from the rough ER, the site of synthesis, to the cis and then the trans-Golgi network, where they are sorted and packaged into the granules of the regulated secretory pathway. It is within these granules that most prohormones are endoproteolytically processed at paired basic residues to the biologically active peptide hormones, which are then released upon stimulation (2). Failure of the prohormones to be sorted and packaged into regulated secretory granules will result in their secretion via the constitutive (default) pathway in an unregulated manner (3, 4). Recent evidence indicates that the mechanism of sorting prohormones and proneuropeptides at the trans-Golgi network into the regulated secretory granules involves the binding of a sorting signal to a receptor (5), although an aggregation step may also be involved for more effective sorting (6–8). Receptor-mediated mechanisms for sorting proteins into different compartments are commonly used by cells (e.g., to target specific enzymes to the lysosomes by the mannose-6-PO4 receptor (9) and to retain proteins in the rough ER by the KDEL receptor (10).
The regulated secretory pathway sorting signals for several proteins [pro-opiomelanocortin (POMC), proenkephalin, and chromogranin B] have been identified (refs. 11 and 12; Bamberger, A., Cool, D. R., Snell, C. R. & Y.P.L., unpublished work). These sorting signals have a consensus motif composed of an amphipathic loop with hydrophobic and charged amino acid residues exposed on the surface of the loop (12, 13). Recently, in vitro binding studies using the sorting signal motif of POMC as a ligand have identified the sorting receptor for POMC as a membrane-associated form of carboxypeptidase E (CPE; also known as carboxypeptidase H) (5), an exopeptidase involved in the removal of C-terminal basic residues from cleaved hormones (14, 15). Moreover, proenkephalin and proinsulin, but not constitutively secreted proteins proalbumin and immunoglobulin, were bound to this receptor (5). Furthermore, in a neuroendocrine cell line (Neuro 2a) in which the synthesis of CPE had been down-regulated by antisense-mRNA, transfected POMC was misrouted and secreted unprocessed, constitutively (5). In view of these findings, we investigated the Cpefat mouse, which has a mutation (Ser202 → Pro202) in the carboxypeptidase E gene and lacks the enzyme (16) to determine whether there was misrouting of prohormones to the constitutive pathway. Misrouting of prohormones could account for some of the endocrinological abnormalities (e.g., hyperproinsulinemia, obesity, and infertility) observed in these animals (16).
In the present study we show that two pituitary hormones, adrenocorticotropin (ACTH) and its precursor, POMC, and growth hormone (GH) were secreted constitutively from anterior pituitary (AP) cells of Cpefat mice. Moreover, POMC and GH were found to be elevated in the anterior pituitary and the circulation of Cpefat mice, as compared with normal mice. These findings provide new insights into the defects in the cellular metabolism, sorting and secretion of POMC and GH associated with the lack of CPE, the regulated secretory pathway sorting receptor in Cpefat mice.
MATERIALS AND METHODS
Materials.
All reagents used were of analytical grade. Collagenase type IV, deoxyribonuclease I type DN-100, bovine serum albumin, RIA grade, and ascorbic acid were from Sigma; [35S]methionine (specific activity 1,000 Ci/mmol; 1 Ci = 37 GBq) was from DuPont/NEN. PBS (pH 7.2), minimum essential medium, DMEM, and 1 M Hepes buffer (pH 7.0) were purchased from Biofluids (Rockville, MD). Rat corticotropin-releasing hormone (CRH) was from Peninsula Laboratories. [125I]ACTH and [125I]GH were custom iodinated by Corning. ACTH antiserum, DP6, DP4, and DP5 specific for the N-terminal, C-terminal, and midportion of the ACTH molecule, respectively, were raised in rabbits in our laboratory. Synthetic human ACTH1–39 and ACTH1–14, used as standards, were purchased from Bachem. Rat GH antiserum was obtained from the National Hormone and Pituitary Program (Rockville, MD).
Animals.
BKS/Cpefat (fat/fat), BKS +/fat, and BKS +/+ female mice (7–11 weeks old) were obtained from Edward Leiter (The Jackson Laboratory). In the BKS/Cpefat mice, the mutation maps to the gene for CPE (16). Mice were kept on a 14 h:10 h light–dark cycle and given food and water ad libitum. Mice were sacrificed according to National Institute of Child Health and Human Development animal protocol (no. 95–020), and the anterior pituitaries were removed for preparation of dissociated cell cultures.
Anterior pituitary cell culture.
Mouse anterior pituitary cells were prepared by enzymatic dispersion as previously described (17). This method yielded more than 90% viable cells. Cells were plated in DMEM containing 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, 100 units per ml to 100 μg/ml penicillin-streptomycin, and 10% (vol/vol) heat-inactivated fetal calf serum at a density of 1.2–1.5 × 105 cells per well in 24-well plastic cluster dishes (Costar). Cell cultures were incubated at 37°C in a humidified atmosphere of 90% air/10% CO2 and used for experimental procedures on the fifth day after plating.
