Abstract
Objectives
The primary goal of this study was to determine if physiological forward-masking patterns in cochlear implants are predictive of psychophysical forward-masking patterns (PFM). It was hypothesized that the normalized amount of physiological masking would be positively correlated with the normalized amount of psychophysical masking for different masker-probe electrode separations. A secondary goal was to examine the relation between the spatial forward-masking patterns and speech-perception performance. It was hypothesized that subjects with less channel interaction overall (either psychophysically or physiologically) would have better speech-perception ability due to better spectral resolution.
Design
Data were collected for 18 adult cochlear implant recipients (N = 9 Clarion CII or HiRes 90K, N = 9 Nucleus 24R[CS]). Physiological spatial forward-masking patterns were obtained with the electrically evoked compound action potential (ECAP) through the implant telemetry system. PFM patterns were obtained using a 3-interval, 2-alternative forced-choice adaptive procedure. Both measures used a fixed probe electrode with varied masker location. For each subject, spatial forward-masking patterns were obtained for three probe electrodes with five masker locations per probe.
Results
On an individual basis, the correlation between ECAP FM and PFM was strong for 10 subjects (r = 0.68 to 0.85, p ≤ 0.02), moderately strong for two subjects (r = 0.54 to 0.55, p = 0.06 to 0.07), and poor for six subjects (r = 0.13 to 0.45, p > 0.14). Results across subjects and electrodes showed a highly significant correlation between ECAP FM and PFM (r = 0.55, p < 0.0001); the correlation was strongest for basal electrodes. There was no significant correlation between speech perception and ECAP FM or PFM. Subjects whose ECAP FM patterns correlated well with PFM patterns generally had the poorest speech perception and subjects with the poorest correlations had the best speech perception.
Conclusions
ECAP FM and PFM patterns correlated well for two-thirds of the subjects. Although the group correlation was statistically significant, ECAP FM patterns only accounted for 30% of the variance in the PFM measures. This suggests that the ECAP measures alone are not sufficient for accurately predicting PFM patterns for individual subjects.
Keywords: cochlear implant, forward masking, electrically evoked compound action potential, channel interaction, speech perception
Introduction
In multi-channel cochlear implants (CIs), spectral representation of the incoming signal is achieved through stimulation of various electrodes located along the longitudinal axis of the cochlea. Current from each electrode creates an electric field that stimulates surrounding neural tissue. Typically, current fields are not completely distinct from electrode to electrode, but rather overlap with one another to some extent. This overlap can cause interactions between the current fields themselves or between neural populations stimulated by different electrodes, which may ultimately affect perception with the implant. These channel interactions can occur with either simultaneous or sequential stimulation. With simultaneous stimulation, current fields can interact by summing or subtracting prior to neural stimulation, potentially resulting in over-stimulation or an inadequate amount of current, respectively (e.g., Abbas & Brown, 1988; Boëx, de Balthasar, Kós, & Pelizzone, 2003a; Middlebrooks, 2004; Shannon, 1983b; White, Merzenich, & Gardi, 1984). With sequential stimulation, interaction can occur due to forward-masking effects within the overlapping neural population, where neurons that respond to the first stimulus are unable to respond to a following stimulus due to neural refractory properties. The perceptual effect of this latter type of channel interaction remains unclear, although it may lead to spectral smearing and/or reduced speech perception (Throckmorton & Collins, 1999). In CI recipients, spatial forward-masking patterns can be measured by presenting masker and probe stimuli to different electrodes along the intracochlear array. Typically one stimulus is fixed in location as the other is varied across the array. This type of forward masking has been measured both physiologically (Abbas, Hughes, Brown, et al., 2004; Cohen, Richardson, Saunders, & Cowan, 2003; Eisen & Franck, 2005; Hughes & Abbas, 2006ba; 2006b) and psychophysically (Boëx, Kós, & Pelizzone, 2003b; Chatterjee, Galvin, Fu, & Shannon, 2006; Chatterjee & Shannon, 1998; Cohen et al., 2003; Kwon and van den Honert, 2006; Lim, Tong, & Clark, 1989; Shannon, 1983b; Throckmorton & Collins, 1999). Results from both psychophysical and physiological forward-masking studies have shown that channel interaction decreases as electrode separation increases. Thus, it can be hypothesized that spatial forward-masking patterns for physiological stimuli are related to or predictive of spatial forward-masking patterns obtained psychophysically. Because the majority of speech-processing strategies utilize sequential stimulation, the present study focuses on the effects of spatial channel interaction obtained with forward masking (i.e., sequential stimulation) for both physiological and psychophysical stimuli.
Cohen et al. (2003) compared spatial forward-masking patterns obtained using the electrically evoked compound action potential (ECAP) to those obtained psychophysically in a group of six Nucleus CI recipients. They found no significant difference in the width of the two functions, suggesting that the same patterns emerged for both physiological and psychophysical measures. Although this finding is encouraging, the results are from a single study with a small subject pool. Additionally, relative stimulus level was not consistent between ECAP and psychophysical measures, psychophysical measures used a fixed masker electrode with varied probe whereas ECAP measures used a fixed probe electrode with varied masker, different ground electrodes were used for ECAP and psychophysical measures, and a subset of data were excluded from the statistical analysis. The present study further investigated the relation between ECAP and psychophysical spatial forward masking patterns in a larger group of subjects with attention to these methodological issues.
One current area of CI research is aimed at reducing channel interaction with the goal of improving speech perception with the implant. Spatial neural excitation patterns are dependent on a number of factors, including neural survival, bone or tissue growth within the cochlea, electrode design and placement, and signal characteristics (e.g., Finley, Wilson, & White, 1990; Frijns, Briaire, & Grote, 2001; Kral, Hartmann, Mortazavi, & Klinke, 1998; Liang, Lusted, & White, 1999; Rebscher, Snyder, & Leake, 2001; Ryan, Miller, Wang, & Wolf, 1990; van den Honert & Stypulkowski, 1987). A common method used to reduce channel interaction is to modify electrode coupling to create more spatially restricted current fields. It is known that spatially restricted electric fields, such as that produced by bipolar or tripolar stimulation, yield selective recruitment patterns of peripheral and central auditory nerve fibers, whereas broader electric fields (e.g., produced by monopolar stimulation) result in broad recruitment patterns (Bierer & Middlebrooks, 2002; Kral et al., 1998; Snyder, Bierer, & Middlebrooks, 2001; van den Honert & Stypulkowski, 1987). It is often assumed that speech-processing strategies that utilize highly selective stimulation modes should yield better spectral resolution than broad modes, ultimately resulting in better speech-perception performance. However, several studies have demonstrated the same or better performance for broader stimulation modes than for more spatially restrictive modes (Mens & Berenstein, 2005; Pfingst, Franck, Xu, et al., 2001; Pfingst, Zwolan, & Holloway, 1997; Zwolan, Kileny, Ashbaugh, & Telian, 1996). Further, virtually all of the newest speech-processing strategies use monopolar stimulation, with recipients achieving higher scores on speech-perception measures than previously (e.g., Dunn, Tyler, Witt, & Gantz, 2006; Parkinson, Arcaroli, Staller, et al., 2002). These results question the assumption that reducing channel interaction unequivocally leads to improved performance.
Spatial forward-masking patterns can be compared with speech perception to evaluate the extent to which peripheral channel interaction affects performance with a CI. The height of the pattern (amount of masking) reflects temporal effects; i.e., greater amount of masking represents temporal interaction between masker and probe. The width of the pattern reflects spatial interactions; i.e., narrower patterns represent better spatial selectivity. The average amount of masking across an entire function reflects both temporal and spatial interactions (see Throckmorton and Collins, 1999). Results from studies comparing spatial forward-masking patterns to speech perception have been mixed. Hughes and Abbas (2006a) found no correlation between width of ECAP spatial forward masking patterns (an index of spatial selectivity) and speech-perception performance on word, phoneme, and sentences-in-noise tests for a group of ten Nucleus-24 recipients using the spectral peak (SPEAK; Seligman & McDermott, 1995; Skinner, Clark, Whitford, et al., 1994) or advanced combination encoder (ACE; Arndt, Staller, Arcaroli, et al., 1999) processing strategy with monopolar stimulation. In contrast, Throckmorton and Collins (1999) reported a significant correlation between average psychophysical forward-masking levels (which reflects both temporal and spatial interactions) and speech recognition for sentences, medial consonants, and phonemes in a group of seven Nucleus-22 recipients using either bipolar-plus-one (BP+1) or BP+2 stimulation. They also reported average widths of the psychophysical functions for three subjects, where the subject with the narrowest function had the highest average speech-perception score. Additionally, Boëx et al. (2003b) found a significant negative correlation between psychophysical forward masking (measured as the ratio of masked to unmasked probe thresholds at a masker-probe separation of 4 mm) and percent correct on a medial consonant test for 12 subjects implanted with either the Ineraid or Clarion device, utilizing either continuous interleaved sampling (CIS; Wilson, Finley, Lawson, et al., 1991) with monopolar coupling or simultaneous analog stimulation (SAS; Battmer, Feldmeier, Kohlenberg, & Lenarz, 1997; Osberger & Fisher, 2000) with bipolar coupling.