Secretion studies.
For ACTH secretion studies, the culture medium was removed and the cells were preincubated in 0.5 ml of DMEM containing 0.25% BSA for 2 h in a humidified atmosphere containing 10% CO2 at 37°C. Following the preincubation period the medium was removed from the cells and 0.2 ml of fresh medium without or with 10 nM CRH and 200 units per ml of aprotinin (ICN) were added to the cells for 3 h. Following the stimulation period, the medium was removed and centrifuged at 500 × g to pellet any cellular material. The supernatant was carefully removed and was added to an equal volume of 2× extraction buffer (10% formic acid/2% trifluoroacetic acid (TFA)/0.4% 2-mercaptoethanol/2 M HCl/2% NaCl) and mixed. The mixture was then passed through a C18 Sep-Pak cartridge (Millipore) and eluted with 80% acetonitrile/0.1% TFA. The eluate was lyophilized for subsequent radioimmunoassay (RIA) for ACTH after HPLC as described previously (12) or Western blot analysis. In the experiments using CRH, ascorbic acid at a final concentration of 10 μM was added to the medium to prevent oxidation of CRH.
After the incubation period, the cells were rinsed twice with cold PBS and collected into 0.5 ml of 0.1 M HCl. The cells were lysed by freezing and thawing 3 times. The extract (100 μl) was saved for protein assay. The rest of the cell extract was spun at 15,000 × g for 15 min to remove cell debris, and the supernatant was collected for Western blots.
Western blot analysis of CPE, ACTH, and GH.
Western blot analysis was performed as previously described (18). Each lane that was to be compared contained an equivalent volume of sample or concentration of protein. Detection of immunoreactive CPE, ACTH, or GH was carried out using the enhanced chemiluminescence (ECL) system (Amersham). The films were scanned into a Macintosh PPC 8100 computer, and the bands were quantitated using the nih-image1.57 program. Statistical analysis of data derived from Western blots was done using the arbitary units for the band quantitated by the scanner. For CPE, each whole pituitary was extracted in 50 μl of 10 mM HCl/0.5% SDS. Antibodies used for Western blots of CPE were CPH7–4, directed against the C-terminal last 11 residues of full-length CPE, and antibody CPH2–4, directed against the first 15 residues of the N-terminal of CPE. Both antisera were generated in our laboratory and used at a dilution of 1:4,000. For ACTH, secretion medium and cell extracts were analyzed by Western blots using antiserum DP4 (1:2,500 dilution) or DP5 (1:8,000 dilution). Both antisera recognize all forms of ACTH and POMC. For GH, secretion medium and cell extracts were analyzed by Western blot using a rat GH antiserum at 1:2,000 dilution.
Pulse-chase labeling and immunoprecipitation of GH in AP cells.
The cells were rinsed twice with methionine-free DMEM (GIBCO) and preincubated with the same medium for 30 min. Following preincubation, the cells were pulse-labeled for 30 min in 0.2 ml methionine-free DMEM containing 1 mCi/ml [35S]methionine. At the end of the pulse labeling, the medium was removed, and, after rinsing the cells as above, they were incubated in 0.25 ml of chase medium I (2.5 mM Hepes, pH 7.4/125 mM NaCl/4.5 mM KCl/1.2 mM KH2PO4/5.6 mM glucose/5 mM MgC12/0.1% BSA/1 mM methionine/200 units per ml aprotinin) or medium II, which contained the same components as medium I except the concentrations of KCl and NaCl were changed to 50 mM and 80 mM, respectively, and 5.2 mM CaCl2 was added. After 3 h of chase incubation, the chase medium was immediately removed and spun to remove any cell debris and then immunoprecipitated with GH antiserum. The cells were rinsed with cold PBS and collected into 0.5 ml of 0.1 M HCl followed by freezing and thawing three times. The lysate (100 μl) was saved for protein assay. The rest of the cell lysate was centrifuged, and the supernatant was immunoprecipitated with GH antiserum after adjustment to pH 7 with NaOH.
The immunoprecipitation was done using a previously described procedure (19), with some modifications. The chase medium and cell lysate were first precleared by incubation with 30 μl Protein A Sepharose CL-4B that had been freshly washed with 1× buffer A (19) for 30 min at 4°C, and the beads were removed by centrifugation. This was followed by incubation overnight with 15 μl GH antiserum (1:16 dilution), 50 μl buffer B (19), appropriate volume of 10 × buffer A to yield a 1 × final concentration, 1 mM AEBSF (ICN) 200 units per ml aprotinin, and 1 μg/ml leupeptin. The immunocomplex was then precipitated by incubation with 40 μl Protein A Sepharose CL-4B beads for 30 min with shaking at 4°C. The beads were spun down and washed three times with 1 × buffer A, once with buffer A containing 1 M NaCl, once with buffer A containing 0.1% SDS, and once with PBS. The washed beads were then resuspended in 60 μl of SDS–urea sample buffer, boiled for 5 min, and spun. An aliquot of the supernatant (5 μl) was counted for radioactivity, and the rest was applied to SDS/PAGE followed by autoradiography. Quantitation of the bands was performed using the nih-image1.57 program.
Radioimmunoassay of ACTH in secretion medium and plasma.
Secretion medium was radioimmunoassayed for ACTH, as described previously, using the DP6 antiserum (20). Plasma was collected and purified through a Sep-Pak C18 cartridge. The eluant was lyophilized and radioimmunoassayed for ACTH, as described previously, using the DP5 antiserum (17).
Western blot analysis of GH in serum.
Serum was collected from normal and Cpefat mice, and GH was immunoprecipitated as described above. The immunoprecipitated sample was then run on SDS/PAGE, and GH was detected by Western blot analysis using a rat GH anti-serum.
Immunocytochemistry.
Immunocytochemistry of the mouse anterior pituitary cells was performed as previously described (12). Images were captured using a TEC-470 CCD color camera (Optronics Engineering, Goleta, CA) on a Nikon Optiphot epifluorescent microscope coupled to a Macintosh 8100/100 PowerPC computer (Apple II+) using Adobe photoshop3.0.5 software (Adobe Systems, Mountain View, CA).
RESULTS
Expression of CPE in Normal and Cpefat Mice.
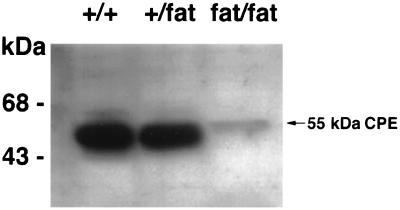
Because the Cpefat mouse carries a mutation in the CPE gene, the expression of CPE was examined by Western blot analysis in the pituitary of Cpefat and normal mice. Fig. 1 shows that mice carrying a mutation of the CPE gene in one allele (+/fat) had similar levels of CPE expression as wild-type animals (+/+). Thus +/+ and +/fat mice were used indiscriminately as normal animals in control studies described below. In contrast, Cpefat (fat/fat) homozygote mice had <10% of the CPE protein in the pituitary compared with +/+ mice.
Figure 1.
Western blot of CPE in BKS/Cpefat (fat/fat), BKS +/fat, and BKS +/+ mouse pituitaries. Equal volumes of pituitary extract were applied to each lane. Western blot analysis with the N-terminal CPE antibody showed a 55-kDa CPE band, which is indicated by the arrow. Identical results were obtained using the C-terminal antibody, indicating that the band represents full-length CPE. Note the marked decrease in CPE in the Cpefat (fat/fat) mouse pituitary compared with the +/+ and +/fat mice. Molecular mass markers are on the left.
Processing and Secretion of POMC from Anterior Pituitary Cells.
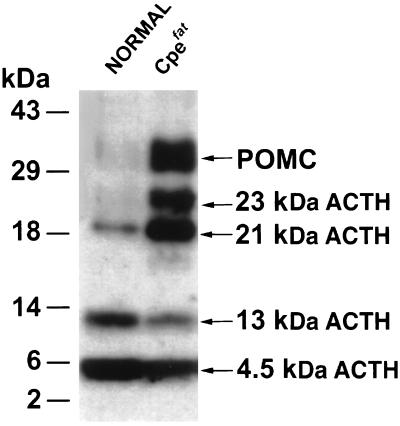
The processing and secretion of POMC was examined in primary cultures of AP cells of normal and Cpefat animals. Fig. 2 shows that although 4.5 kDa ACTH (ACTH1–39, the final processed product) and 13 kDa ACTH (glycosylated 4.5 kDa ACTH) were the major products found in the AP cells of normal animals, POMC and intermediate forms of ACTH (21–23 kDa) constituted the major ACTH-related products found in Cpefat mice. Quantitative analysis of the Western blots showed 24 ± 10-fold (n = 3) more POMC in Cpefat mice compared with normal mice. The POMC/ACTH ratio was 0.82 in Cpefat mice versus 0.04 in normal mice (Table 1). These results indicate enhanced synthesis of POMC in the AP cells of Cpefat mice that was poorly processed to ACTH because there was not large amounts of ACTH (13 kDa and 4.5 kDa) secreted in the medium with or without stimulation (Fig. 3).
Figure 2.