Electrode discrimination and pitch ranking, which can be considered indices of spectral overlap between electrodes, have also been compared with speech perception in a number of studies. Some studies have found a positive correlation (Donaldson & Nelson, 2000; Henry, McKay, McDermott, & Clark, 2000; Nelson, Van Tassell, Schroder, et al., 1995; Throckmorton & Collins, 1999) and others have found no correlation (Hughes & Abbas, 2006a; Zwolan, Collins, & Wakefield, 1997). Given the differences in methodology, stimulation modes, and processing strategies across studies, the extent to which channel interaction affects speech perception with a CI remains unclear.
The primary purpose of this study was to compare ECAP spatial forward-masking patterns with psychophysical masking patterns to determine whether ECAP measures can provide a quick, objective alternative to time-consuming psychophysical measures. It was hypothesized that the relative masking with the ECAP would be correlated with the relative masking in the psychophysical task for different masker-probe electrode separations. The secondary purpose of this study was to compare physiological and psychophysical spatial forward-masking measures with speech-perception ability to determine how channel interaction relates to performance with a CI. It was hypothesized that subjects with less channel interaction (either psychophysically or physiologically) would have better speech-perception ability due to improved spectral resolution.
Materials and Methods
Subjects
Data were collected for 18 adult CI recipients. Demographic information for participants is listed in Table 1. Nine subjects were implanted with the Nucleus 24R(CS) Contour array (Cochlear Americas, Englewood, CO; subject number beginning with “R”) and nine were implanted with Clarion devices (Advanced Bionics Corporation, Valencia, CA; N = 4 Clarion CII, N = 5 HiRes 90K; subject number beginning with “C”). Subject C1 was the only subject with an electrode positioner. Subject R6 received sequential bilateral CIs, with a Nucleus 22 in the left ear followed six years later by a Nucleus 24R(CS) in the right ear. This subject was tested using the right-ear 24R(CS) implant only, as the Nucleus 22 lacks telemetry capabilities necessary for ECAP measures. Impedance measures were made for all subjects prior to data collection to identify any short or open circuits. Subject R1 had open circuits on electrodes 10, 13, 15, 17, and 20. These electrodes were excluded from testing for this subject. This study was approved by the Boys Town National Research Hospital Institutional Review Board under protocol number 03–07.
Table 1.
Demographic information for participating subjects.
| Subject | CI ear | Device | Age at CI (yrs, mos) | Duration deafness, CI ear (yrs, mos) | Duration CI use (yrs, mos) | Processing strategy | Contralateral ear amplification |
|---|---|---|---|---|---|---|---|
| R1 | R | 24R(CS) | 60, 10 | 4, 0 | 3, 2 | ACE | HA |
| R2 | R | 24R(CS) | 51, 10 | 2, 0 | 2, 3 | ACE | None |
| R3 | R | 24R(CS) | 56, 1 | 50, 0 | 3, 0 | ACE | HA |
| R4 | L | 24R(CS) | 41, 11 | 1, 0 | 2, 8 | ACE | None |
| R5 | R | 24R(CS) | 49, 5 | 16, 0 | 4, 7 | ACE | None |
| R6 | R | 24R(CS) | 44, 6 | 0, 10 | 1, 1 | ACE | Nuc22 CI |
| R7 | R | 24R(CS) | 62, 2 | 5, 0 | 0, 6 | ACE | HA |
| R9 | L | 24R(CS) | 40, 7 | 29, 0 | 2, 10 | ACE | HA |
| R10 | R | 24R(CS) | 61, 10 | 2, 0 | 2, 6 | ACE | HA |
| C1 | R | CII | 18, 4 | 18, 4 | 2, 9 | HiRes-P | None |
| C2 | L | 90K | 69, 1 | 0, 6 | 1, 0 | HiRes-P | HA |
| C4 | R | 90K | 57, 0 | 19, 0 | 0, 5 | HiRes-P | HA |
| C5 | R | 90K | 42, 11 | 42, 0 | 0, 7 | HiRes-P | HA |
| C6 | L | CII | 57, 3 | 5, 0 | 2, 3 | HiRes-P | None |
| C7 | L | CII | 64, 4 | 21, 0 | 2, 0 | HiRes-P | HA |
| C8 | R | CII | 55, 7 | 3, 0 | 1, 11 | HiRes-P | HA |
| C9 | R | 90K | 41, 11 | 4, 0 | 1, 2 | HiRes-P | None |
| C10 | R | 90K | 51, 10 | 35, 0 | 0, 7 | HiRes-S | HA |
R = right, L = left, ACE = Advanced Combination Encoder, HiRes-P = High Resolution Paired, HiRes-S = High Resolution Sequential, HA = hearing aid, Nuc22 CI = Nucleus 22 cochlear implant.
ECAP Stimuli and Procedure
ECAP measures were obtained prior to psychophysical testing for all subjects. There was no ECAP response on electrode 16 (E16) in subject R9, so that electrode was not tested in the psychophysical portion of the experiment. ECAPs were obtained using a traditional forward-masking technique described previously (e.g., Abbas, Brown, Shallop, et al., 1999; Abbas et al., 2004; Brown, Hughes, Luk, et al., 2000; Cohen et al., 2003; Dillier, Lai, Almqvist, et al., 2002). For Nucleus subjects, ECAPs were measured using the Neural Response Telemetry (NRT, v. 3.0) software. With NRT, stimuli were delivered through a SPrint body-worn speech processor interfaced with a Processor Control Interface (PCI). The following default parameters were used: 80-Hz rate, 60-dB gain, 500-μsec masker-probe interval, MP1 (extracochlear monopolar ball electrode) for masker/probe reference electrode, and MP2 (extracochlear monopolar plate electrode) for recording reference electrode. Responses were recorded using 50–200 averages, depending on overall amplitude and noise level of each waveform. For all but one of the Clarion subjects (C1), ECAPs were measured using the Bionic Ear Data Collection System (BEDCS, v. 1.16.191) with the following parameters: 20-Hz rate, 1000 gain, 500-μsec masker-probe interval, case ground (IE1 in the CII device and IE2 in the 90K device) for masker/probe reference electrode, and case ground (IE1) for CII subjects or ring ground (IE1) for 90K subjects for recording reference electrode. Responses were recorded using 60–100 averages, depending on overall amplitude and noise level of each waveform. ECAP measures for the first Clarion subject (C1) were obtained using a test-bench version of the Neural Response Imaging (NRI) software. The only parameter difference between NRI and BEDCS was that a gain of 300 was used for NRI. Masker and probe stimuli for ECAP measures each consisted of a single biphasic current pulse. Pulse duration was 25 μsec/phase for the first three Nucleus subjects and the first two Clarion subjects enrolled in the study (R1, R2, R3, C1, and C2); all subsequent subjects used 50 μsec/phase. This change allowed greater headroom for maximum comfort levels to be achieved without reaching voltage compliance limits, as some of the early subjects were very close to these limits. For each subject, the same pulse duration was used for both ECAP and psychophysical measurements to allow direct comparison between the two measures. All pulses for both measures (ECAP and psychophysics) and device types (Nucleus and Clarion) had an inter-phase gap of 8–10 μsec.
Behavioral dynamic range for the ECAP stimulus was determined using an ascending procedure. Subjects used a visual rating scale that ranged from 0 (no sound) to 10 (stop—too loud) to indicate the current level at which the sound was first heard (rating of 1) and the highest level at which the sound was loud but still tolerable (rating of 8). The current level for a rating of 1 represented behavioral threshold and the current level for a rating of 8 was taken as the upper limit of the behavioral dynamic range.
For ECAP data collection, masker and probe stimulus levels were fixed at 80% of the behavioral dynamic range using the ECAP stimulus. For three subjects, higher levels were necessary to elicit measurable ECAP responses: subject R5, E16 at 100%; subject R9, E7 at 90%, E11 at 100% and no response on E16; subject C6, E12 at 90%. The probe pulse was fixed on one electrode, while the masker varied in location from +4, +2, 0, −2, and −4 electrode positions relative to the probe electrode for Clarion subjects and +6, +3, 0, −3, and −6 positions for Nucleus subjects, so as to maintain relatively consistent radial distance between electrodes for the two device types. (An electrode spacing of two in the Clarion approximately equals an electrode spacing of three in the Nucleus.) Masker-probe electrode pairs tested for each device are listed in Table 2. Clarion devices are numbered such that E1 is most apical, and Nucleus devices are numbered such that E1 is most basal. E13 was initially chosen for the basal probe electrode in the first Clarion subject (C1); E12 was chosen for all subsequent Clarion subjects to accommodate four electrode spacings in a basal direction for the masker. Subject R1 had open circuits on E10, E13, and E17, so E9, E12/14, and E16, respectively, were used instead.