Western blot of immunoreactive ACTH in anterior pituitary cells of normal and Cpefat mice. Protein (18 μg) from the cell extracts was applied in each lane for SDS/PAGE and Western blot analysis with DP4 antiserum. Molecular mass markers are shown on the left, and the various forms of ACTH are indicated on the right.
Table 1.
Forms of immunoreactive ACTH in anterior pituitary (% total immunoreactive ACTH)
| Mice | POMC | 23 kDa | 21 kDa | 13 kDa | 4.5 kDa | POMC/ACTH* |
|---|---|---|---|---|---|---|
| Normal | 3.6 ± 1.6 | 1.05† | 8.3 ± 4.2 | 22 ± 7.0 | 64 ± 11 | 0.04 |
| Cpefat | 25 ± 0 | 17 ± 1.8 | 27 ± 3.0 | 7.6 ± 1.4 | 23 ± 0.3 | 0.82 |
Values are mean ± SEM from three different western blots (see Fig. 2).
ACTH includes 4.5 kDa and 13 kDa ACTH only.
23 kDa ACTH was present in one of the Western blots.
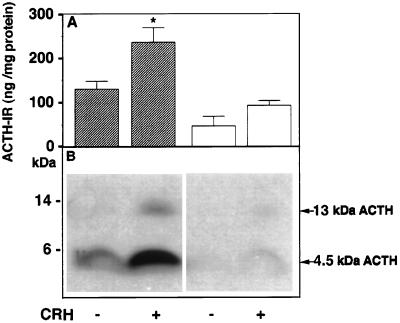
Figure 3.
Radioimmunoassay and Western blot of immunoreactive ACTH in the secretion medium of anterior pituitary cells with and without CRH stimulation. (A) Secretion medium was subjected to HPLC and fractions were radioimmunoassayed using DP6 ACTH antiserum. Immunoreactive ACTH values corresponding to the retention times for ACTH1–39, including oxidized and basic-residue extended forms, were summed and expressed as ng ACTH-IR/mg cell protein, from which the medium was derived. The solid and open bars show the mean value ± SEM from three HPLC runs of medium from normal and Cpefat mouse pituitary cells, respectively, with and without CRH stimulation. ∗, P < 0.05 relative to control in normal mice. (B) Western blot analysis of secretion medium showing stimulated secretion of 13 kDa and 4.5 kDa ACTH with CRH in normal mice and low levels of 4.5 kDa that was not stimulated by CRH in Cpefat mice.
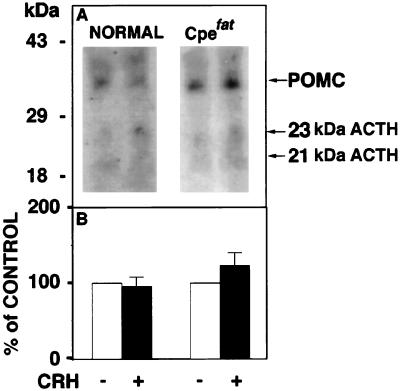
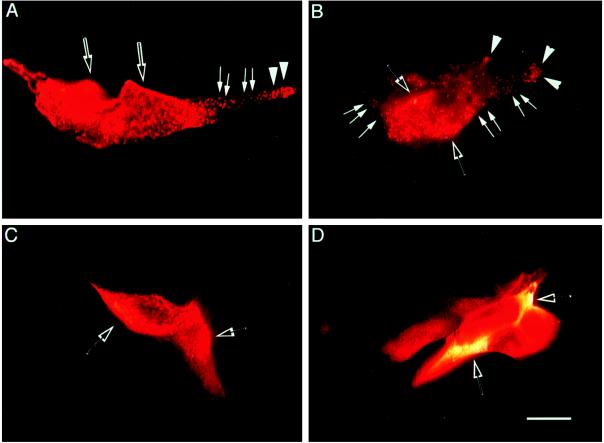
Radioimmunoassay of ACTH1–39 following HPLC of the secretion medium from AP cells revealed a 1.8-fold stimulation of ACTH1–39 with CRH treatment in normal mice. However, no significant stimulation with CRH was observed in Cpefat mice (Fig. 3A), indicating constitutive secretion. Moreover, there was significantly less ACTH (4.5 kDa and 13 kDa) released from the AP cells of Cpefat versus normal mice (P < 0.05) as revealed by radioimmunoassay (Fig. 3A), and this was also evident in the Western blot (Fig. 3B), consistent with lower amounts of ACTH (4.5 kDa and 13 kDa) synthesized in the cells (Fig. 2). In contrast, analysis of POMC secretion by Western blot (Fig. 4A) showed constitutive release of POMC and some 21–23 kDa ACTH into the medium from AP cells of Cpefat mice, as evidenced by the lack of stimulation by CRH (Fig. 4B). A small amount of POMC was sometimes also seen secreted constitutively from AP cells of normal mice (Fig. 4A) that was not stimulated by CRH (Fig. 4B). The amount of POMC released into the medium of Cpefat mice ranged from 3- to 60-fold greater than that in normal mice. Immunocytochemical localization of POMC/ACTH in a subpopulation of Cpefat mouse anterior pituitary corticotrophs showed accumulation of the immunoreactivity in the perinuclear region, characteristic of distribution in the ER/Golgi (Fig. 5 C and D). No punctate POMC/ACTH immunostaining was observed in these Cpefat mouse AP cells, unlike corticotrophs of normal mice (Fig. 5 A and B), indicating lack of sorting of POMC to the regulated secretory pathway.
Figure 4.
Western blot of POMC and 23 kDa and 21 kDa immunoreactive ACTH in secretion medium. (A) Western blot showing POMC and 21–23 kDa ACTH in the secretion medium from anterior pituitary cells of normal and Cpefat mice, with and without CRH treatment. Molecular mass markers are on the left, and the forms of ACTH are indicated by arrows on the right. (B) Western blots similar to that shown in A were quantitated. The sum of these three forms of ACTH from four Western blots of control (without CRH) secretion medium were averaged and made to equal 100% for normal and Cpefat animals (open bars). The sum of the three forms of ACTH in the secretion medium with CRH treatment was expressed as a percentage of control for normal and Cpefat mice. The mean percentage of control ± SEM (n = 4) is shown (solid bars). No significant difference was found between control and CRH treatment for both normal and Cpefat animals.
Figure 5.
Immunocytochemical localization of POMC in mouse anterior pituitary cells. Rhodamine staining for ACTHi is shown for normal mouse anterior pituitary cells (A and B) or for anterior pituitary cells from Cpefat mice grown in culture (C and D). Punctate granules in A and B are indicated by white arrows, and punctate granules accumulating in the tips of the cell extensions are indicated by large white arrowheads in A and B. ACTHi staining in the Golgi and ER is indicated in A–D by open arrows. There are no punctate granules found in the cells stained in C and D. (Bar = 10 μM.)
Secretion of Growth Hormone from Anterior Pituitary Cells.
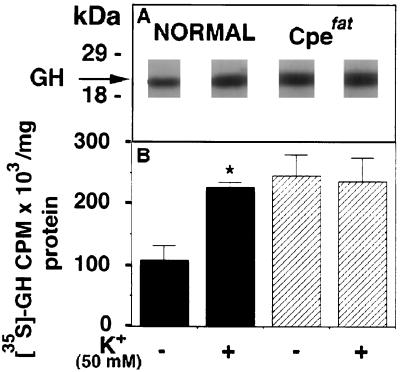
The secretion of GH from AP cells was examined in normal and Cpefat mice by pulse-labeling studies. Fig. 6A shows the labeled band of [35S]GH in the medium from normal and Cpefat mice. GH release was stimulated 2.1-fold by high K+ in normal mice (Fig. 6B). In Cpefat mice, high K+ did not stimulate release of GH from AP cells, but there was a 2.2-fold increase in basal secretion of GH into the medium as compared with normal mice (Fig. 6). Western blot analysis showed that GH levels in the Cpefat mouse AP cells were 1.53 ± 0.07-fold (n = 3, P < 0.01) higher than in normal mice.
Figure 6.
(A) Autoradiograph showing immunoprecipitated [35S]GH in the secretion medium from control (without high K+ treatment) and high-K+-treated anterior pituitary cells from normal and Cpefat mice. The molecular mass markers are shown on the left. (B) Solid bars show the mean ± SEM (n = 3) cpm [35S]GH/mg cell protein, in the medium from normal cells without and with high K+ treatment. Cross-hatched bars show the mean ± SEM (n = 3) cpm [35S]GH/mg cell protein in the medium from Cpefat mouse cells without and with 50 mM K+ treatment. ∗, P < 0.05 relative to control.
Circulating Levels of ACTH and GH in Normal and Cpefat Mice.