Table 2.
Masker and probe electrode combinations for psychophysical and physiological testing for each device type. Electrode number 1 is basal for Nucleus and apical for Clarion.
| Nucleus 24R(CS) | Clarion CII/90K | ||
|---|---|---|---|
| Probe Electrode | Masker Electrodes | Probe Electrode | Masker Electrodes |
| 7 | 1, 4, 7, 10, 13 | 5 | 1, 3, 5, 7, 9 |
| 11 | 5, 8, 11, 14, 17 | 9 | 5, 7, 9, 11, 13 |
| 16 | 10, 13, 16, 19, 22 | 12 | 8, 10, 12, 14, 16 |
ECAP Data Analysis
Peak-to-peak ECAP amplitudes were measured from the leading negative peak (N1) to the following positive peak or plateau (P2) using a custom analysis program written in Matlab. With the forward-masking subtraction paradigm, the ECAP amplitude will generally decrease as the masker is placed farther away from the probe (Abbas et al., 2004; Cohen et al., 2003; Eisen & Franck, 2005; Hughes & Abbas, 2006a). As a result, the largest ECAP amplitude tends to occur when masker and probe stimuli are on the same electrode. This trend is illustrated in Figure 1A with open circles, which show ECAP amplitudes as a function of masker electrode for the three probe electrodes (P7, P11, and P16) tested in Nucleus subject R3. These amplitudes were then normalized (Figure 1B, open circles) relative to the amplitude of the ECAP obtained when the masker and probe were presented on the same electrode, where maximal masking typically occurs. Normalized amplitudes represent the relative amount of masking (or overlap) across masker electrodes. For example, a normalized masking value of 1.0 represents 100% overlap between masker and probe and 0.2 represents a 20% overlap. Changes in ECAP amplitude as a function of masker electrode location therefore reflect changes in the relative amount of masking across electrodes. The normalized masking for the ECAP (ECAP forward masking, or ECAP FM) was then compared to psychophysical data.
Fig. 1.
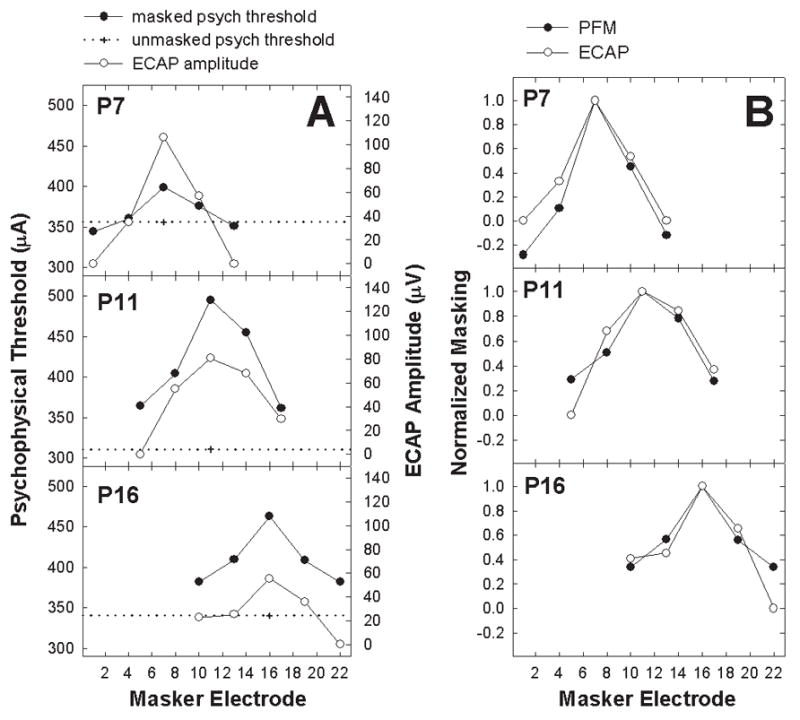
A: Raw psychophysical (left ordinate) and physiological (electrically evoked compound action potential [ECAP]; right ordinate) data from Nucleus subject R3. Each panel represents data from a different probe electrode (electrode number indicated on each panel). Plus symbols with horizontal dotted lines represent unmasked psychophysical probe thresholds. Filled circles show masked psychophysical forward-masking (PFM) thresholds as a function of masker electrode. Open circles indicate ECAP amplitude as a function of masker electrode. B: Data from Figure 1A normalized to the masker-equals-probe-electrode condition.
Psychophysical Stimuli and Procedure
For each subject, the same monopolar masker-probe electrode pairs were used for both the ECAP and psychophysical portions of the experiment (see Table 2). Ideally, spatial forward masking should utilize a narrow electrode configuration for the probe to obtain the most precise measures. However, because the goal was to examine interactions that may occur with typical device use, monopolar electrode coupling was used for all measures in this study. Psychophysical masker and probe stimuli consisted of a 1000-pps train of 50-μsec/phase biphasic current pulses (25-μsec/phase for the first five subjects, as noted previously). Masker duration was 300 ms and probe duration was 20 ms. The masker-probe delay was 2 ms from offset of the masker to onset of the probe. Behavioral dynamic range was measured for each masker electrode with the 300-ms stimulus and for each probe electrode with the 20-ms stimulus using the procedure described above for the ECAP stimulus. Dynamic range measures for the different stimuli were necessary to determine appropriate presentation levels for the psychophysical masker, psychophysical probe, and ECAP stimuli because threshold and maximum loudness vary with stimulus rate and duration because of temporal integration. Threshold and upper-comfort current levels obtained with the fast-rate psychophysical stimuli were generally significantly lower than the behavioral dynamic range measures obtained with the slow-rate ECAP stimulus. If a fixed current level was used for both psychophysical and ECAP measures, ECAP thresholds would often be at or above current levels that would be optimal for psychophysical masking, making it difficult to obtain measurable ECAP responses. Therefore, psychophysical and ECAP masker levels were fixed at the same percentage of the dynamic range for each respective stimulus, rather than at a fixed current level across measures.
Unmasked probe thresholds were obtained for a basal, middle, and apical electrode (see Table 2) in each subject using an adaptive 3-interval, 2-alternative forced-choice (3I-2AFC) task in which the subject indicated the interval (2 or 3) in which they heard the sound.1 Initial stimulus level for the probe was selected individually for each subject based on the dynamic range of the probe stimulus to ensure that starting level was sufficiently audible but not too loud. Masked thresholds were then obtained using the adaptive 3I-2AFC task. Masker levels were fixed at 80% of the dynamic range for the 300-ms masker stimulus on each electrode. The masker was presented in all three intervals, with the probe randomly occurring in either interval 2 or interval 3. The subject indicated which interval (2 or 3) contained the sound that was different from the standard (masker alone) in interval 1.
For Nucleus subjects, psychophysical testing was conducted with a custom program that implemented Nucleus Implant Communicator (NIC) routines; Clarion subjects used a custom program through BEDCS. For both device types, the equipment setup was identical to that used for the respective ECAP measures. Nucleus subjects entered their responses via a touch-screen monitor. Clarion subjects entered their responses using numeric keys on the laptop computer that ran the BEDCS program. Response feedback was not provided. Thresholds were determined using a 3-down, 1-up adaptive procedure, which estimates 79.4% correct on the psychometric function (Levitt, 1971). Each run consisted of 11 reversals. For Nucleus subjects, the initial step size was 10 current level units (CL) until the first reversal, then 5 CL until the second reversal, and finally 2 CL for the remaining reversals. For Clarion subjects, the initial step size was 3 dB for the first three reversals, then 1.5 dB for the next 2 reversals, and finally 0.75 dB for the remaining reversals. The mean of the last four reversals was taken as threshold for that block. Final threshold for each electrode condition was determined as the average of 3–5 blocks.
Psychophysical Data Analysis
Psychophysical data for Nucleus recipients were collected using logarithmic CL units, whereas all remaining data (ECAP amplitudes for both devices and Clarion psychophysical thresholds) were in linear units (microvolts for ECAP or clinical units [CU] for Clarion psychophysics). Therefore, all masked and unmasked Nucleus psychophysical thresholds were first converted from CL to microamperes using conversion charts provided by Cochlear Corporation so that all data were in linear units. Figure 1A shows an example of converted unmasked (plus sign, with dotted reference line) and masked (filled circles) probe thresholds for the three probe electrodes in Nucleus subject R3. In this example, masked psychophysical threshold approximates unmasked threshold for probe electrode 7 (P7) when the masker was spaced three electrode positions away in a basal direction (E4) and six electrodes in an apical direction (E13). This suggests that there was little effect of the masker beyond the probe electrode. In contrast, residual masking remained for P11 and P16 for even the most distant masker electrodes (i.e., the filled circles never reach the dotted line).
For all subjects, the amount of psychophysical forward masking (PFM) for each masker-probe pair was calculated by subtracting the unmasked probe threshold from the masked threshold. As with the ECAP measures, the amount of masking was then normalized to the amount of masking obtained in the masker-equals-probe-electrode condition and then plotted as a function of masker electrode (Figure 1B, filled circles).
Speech-Perception Measures
Speech-perception ability was tested using the Consonant-Nucleus-Consonant (CNC) monosyllabic word test (Peterson & Lehiste, 1962) and the Bamford-Kowal-Bench Sentences in Noise (BKB-SIN) test (Beta-2 Trial Version, Etymotic Research, 2001). Two CNC lists were presented to each subject at 60 dB SPL and were scored as percent correct for words (total of 100 items) and phonemes (total of 300 items). Three lists of BKB-SIN sentences were presented to each subject with the target sentences fixed at 70 dB SPL. Each list in the BKB-SIN test consists of 8–10 sentences presented at signal-to-noise ratios (SNRs) that progress in −3-dB steps from +21 dB to 0 dB for the 8-sentence lists or to −6 dB for the 10-sentence lists. The presentation level of the target sentences is fixed while the level of the multi-talker babble background systematically increases. The test is scored as the SNR at which the subject achieves 50% correct on the entire list. Thus, a high SNR represents poor performance, whereas a low SNR represents good performance. Scores from three BKB-SIN lists were averaged for the final scores reported here. Speech-perception materials were presented in a sound-treated booth with the subject seated directly in front of a loudspeaker at a distance of 4.5 feet. Subjects were tested using their most-used speech-processor program, volume, and microphone sensitivity settings. Subjects who normally wore either a hearing aid or a second CI in the non-test ear (see Table 1) removed that device so that all testing was conducted with the unilateral CI only. Those subjects were allowed to adjust the volume on their speech processor to a comfortable listening level to compensate for reduced volume settings that are typically used in each ear in the binaural aided/implanted condition. Speech materials were attenuated through an audiometer and were calibrated individually at the subject’s CI microphone. All lists were randomly selected for each subject.
Results
Physiological versus Psychophysical Forward Masking
Figure 1B shows an example of normalized ECAP FM (open circles) and PFM (filled circles) patterns for all three probe electrodes (P7, P11, and P16) for subject R3 (data from Figure 1A normalized). For both physiological and psychophysical measures, the greatest amount of masking occurred when masker and probe were on the same electrode, with less masking at larger masker-probe separations. Normalized masking values of zero represent no measurable masking.
To determine whether the ECAP and PFM patterns were related, normalized PFM was plotted relative to normalized ECAP FM for each masker-probe electrode pair tested in each subject. The normalization points (masker-equals-probe-electrode conditions, which were always 1.0) were removed to avoid bias and a correlation coefficient was obtained for the 12 data points in each subject. Figure 2 shows individual scatter plots for all 18 subjects. Subject number and correlation coefficient are shown in each graph. The dashed diagonal line represents the point where the two variables are equal. Linear regression results are indicated by solid lines. Ten of the 18 subjects showed a statistically significant correlation (r = 0.68 to 0.85, p ≤ 0.02) between the ECAP and PFM functions and two subjects showed a moderately strong correlation (r = 0.54 to 0.55, p = 0.06 to 0.07). These subjects are shown in the top two rows of Figure 2, with the two moderate-correlation subjects (R7 and C6) offset at right. The remaining six subjects, shown in the bottom row of Figure 2, demonstrated a poor correlation (r = 0.13 to 0.45, p > 0.14) between ECAP FM and PFM. Data points below the dashed unity line represent more masking with the ECAP (i.e., broader functions); whereas data points above the unity line represent more masking (broader functions) psychophysically. Only one subject, C1, had consistently more normalized masking psychophysically than physiologically. Table 3 lists the correlation coefficient, p-value, and slope of the regression line for each subject.
Fig. 2.
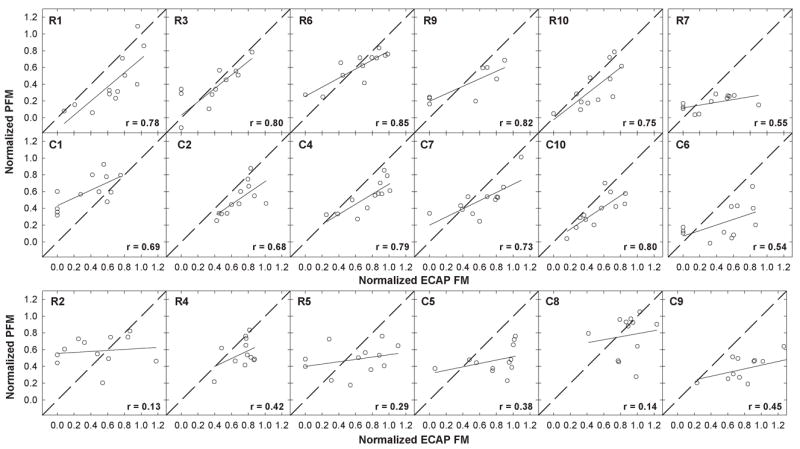
Individual-subject plots showing normalized psychophysical forward masking (PFM) plotted relative to normalized electrically evoked compound action potential forward masking (ECAP FM) for all masker-probe electrode pairs tested within a subject. Each panel represents data from a different subject; subject numbers are indicated in each panel. Normalization points (masker-equals-probe electrode) were removed and a correlation coefficient (indicated in each panel) was obtained. Solid lines represent results from linear regression analyses. Diagonal dashed lines represent unity. The top row shows data from Nucleus subjects with strong and moderate correlations. The middle row shows data from Clarion subjects with strong and moderate correlations (both subjects with moderate correlations are offset at right). The bottom row shows data from the remaining six subjects with poor correlations.
Table 3.
Correlation coefficients, p-values, and slopes of linear regression analyses for the ECAP-PFM comparison; and speech perception scores for each subject.
| Subject | r-value | p-value | Linear regression slope | CNC words (% Correct) | CNC phonemes (% Correct) | BKB-SIN (dB SNR) |
|---|---|---|---|---|---|---|
| R1 | 0.78 | <0.01* | 0.84 | 46 | 68 | 15.2 |
| R2 | 0.13 | 0.70 | 0.06 | 83 | 93 | 4.2 |
| R3 | 0.80 | <0.01* | 0.80 | 42 | 64 | 13.5 |
| R4 | 0.42 | 0.18 | 0.48 | 68 | 86 | 4.8 |
| R5 | 0.29 | 0.37 | 0.14 | 78 | 90 | 6.5 |
| R6 | 0.85 | <0.01* | 0.54 | 17 | 42 | 13.8 |
| R7 | 0.55 | 0.06 | 0.16 | 39 | 64 | 6.8 |
| R9 | 0.82 | 0.01* | 0.45 | 1 | 20 | 22.5 |
| R10 | 0.75 | <0.01* | 0.78 | 35 | 58 | 12.7 |
| C1 | 0.69 | 0.01* | 0.46 | 21 | 43 | 19.2 |
| C2 | 0.68 | 0.02* | 0.68 | 39 | 62 | 14.8 |
| C4 | 0.79 | <0.01* | 0.60 | 69 | 84 | 14.5 |
| C5 | 0.38 | 0.23 | 0.20 | 37 | 64 | 17.2 |
| C6 | 0.54 | 0.07 | 0.33 | 67 | 81 | 15.2 |
| C7 | 0.73 | <0.01* | 0.49 | 8 | 25 | 21.8 |
| C8 | 0.14 | 0.66 | 0.18 | 75 | 89 | 5.8 |
| C9 | 0.45 | 0.14 | 0.22 | 37 | 63 | 3.5 |
| C10 | 0.80 | <0.01* | 0.67 | 5 | 23 | 22.2 |
CNC = Consonant-Nucleus-Consonant, BKB-SIN = Bamford-Kowal-Bench Speech in Noise, SNR = signal-to-noise ratio.
Statistically significant at alpha = 0.05.