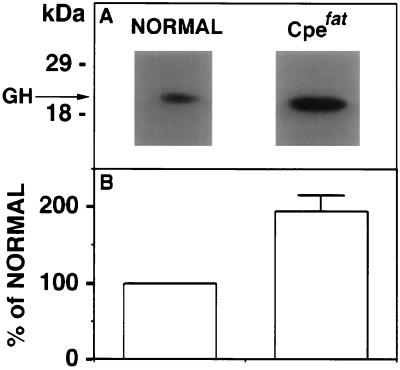
Plasma levels of immunoreactive ACTH were determined by radioimmunoassay in Cpefat and normal mice. The mean (± SEM) levels of circulating immunoreactive ACTH in Cpefat mice (2.63 ± 0.25 ng/ml, n = 7) were above normal mice (1.96 ± 0.28 ng/ml, n = 5). Although the values were not statistically significant (P = 0.1) between normal and Cpefat mice, due to the low number of animals, there could be qualitative differences because the antiserum, DP5, at the dilution used for the radioimmunoassay cross-reacts with all forms of ACTH. By extrapolation to that present in the medium from in vitro release experiments, the ACTH immunoreactivity observed in plasma from Cpefat mice should represent primarily POMC, some 21–23 kDa ACTH, and only a small amount of ACTH (Figs. 3 and 4). Indeed, a Western blot of plasma from Cpefat mice confirmed the presence of POMC as a major immunoreactive component (data not shown). GH levels in the plasma were determined by Western blot analysis (Fig. 7) in Cpefat and normal mice. Cpefat mice showed 1.94 ± 0.21-fold (n = 4, P < 0.05) higher levels of GH in the circulation when compared with normal mice.
Figure 7.
(A) Western blot of GH in normal and Cpefat mice serum. GH is indicated by the arrow and molecular mass markers are shown on the left. (B) Bar graphs show the quantitation of serum GH in Cpefat mice from the Western blots expressed as a percentage of that present in serum of normal mice, which was made equal to 100%. The bar graph for Cpefat mice shows the mean percentage ± SEM of normal (194 ± 21; n = 4, P < 0.05).
DISCUSSION
The Cpefat (fat/fat) mouse is obese and has a mutation in the CPE gene (16). While the levels of the mutant CPE mRNA in the pituitary of Cpefat mice were comparable to wild-type (+/+) animals, the CPE protein levels and enzymatic activity were greatly diminished (16). However, the +/fat animals have as much CPE in the pituitary as wild-type animals (Fig. 1), and they are lean. This indicates that a mutation in both alleles is necessary for expression of the obese phenotype. The decreased cellular content of CPE with the Ser202 → Pro202 substitution in the Cpefat mouse was shown to be due to premature degradation of the enzyme, either in the rough ER or Golgi (21). The Cpefat mouse was reported to have hyperproinsulinemia and accumulation of high levels of proinsulin in the pancreas (16). It was hypothesized that proinsulin was poorly processed to insulin in these mice due to inactive PC2, a prohormone convertase that processes proinsulin (22). CPE has been shown in vitro to be able to cleave the C-terminal basic residues of a fragment of 7B2, a PC2 inhibitor, causing it to release from binding to PC2 and thereby activating the enzyme (23). While this phenomenon may also be occurring, our present study indicates a defect in the intracellular routing of peptide hormones to the regulated secretory pathway in these mice, which can account for the lack of processing and secretion of large amounts of proinsulin into the circulation (16). This is consistent with our recent identification of CPE as a sorting receptor for the regulated secretory pathway proteins (5). To provide evidence for a defect in (pro-) hormone sorting in Cpefat mice, we studied the intracellular trafficking of two hormone systems, ACTH and GH in the anterior pituitary. ACTH is generated from processing of POMC by the enzyme PC1 (24, 25), whereas GH is not derived from a precursor and does not require endoproteolytic processing, except for the removal of the “pre-” sequence.
In the Cpefat mouse, POMC was poorly processed as reflected by its content in the anterior pituitary cells, which was ≈20-fold greater than in normal mice. However, the final processed product, ACTH (4.5 kDa and 13 kDa), was only ≈30% of that in normal mice. This result is similar to the low processing of proinsulin observed in the pancreas of these animals. Because POMC is processed by PC1, the inefficient processing of this prohormone cannot be attributed to the lack of activation of PC2, as was hypothesized for proinsulin. Some processing of POMC occurred probably due to the action of PC1 that is active in the trans-Golgi network (26). POMC was released at high levels, constitutively, from the Cpefat mouse AP cells into the medium. However, there was still a large amount of POMC remaining in the Cpefat mouse AP cells that was localized by immunocytochemistry to the ER/Golgi, rather than in punctate granules, consistent with the lack of targeting of POMC to the regulated secretory granules. In contrast, normal mouse AP cells released little or no POMC. There was 64% less ACTH1–39 released from AP cells of Cpefat mice compared with normal mice under CRH-stimulated conditions, and analysis by HPLC followed by carboxypeptidase B treatment indicated they were basic-residue extended forms (unpublished data). This is consistent with the finding of C-terminal basic-residue extended forms of insulin (16) in islet cells and neurotensin in the brain of Cpefat mice (27). Circulating levels of POMC/ACTH immunoreactivity in the Cpefat mice represents primarily POMC. The difference in immunoreactive ACTH levels in the plasma of Cpefat and normal mice would have been greater if not for the unusually high levels of ACTH in the normal mice, probably due to stress from the transportation and handling, because the mice were sacrificed <24 h after arrival. Because Cpefat mice secrete POMC/ACTH constitutively in an unregulated manner, their levels were not expected to change with stress. Higher circulating POMC levels in Cpefat mice may not compensate for the decreased ACTH1–39 because POMC is 300-fold less effective than ACTH1–39 in stimulating corticosterone secretion (28). The finding of 45% less ACTH binding sites observed in adrenal glands of Cpefat mice compared with normal animals (unpublished data) is also consistent with decreased plasma ACTH, because ACTH has been shown to induce up-regulation of ACTH receptor mRNA in mouse and human adrenal cell lines (29). ACTH is a potent lipolytic hormone (30), and MC2 ACTH receptors have been found in murine adipose tissues, similar to those present in adrenal cells (31). Thus, lower levels of plasma ACTH in Cpefat mice may also contribute to the etiology of the obesity observed in these animals due to decreased lipolysis in adipose tissue.