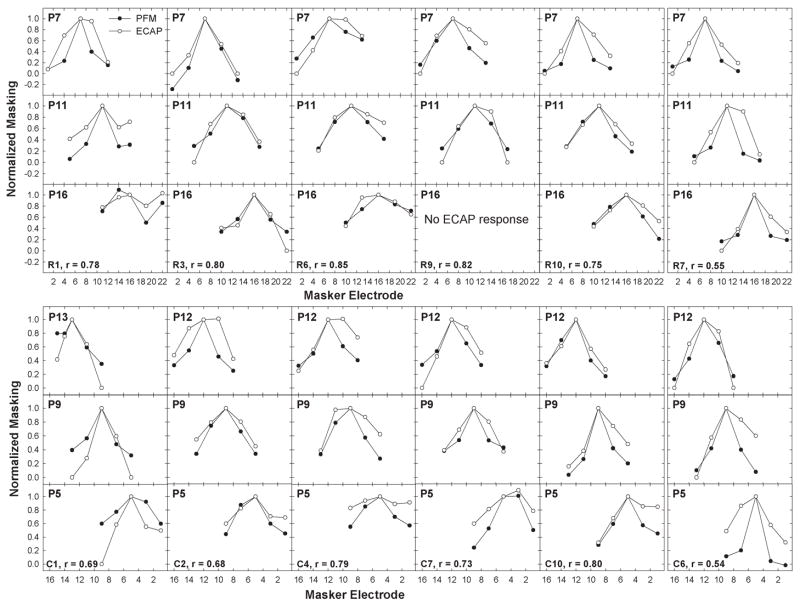
Figure 3 shows normalized ECAP and PFM patterns for all 12 subjects with moderate to strong correlations. Data are plotted as in Figure 1B, where each column within a grouping represents data from a single subject (subject number and correlation coefficient are noted at the bottom of each column set). Nucleus subjects are in the top grouping and Clarion subjects are in the bottom grouping. Basal probe electrodes are on the top row of each grouping, middle probe electrodes are in the middle row, and apical probe electrodes are on the bottom row. For ease of comparison across the two device types, masker electrodes are plotted from base to apex for both devices (i.e., 1 to 22 for Nucleus and 16 to 1 for Clarion). Although there are slight differences between the devices in array length and modiolar proximity, data are plotted so that masker electrode separations are as comparable as possible. Data from the two subjects with the moderate correlations are offset at the right of each grouping. P16 in subject R1 shows a significant nonmonotonicity in both ECAP and PFM functions. This subject’s postoperative x-ray revealed the apical portion of the intracochlear electrode array was curled back on itself.
Fig. 3.
Normalized electrically evoked compound action potential (ECAP; open circles) and psychophysical forward masking (PFM; filled symbols) functions for all three probe electrodes in the 12 subjects with strong to moderate correlations between these two measures. Data are plotted as in Figure 1B. Top grouping: Nucleus subjects. Bottom grouping: Clarion subjects. Each column within a grouping represents data from a different subject (subject number and correlation coefficient are noted in the bottom panel of each column set). Each panel in a column set represents a different probe electrode (indicated in each panel). Within each grouping, basal probe electrodes are on the top row, middle electrodes in the middle row, and apical electrodes on the bottom row. Offset columns at right show data from the two subjects with moderate correlations that were just outside of statistical significance.
Figure 4 shows normalized masking functions for the remaining six subjects who demonstrated a poor correlation between PFM and ECAP FM functions (p > 0.1). Data are plotted as in Figure 3. Five of the six subjects in this group (R2, R5, C5, C8, and C9) had at least one probe electrode with a displaced peak in the ECAP masking function and one subject (C8) also had a displaced peak in the PFM function. In other words, for these subjects, maximum amount of masking did not occur with the masker and probe on the same electrode. A displaced peak in only one of the two functions would clearly contribute to a poor correlation between the two measures. Overall, 39% (7/18) of all ECAP masking functions in the poor-correlation group had displaced peaks. In contrast, only 11% (4/35) of all ECAP masking functions in the significant-correlation group (Figure 3) exhibited displaced peaks. In two of those four subjects (R1 and C7), the PFM function was also similarly displaced, lending to the positive correlation for each subject. Table 4 provides a summary of all functions with displaced peaks. Of the nine subjects with displaced peaks, six were Clarion and three were Nucleus users. The direction of displacement was evenly split: seven functions were skewed in a basal direction and seven toward the apex. There was, however, a bias toward location of the probe electrodes with displaced peaks: nine of the functions with displaced peaks occurred on apical electrodes, whereas four were on basal electrodes, and one was on a middle electrode.
Fig. 4.
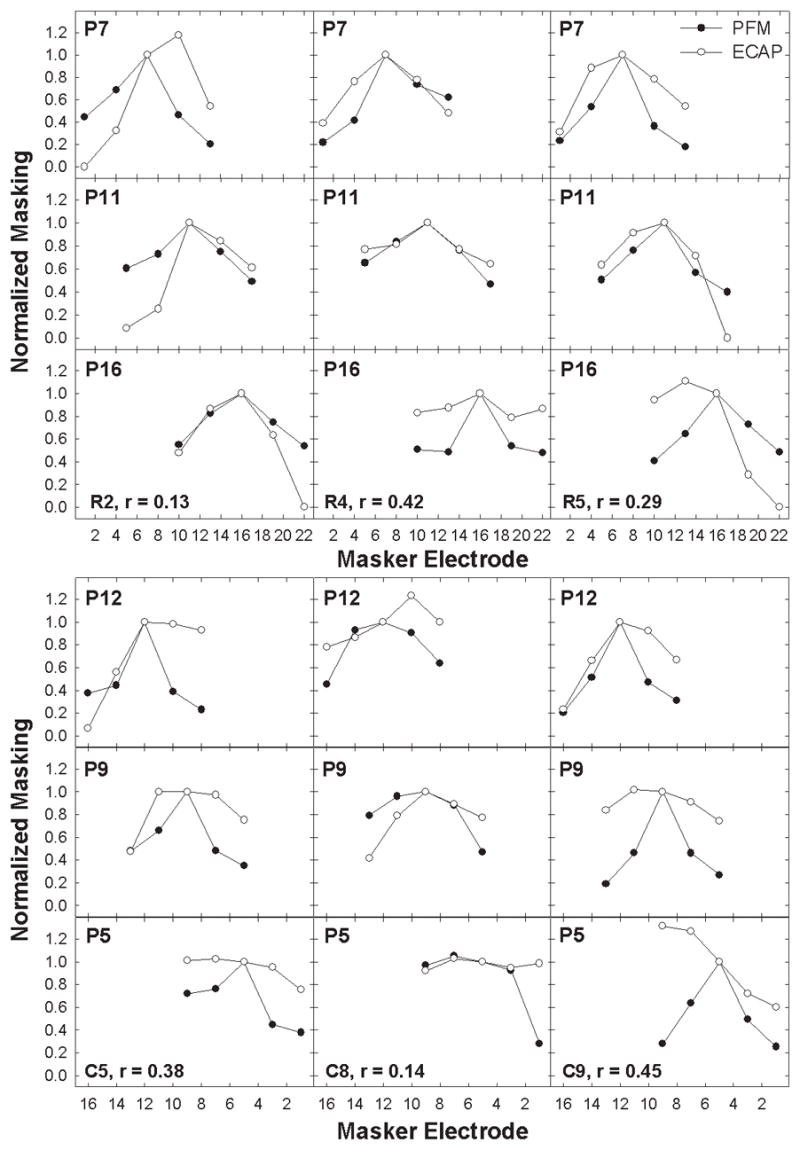
Normalized electrically evoked compound action potential (ECAP; open circles) and psychophysical forward masking (PFM; filled symbols) functions for all three probe electrodes in the six subjects with poor correlations between these two measures (p > 0.1). Data are plotted as in Figure 3. Five of the six subjects (R2, R5, C5, C8, and C9) exhibited a displaced peak in the ECAP function for at least one probe electrode.
Table 4.
Summary of forward-masking functions with displaced peaks.
| Subject | Probe Electrode | Measure | Location of Peak | Recording Location |
|---|---|---|---|---|
| R1* | P16 | ECAP | E22 | E11 |
| R1* | P16 | PFM | E14 | n/a |
| R2 | P7 | ECAP | E10 | E9 |
| R5 | P16 | ECAP | E13 | E21 |
| C2* | P12 | ECAP | E10 | E11 |
| C4* | P12 | ECAP | E10 | E11 |
| C5 | P5 | ECAP | E7 | E2 |
| C7* | P5 | ECAP | E3 | E8 |
| C7* | P5 | PFM | E3 | n/a |
| C8 | P5 | ECAP | E7 | E2 |
| C8 | P5 | PFM | E7 | n/a |
| C8 | P12 | ECAP | E10 | E9 |
| C9 | P5 | ECAP | E7 & E9 | E2 |
| C9 | P9 | ECAP | E11 | E6 |
Significant ECAP-PFM correlation (p < 0.05).
Figure 5 shows normalized PFM plotted as a function of normalized ECAP masking for all masker-probe electrode pairs (excluding the normalization points) from all subjects. The diagonal dashed line indicates equal psychophysical and physiological values. The solid line shows results of linear regression analysis, with a slope of 0.42. Group data showed a highly significant correlation between physiological and psychophysical forward masking (r = 0.55, p < 0.0001); however, only 30% of the variance in PFM is accounted for by the ECAP measures. Because the majority of data points fell below the diagonal unity line, a paired t-test was applied to determine whether there was a significant difference in normalized masking between the two measures. Results indicated significantly more normalized masking for ECAP measures than for psychophysical measures (p < 0.001, t = 7.14, d.f. = 211). These results suggest that ECAP masking patterns were generally broader than PFM patterns.
Fig. 5.
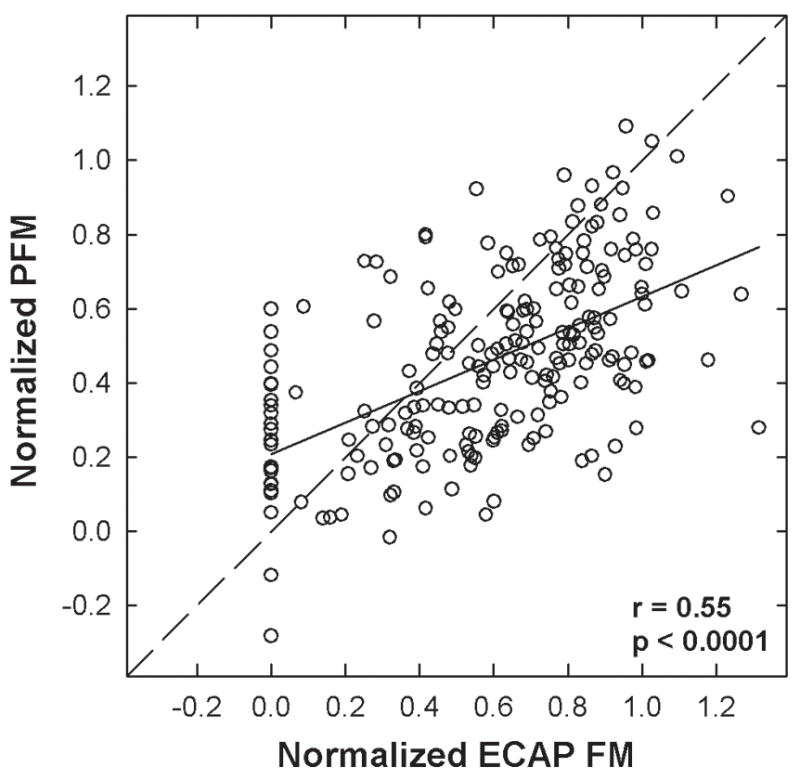
Normalized psychophysical forward masking (PFM) plotted relative to the normalized electrically evoked compound action potential forward masking (ECAP FM) for all electrodes in all subjects, excluding normalization points. The dashed diagonal line indicates unity. The solid line represents results from linear regression analysis. Correlation coefficient and p-value are indicated on the graph.
Figure 6 shows data from Figure 5 separated as a function of probe electrode location. Basal probe electrodes were P7 for Nucleus and P12 for Clarion, middle probe electrodes were P11 for Nucleus and P9 for Clarion, and apical probe electrodes were P16 for Nucleus and P5 for Clarion. The correlation between PFM and ECAP FM remained significant for all three electrode positions (see r- and p-values in each panel), with the strongest correlation for basal electrodes and weakest correlation for apical electrodes. Regression slopes were 0.44, 0.40, and 0.34 for basal, middle, and apical regions, respectively. Results from paired t-tests showed significantly more normalized masking for ECAP measures than for psychophysical measures for all three electrode positions (p < 0.0001, t = 4.5, d.f. = 71 for basal; p = 0.0003, t = 3.8, d.f. = 71 for middle; p = 0.0001, t = 4.0, d.f. = 67 for apical).
Fig. 6.
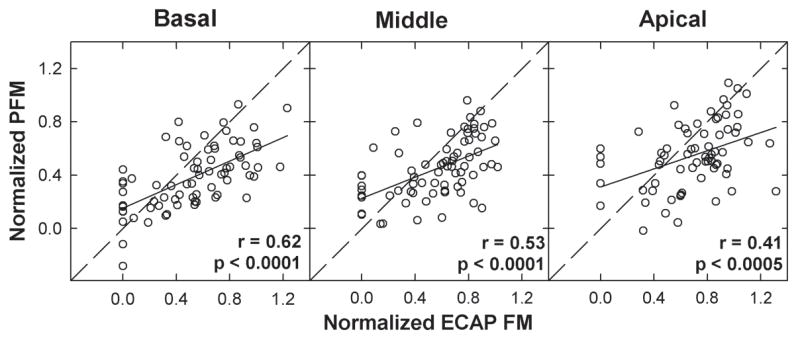
Data from Fig. 5 separated by probe electrode location. From left to right, each panel shows group data for basal probe electrodes (P7 for Nucleus, P12 for Clarion), middle probe electrodes (P11 for Nucleus, P9 for Clarion), and apical probe electrodes (P16 for Nucleus, P5 for Clarion), respectively. Solid lines represent linear regression results; diagonal dashed lines represent unity. Correlation coefficients and p-values are reported in each panel.
Forward Masking versus Speech Perception
Several comparisons were made to determine whether speech perception was related to either PFM or to ECAP FM. Speech-perception performance on each of the three measures (words, phonemes, and sentences in noise) for each subject was compared with: (1) the averaged amount of normalized PFM across all masker-probe pairs within a subject (which represents an index of spatial selectivity), (2) the averaged amount of raw PFM (masked minus unmasked threshold, un-normalized) across all masker-probe pairs within a subject (which represents temporal and spatial effects), (3) the peak amount of raw PFM averaged across all three probe electrodes within a subject (representing temporal effects), and (4) the averaged amount of normalized ECAP FM across all masker-probe pairs within a subject (representing spatial selectivity). The hypothesis was that subjects with better speech-perception performance would have less PFM (i.e., either more sharply defined masking functions or very little masking overall) and/or less ECAP FM as compared with subjects who were poorer performers. Specifically, more overall masking should adversely affect temporal cues in speech perception and greater spatial masking should affect spectral aspects of speech perception.
Pearson correlation analyses were used for all comparisons. Results for the psychophysical comparisons showed no significant correlation between any of the three speech-perception measures and any of the three PFM measures (p > 0.05 for all comparisons). Like the psychophysical data, there was no significant correlation between speech perception and the ECAP FM measures (p > 0.1).
Because there was no significant correlation between either PFM or ECAP FM and speech perception, we would not necessarily expect that some aspect of the ECAP-PFM relationship would correlate with speech perception. However, due to the wide range of correlation coefficients across subjects for the ECAP-PFM comparison and the wide range of speech-perception scores (see Table 3), it was of interest to determine whether subjects whose ECAP FM and PFM functions matched well also had better speech-perception performance. (It should be noted that the correlation coefficient is a goodness-of-fit measure between two variables, not a direct measure of the data.) Figure 7 shows speech-perception performance for BKB-SIN, CNC words, and CNC phonemes plotted relative to the correlation coefficient for the ECAP-PFM comparison for each subject. Subject numbers represent the respective data points. ECAP-PFM correlation coefficients and speech perception scores are listed in Table 3. Results showed an interesting trend, in that subjects with the strongest correlation between ECAP and PFM had the poorest speech-perception performance, and subjects with the weakest correlation had the best speech-perception performance. This result was consistent across all three speech-perception measures (r = −0.71, p < 0.001 for BKB-SIN; r = −0.71, p = 0.001 for CNC words; r = −0.69, p = 0.002 for CNC phonemes). These correlations remained significant (p < 0.01) after applying a Bonferroni correction for the multiple correlations performed with speech-perception data.
Fig. 7.
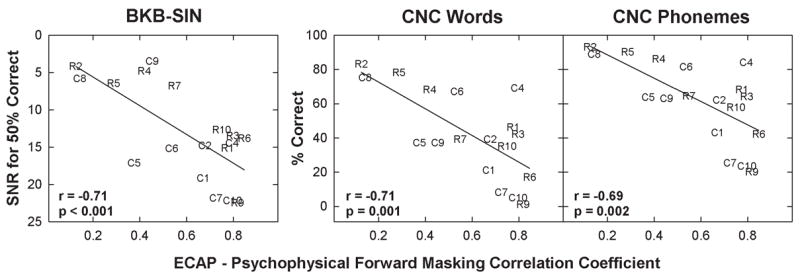
Speech-perception performance plotted relative to the correlation coefficient for the electrically evoked compound action potential forward masking (ECAP FM) versus psychophysical forward masking (PFM) comparison for all 18 subjects. Each panel represents data from a different speech perception measure: from left to right, Bamford-Kowal-Bench Sentences in Noise (BKB-SIN), Consonant-Nucleus-Consonant (CNC) words, and CNC phonemes. Solid lines represent linear regression results. Correlation coefficient and p-values are reported in each panel. SNR: signal-to-noise ratio.
As stated previously, the majority of subjects with a poor correlation between PFM and ECAP FM had at least one function with a displaced peak (hence contributing to the poor correlation). In order to determine whether there was a relation between displaced peaks and speech-perception performance, speech-perception scores for subjects with displaced peaks in either the ECAP or PFM function were compared with scores for subjects without displaced peaks. There was no statistically significant difference in performance between the two groups for any of the three speech-perception measures (t-test, p > 0.1 for all tests); however, the statistical comparison was under-powered. These results tentatively suggest that the presence of displaced peaks in either the ECAP or PFM function do not necessarily equate to better speech perception.