Analysis of the secretion of GH showed that while high K+ stimulated release 2.1-fold in AP cells of normal animals, similar to that observed for rat (32, 33), it had no effect on release of GH from AP cells of the Cpefat mice, indicating constitutive secretion. The level of constitutive secretion of GH from cultured AP cells of Cpefat mice was 2.2-fold higher than basal release in normal mice, and this elevated secretion was also found for circulating levels of GH in Cpefat mice. GH level in Cpefat AP cells was elevated only 1.5-fold, which is much less than POMC. This may be due to higher basal secretion of GH.
In conclusion, this study has demonstrated that two peptide hormones, POMC/ACTH and GH were secreted via the constitutive pathway in an unregulated manner from the anterior pituitary cells of Cpefat mice, indicating that they were not correctly sorted to the granules of the regulated secretory pathway. This misrouting resulted in POMC being poorly processed in the anterior pituitary of the Cpefat mice. The intracellular misrouting defect also led to elevated levels of POMC and GH in the circulation of the Cpefat mice. Since our binding studies indicate that CPE may be a general sorting receptor for many prohormones and proneuropeptides destined for the regulated secretory pathway, including proinsulin (5), it is predicted that processing and secretion of many peptide precursors in the endocrine and nervous systems would be affected in these Cpefat mice, which could lead to various neuro-/endocrine disorders.
Acknowledgments
We thank Dr. Ed Leiter (The Jackson Laboratory) for the gift of Cpefat mice and Dr. David Cool for his help with the immunocytochemistry of the anterior pituitary cells and critical reading of the manuscript. We also thank Dr. Gretti Aguilera for helpful discussions and Marie Nicolas for typing the manuscript.
ABBREVIATIONS
- POMC
pro-opiomelanocortin
- CPE
carboxypeptidase E
- ACTH
adrenocorticotropin
- GH
growth hormone
- AP
anterior pituitary
- CRH
corticotropin-releasing hormone
- ER
endoplasmic reticulum
References
- 1.Loh Y P, Brownstein M J, Gainer H. Annu Rev Neurosci. 1984;7:189–222. doi: 10.1146/annurev.ne.07.030184.001201. [DOI] [PubMed] [Google Scholar]
- 2.Orci L, Ravazzola M, Storch M-J, Anderson R G W, Vassalli J-D, Perrelet A. Cell. 1987;49:865–868. doi: 10.1016/0092-8674(87)90624-6. [DOI] [PubMed] [Google Scholar]
- 3.Kelly R B. Science. 1985;230:25–32. doi: 10.1126/science.2994224. [DOI] [PubMed] [Google Scholar]
- 4.Burgess T L, Kelly R B. Annu Rev Cell Biol. 1987;3:243–293. doi: 10.1146/annurev.cb.03.110187.001331. [DOI] [PubMed] [Google Scholar]
- 5.Cool D R, Normant E, Shen F-S, Chen H-C, Pannell L, Zhang Y, Loh Y P. Cell. 1997;88:73–83. doi: 10.1016/s0092-8674(00)81860-7. [DOI] [PubMed] [Google Scholar]
- 6.Yoo S H, Lewis M S. J Biol Chem. 1992;267:11236–11241. [PubMed] [Google Scholar]
- 7.Colomer V, Kicska G A, Rindler M J. J Biol Chem. 1996;271:48–55. doi: 10.1074/jbc.271.1.48. [DOI] [PubMed] [Google Scholar]
- 8.Chanat E, Huttner W B. J Cell Biol. 1991;115:1505–1519. doi: 10.1083/jcb.115.6.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kornfeld S. Biochem Soc Trans. 1990;18:367–374. doi: 10.1042/bst0180367. [DOI] [PubMed] [Google Scholar]
- 10.Vaux D, Tooze J, Fuller S. Nature (London) 1990;345:495–502. doi: 10.1038/345495a0. [DOI] [PubMed] [Google Scholar]