Discussion
Physiological versus Psychophysical Forward Masking
The primary purpose of this study was to examine the relation between physiological and psychophysical spatial forward-masking patterns. For both measures, there was generally one peak in the masking function that occurred when the masker and probe were on the same electrode, indicating the maximum amount of masking. As the spatial separation between masker and probe electrodes increased, the relative amount of masking decreased. This pattern represents a reduction in overlap between stimulated neural populations as the distance between masker and probe increases. When the relative amount of masking reaches zero, we conclude that there is no measurable overlap between neural populations stimulated by masker and probe electrodes. These patterns are consistent with previous findings for both ECAP FM (Abbas et al., 2004; Cohen et al., 2003; Eisen & Franck, 2005) and PFM (Boëx et al., 2003b; Chatterjee et al., 2006; Chatterjee & Shannon, 1998; Cohen et al., 2003; Lim et al., 1989; Shannon, 1983b; Throckmorton & Collins, 1999).
It was hypothesized that physiological forward masking would be correlated with psychophysical forward masking as a function of spatial separation between masker and probe electrodes. Results from this study showed a significant correlation between the two measures across subjects, consistent with this hypothesis and with previous results from a smaller group of subjects reported by Cohen et al. (2003). On an individual basis, the correlation between ECAP FM and PFM was strong for 10 subjects, moderately strong for two subjects, and poor for six subjects. Although the group correlation was statistically significant, only 30% of the variance in PFM was accounted for by the ECAP measures. This suggests that the ECAP measures alone are not sufficient to accurately predict PFM patterns for individual subjects.
Several factors may have precluded a stronger correlation between ECAP and PFM patterns. First, masker-probe delays were slightly different between ECAP (0.5 ms) and psychophysical (2 ms) measures. Peripheral masking at the level of the auditory nerve has been shown to peak at masker-offset to probe-onset delays of approximately 0.5–1 ms and persist for up to about 4–5 ms for monopolar stimulation (Abbas et al., 1999; Brown, Abbas, Borland, & Bertschy, 1996; Brown, Abbas, & Gantz, 1998; Shpak, Berlin, & Luntz, 2004). Similarly, short recovery time constants have also been reported for psychophysical forward masking (i.e., < 7 ms) when single pulses were used (Brown et al., 1996; Nelson & Donaldson, 2001). For pulse-train stimuli, two-component or exponential psychophysical recovery functions consisting of a fast process and a slow or residual process have been reported (Chatterjee, 1999; Nelson & Donaldson, 2002). The break point between the two processes occurred at approximately 30–50 ms. Short delays (such as 2 ms) are thus hypothesized to represent effects of more peripheral refractory processes. Lastly, Chatterjee and Shannon (1998) observed sharper psychophysical spatial masking patterns for a 1-ms delay that broadened when the delay was increased to 10 ms; beyond those results, little is known about the effect of masker-probe delay on spatial forward masking patterns. It is not clear whether a stronger correlation between ECAP FM and PFM would have been obtained if the same masker-probe delay had been used for both measures in the present study.
A second potential factor is that different stimuli were used for ECAP and PFM measures. For this study, ECAP measures were obtained with traditional single-pulse stimuli whereas PFM measures were obtained with pulse-train stimuli that were generally similar in duration to those used in previous spatial PFM studies (Boëx et al., 2003b; Chatterjee et al., 2006; Chatterjee & Shannon, 1998; Cohen et al., 2003; Dingemanse, Frijns, & Briaire, 2006; Kwon & van den Honert, 2006; Throckmorton & Collins, 1999). In a subsequent study testing a subset of subjects from the present study, we evaluated the relation between ECAP FM patterns and PFM patterns using single pulses for both measures (manuscript in preparation). Results showed a much poorer relation between ECAP and single-pulse PFM than in the present study, suggesting that pulse-train stimuli produce more selective PFM patterns than single pulses.
A third factor contributing to the group ECAP-PFM correlation not being stronger is the number of functions with displaced peaks. As shown in Figure 6, apical electrodes exhibited the lowest correlation between ECAP FM and PFM. Table 4 shows a high incidence of shifted peaks for apical probe electrodes. Nerve fibers in the apical region of the human cochlea are closer to the medial wall of the scala tympani than in the base. Cochlear cross-sectional area also decreases toward the apex, potentially putting the electrode contacts in closer proximity to neural elements. It may be that cross-turn stimulation contributed to the higher incidence of peak displacement in apical electrodes. However, if cross-turn stimulation produced the displaced ECAP peaks, one would expect corresponding peak displacement in the PFM function as well. As can be seen from Table 4, this was typically not the case. Only three of the nine subjects (R1, C7, and C8) had a displaced peak in both the ECAP and PFM functions. As stated previously, the apical portion of R1’s electrode array was curled back on itself, which is consistent with the two-peaked pattern seen in both the ECAP and psychophysical data for P16.
Another explanation for the shifted ECAP peaks in the other subjects may be longitudinal spread of current and/or relative location of the recording electrode (Abbas et al., 2004), which could account for the presence of a shifted peak in the ECAP function and lack of one in the PFM function. Psychophysical responses are obtained by the participant’s subjective response, whereas ECAP measures are measured from an intracochlear recording electrode. Location of the recording electrode affects the measured ECAP amplitude, with increased distance between stimulating and recording electrodes typically resulting in a decrease in ECAP amplitude (Abbas et al., 1999; Cohen, Saunders, & Richardson, 2004; Frijns, Briaire, de Laat, & Grote, 2002). In some cases, however, ECAP amplitude can be inflated due to excessive artifact in the response if the recording electrode is too close to the stimulating electrode. It may be difficult to visualize these artifact effects, especially for very large ECAP responses. As indicated in Table 4, there were four instances in the present study (subject R2, P7; subject C2, P12; subject C4, P12; and subject C8, P12) where the recording electrode was adjacent to the masker electrode on which the shifted peak occurred.
As shown in Figure 5, there was significantly more normalized masking overall (i.e., broader patterns) for ECAP measures than for PFM. These results indicate that masking decreased more for psychophysical measures than for physiological measures as the spatial separation between masker and probe electrodes increased. It could be hypothesized that the few subjects with more PFM than ECAP FM would have poorer speech-perception ability due to broader perceptual masking patterns. Only two subjects (R2 and C1) demonstrated more psychophysical masking than physiological masking. As shown in Table 3, subject R2 demonstrated very good speech-perception performance, whereas subject C1 demonstrated low performance levels. Thus, it does not appear that individuals with more PFM than ECAP FM necessarily have poorer speech-perception ability.
One possible explanation for the greater amount of normalized ECAP FM versus PFM seen in Figure 5 may be that differences in current level used for ECAP measures versus PFM measures resulted in different amounts of spatial spread. Both measures were made at the same percentage of dynamic range (in most cases 80%); however, dynamic range boundaries differ with stimulus rate and duration because of temporal integration. The ECAP stimulus was a single pulse presented at a slow rate, whereas the psychophysical masker was a 300-ms pulse train presented at a fast rate. Thus, the dynamic range boundaries occurred at much higher current levels for the ECAP than for the psychophysical masker. In the Nucleus subjects, 80% ECAP masker levels averaged 194 CL and 80% psychophysical masker levels averaged 179 CL. In the Clarion subjects, 80% ECAP masker levels averaged 748 μA and 80% psychophysical masker levels averaged 397 μA. The higher ECAP masker current levels possibly yielded more spread of excitation and thus more overall masking than the psychophysical patterns. The literature reports little effect of masker level on the shape of psychophysical spatial forward masking patterns for BP+1 or BP+2 stimulation (Chatterjee et al., 2006; Chatterjee & Shannon, 1998; Kwon & van den Honert, 2006) and for monopolar stimulation (Kwon & van den Honert, 2006), although a few individual examples of masker-level effects were reported (Chatterjee & Shannon, 1998; Kwon & van den Honert, 2006; Lim et al., 1989). For ECAP measures with monopolar stimulation, Abbas et al. (2004) and Cohen et al. (2003) reported individual examples of wider spatial forward-masking patterns for higher masker levels, as well as examples where masker level had little effect. Eisen and Franck (2005) examined the width of ECAP masking patterns for three intensity levels (roughly 2, 3, and 4 dB above ECAP threshold) in a group of 27 children. They found significant width differences between low and medium intensities as well as between low and high intensities, with wider functions obtained at higher stimulus levels. Thus, there is evidence that increased current level can produce wider ECAP spatial masking patterns, more so than for PFM. It is therefore possible that masker current level contributed to the difference in masking between ECAP and psychophysical measures in the present study, given the higher ECAP current levels used.