- 11.Chanat E, Weiss U, Huttner W B, Tooze S A. EMBO J. 1993;12:2159–2168. doi: 10.1002/j.1460-2075.1993.tb05864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cool D R, Fenger M, Snell C R, Loh Y P. J Biol Chem. 1995;270:8723–8729. doi: 10.1074/jbc.270.15.8723. [DOI] [PubMed] [Google Scholar]
- 13.Cool D R, Loh Y P. Biochimie. 1994;76:265–270. doi: 10.1016/0300-9084(94)90156-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hook V Y H, Loh Y P. Proc Natl Acad Sci USA. 1984;81:2776–2780. doi: 10.1073/pnas.81.9.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fricker L. Annu Rev Physiol. 1988;50:309–321. doi: 10.1146/annurev.ph.50.030188.001521. [DOI] [PubMed] [Google Scholar]
- 16.Naggert J K, Fricker L D, Varlamov O, Nishina P M, Rouille Y, Steiner D F, Carroll R J, Paigen B J, Leiter E H. Nat Genet. 1995;10:135–142. doi: 10.1038/ng0695-135. [DOI] [PubMed] [Google Scholar]
- 17.Loh Y P, Castro M G, Zeng F-J, Patel-Vaidya U. J Mol Endocrinol. 1988;1:39–48. doi: 10.1677/jme.0.0010039. [DOI] [PubMed] [Google Scholar]
- 18.Cool D R, Louie D Y, Loh Y P. Endocrinology. 1996;137:5441–5446. doi: 10.1210/endo.137.12.8940369. [DOI] [PubMed] [Google Scholar]
- 19.Birch N P, Loh Y P. In: Proteolytic Enzymes. Beynon R J, Bond J S, editors. Oxford: IRL; 1989. pp. 211–230. [Google Scholar]
- 20.Estivariz F E, Friedman T C, Chikuma T, Loh Y P. J Biol Chem. 1992;267:7456–7463. [PubMed] [Google Scholar]
- 21.Varlamov O, Leiter E H, Fricker L. J Biol Chem. 1996;271:13981–13986. doi: 10.1074/jbc.271.24.13981. [DOI] [PubMed] [Google Scholar]
- 22.Smeekens S, Montag A, Thomas G, Albiges-Rizo C, Carroll R, Benig M, Phillips L, Martin S, Ohagi S, Gardner P, Swift H, Steiner D. Proc Natl Acad Sci USA. 1992;89:8822–8826. doi: 10.1073/pnas.89.18.8822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu X, Rouille N S, Lamango N S, Steiner D F, Lindberg I. Proc Natl Acad Sci USA. 1996;93:4919–4924. doi: 10.1073/pnas.93.10.4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benjannet S, Rondeau N, Day R, Chretien M, Seidah N G. Proc Natl Acad Sci USA. 1991;88:3564–3568. doi: 10.1073/pnas.88.9.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas L, Leduc R, Thorne B, Smeekens S, Steiner D, Thomas G. Proc Natl Acad Sci USA. 1991;88:5297–5301. doi: 10.1073/pnas.88.12.5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindberg I, Ahn S C, Breslin M B. Mol Cell Neurosci. 1994;5:614–622. doi: 10.1006/mcne.1994.1075. [DOI] [PubMed] [Google Scholar]
- 27.Rovere C, Viale A, Nahon J, Kitabgi P. Endocrinology. 1996;137:2954–2958. doi: 10.1210/endo.137.7.8770919. [DOI] [PubMed] [Google Scholar]
- 28.Gasson J. Biochemistry. 1979;18:4215–4224. doi: 10.1021/bi00586a028. [DOI] [PubMed] [Google Scholar]
- 29.Mountjoy K G, Bird I M, Rainey W E, Cone R D. Mol Cell Endocrinol. 1994;99:R17–20. doi: 10.1016/0303-7207(94)90160-0. [DOI] [PubMed] [Google Scholar]
- 30.Ramachandran J, Farmer S W, Liles S, Li C H. Biochim Biophys Acta. 1976;428:347–354. doi: 10.1016/0304-4165(76)90042-8. [DOI] [PubMed] [Google Scholar]
- 31.Boston B A, Cone R D. Endocrinology. 1996;137:2043–2050. doi: 10.1210/endo.137.5.8612546. [DOI] [PubMed] [Google Scholar]
- 32.Stojilkovic S S, Izumi S-I, Catt K J. J Biol Chem. 1988;263:13054–13061. [PubMed] [Google Scholar]
- 33.Lussier, B. T., French, M. B., Moor, B. C. & Kraicer, J. (1991) 128, 592–603. [DOI] [PubMed]