In general, monopolar stimulation has been shown to produce very broad, non-selective physiological patterns in animal models (e.g., Bierer & Middlebrooks, 2002; Ryan et al., 1990; van den Honert & Stypulkowski, 1987). Results from the present study show that monopolar stimulation can produce relatively selective patterns of excitation for both psychophysical and ECAP measures in humans. Although this could be a species difference, several animal studies have also shown cases where good selectivity was achieved for monopolar stimulation, particularly at low current levels (e.g., 50-μA condition, Figure 2, Ryan et al., 1990; preparation C-239, Figure 3, van den Honert & Stypulkowski, 1987; +1-dB condition, Figure 3, Bierer & Middlebrooks, 2002). The relatively selective patterns in the present study are qualitatively similar to human psychophysical results reported for BP+1 stimulation (Chatterjee et al., 2006; Chatterjee & Shannon, 1998; Cohen, Saunders, & Clark, 2001; Lim et al., 1989; Throckmorton & Collins, 1999). This observation is also supported by results from a recent study by Kwon and van den Honert (2006), who compared psychophysical forward masking patterns obtained with monopolar and either BP+1 or BP+2 modes within subjects. They found no significant difference in the normalized amount of masking for the two modes, and attributed this finding to the difference in current level needed to achieve equal loudness between modes. Narrow stimulation modes (such as BP+1) typically require higher current levels to reach threshold and maximum comfort levels than broader stimulation modes (e.g., Pfingst et al., 1997; Shannon, 1983a). As demonstrated by electrophysiological and computer-modeling studies, higher current levels also produce greater spread of excitation (e.g., Abbas et al., 2004; Eisen & Franck, 2005; Frijns, de Snoo, & Schoonhoven, 1995; Frijns, de Snoo, & ten Kate, 1996). Results from the present study concur with those from Kwon and van den Honert (2006), showing that selective spatial excitation patterns can be measured in human CI users with monopolar stimulation.
Forward Masking versus Speech Perception
It was hypothesized that subjects with less PFM would have better speech-perception performance compared to subjects with more PFM. Results from this study showed no significant correlation between the amount of PFM (either in a temporal, spatial, or temporal-spatial domain) and speech-perception performance. This finding contrasts with results of Throckmorton and Collins (1999), who found a significant correlation between averaged PFM (representing spatial and temporal masking) and three different speech perception tests (Iowa Medial Consonants, NU-6 phonemes, and CID Everyday Sentences) for seven subjects. Several differences between the two studies may have collectively contributed to the difference in findings: different speech-perception tests were used (e.g., a consonant test may be more sensitive than a word test); the range of speech-perception scores was very different between the studies, which could affect the correlation with PFM measures; their study had a relatively small number of subjects; PFM measures in their study were based on a single adaptive run for each masker-probe electrode combination as opposed to averaging 3–5 runs as in the present study; and subjects in their study used BP+1 or BP+2 stimulation modes, whereas the present study used monopolar stimulation for both measures. Although Kwon and van den Honert (2006) showed no significant difference in PFM patterns between monopolar and bipolar modes, a number of investigators have shown better speech perception for broader stimulation modes (Pfingst et al., 1997, 2001; Zwolan et al., 1996). It is possible that differences in stimulation mode affected speech-perception results, which in turn possibly affected the relation between PFM and speech perception.
The present study also compared the averaged amount of normalized ECAP FM to speech perception. It was hypothesized that subjects with less ECAP FM (i.e., more spatially selective patterns) would have better speech-perception performance compared with subjects who exhibited greater ECAP FM. Results showed no significant correlation between speech-perception performance and the averaged amount of normalized ECAP FM across all masker-probe pairs within a subject. These results are consistent with previous research that found no statistically significant correlation between the width of the ECAP FM function at 75% normalized amplitude and performance on HINT sentences and CNC words and phonemes (Hughes & Abbas, 2006a).
As shown in Table 1, 12 of the 18 subjects wore contralateral amplification in their daily lives. Speech-perception testing was conducted without contralateral amplification in order to relate psychophysical and ECAP measures to performance specifically with the implanted ear. Due to time restrictions, subjects were not given an extended opportunity to acclimate to listening in the CI-alone condition. Therefore, it is reasonable to conclude that the performance levels in Table 1 for these 12 subjects may be lower than what might have been obtained in the binaural condition. A comparison between the 12 subjects who normally wore contralateral amplification and the 6 subjects who did not revealed no significant difference in CNC word or phoneme scores (T = −2.06, p = 0.056 and T = −1.91, p = 0.074, respectively). There was, however, a significant difference in BKB-SIN scores between the two groups (T = 2.13, p = 0.049), where the unilateral CI group scored better than the group who normally wore binaural amplification. Perhaps this difference reflects the loss of added benefit in noise that the binaural group was accustomed to for their everyday listening.
Results from this study showed that individuals whose ECAP FM patterns correlated well with PFM patterns were the poorest performing subjects on tests of word/phoneme recognition and sentences in noise, and subjects with the poorest correlations had the best speech perception. Although a displaced peak in one function (typically the ECAP function) can certainly contribute to a poor correlation between ECAP FM and PFM measures, it does not logically follow that a poor correlation would be linked to better speech perception. It may be that for these better-performing subjects, higher-level auditory mechanisms involved in the psychophysical task are better able to compensate for an abnormal peripheral representation (i.e., shifted peak in the ECAP function), leading to better speech perception with the implant.
Another potential explanation for the results shown in Figure 7 may be temporal integration differences across subjects. CI users with greater temporal integration have been shown to have better speech-perception performance (Chatterjee, 1999; Firszt, Chambers, & Kraus, 2002). Subjects with greater temporal integration will be more likely to have large differences in current level between psychophysical and physiological measures due to the different stimuli used. That is, the slow-rate, short-duration ECAP measures would produce relatively high current levels for threshold and maximum comfort and the fast-rate, longer-duration PFM measures would produce relatively low current levels. Higher current levels have been shown to produce greater spread in ECAP spatial masking patterns (Abbas et al., 2004; Eisen & Franck, 2005). Thus, we might expect a poorer correlation between ECAP and psychophysical masking patterns for subjects with greater temporal integration ability due to more spatial masking (greater spread) in the ECAP functions for higher stimulus current levels. To test this hypothesis, the differences between behavioral threshold obtained with the ECAP stimulus (short duration, slow rate) and the psychophysical masker (long duration, fast rate) were averaged across the three probe electrodes for each subject. The averaged difference values were then correlated with each of the three speech-perception scores across subjects. Results showed no significant correlation between this measure of temporal integration and speech perception (p > 0.5 for all three speech perception tests), which does not support the hypothesis. However, it should be emphasized that these temporal integration values were calculated from the behavioral thresholds; temporal integration was not explicitly measured in this study. It is possible that temporal integration may play a role in the results shown in Figure 7, but its effects are too small to be reflected by the approximate measures used to estimate behavioral thresholds.
Finally, the possible effects of electrode insertion angle and/or insertion depth on the forward-masking and speech-perception data in the present study should be considered. We might expect that electrodes in closer proximity to the modiolus would require less current and thus yield more selective masking patterns for both ECAP and psychophysical measures. Cohen et al. (2003) and Hughes and Abbas (2006b) found narrower ECAP masking pattern widths for subjects with a perimodiolar array (Nucleus 24R[CS] Contour) than for a straight array (Nucleus 24M). However, Cohen et al. (2003) found no significant difference in width of the PFM pattern between the two device types. Likewise, Boëx et al. (2003b) found no significant difference in PFM between devices with and without a perimodiolar electrode positioner. Although all Nucleus subjects in the present study were implanted with the perimodiolar Contour device, large variability in electrode proximity across the Contour array has been reported (e.g., Huang, Reitzen, Marrinan, et al., 2006; Saunders, Cohen, Aschendorff, et al., 2002), which could account for some of the variability seen in the present data. All of our subjects had post-operative x-rays done as part of their routine clinical care; however, the views obtained were not sufficient for estimating insertion depth or insertion angle according to methods described in the literature (Cohen, Xu, Xu, & Clark, 1996; Xu, Xu, Cohen, & Clark, 2000).
Conclusions
This study showed a significant positive correlation between physiological and psychophysical measures of channel interaction obtained with a forward-masking technique. Correlations were significant for basal, middle, and apical electrode regions. However, correlation coefficients were not strong enough for the ECAP to be used as a sole predictor of PFM patterns on an individual basis. Overall, there was more channel interaction physiologically than psychophysically, although this finding may possibly be attributed to the use of higher current levels for the physiological measures. There was no significant correlation between speech perception and ECAP FM or PFM. Subjects whose ECAP FM patterns correlated well with PFM patterns had the poorest speech perception and subjects with the poorest correlations had the best speech perception. It is possible that ECAP recording electrode location contributed to the presence of displaced peaks in the ECAP FM function for some individuals. Further investigations are necessary to evaluate how spatial selectivity measures can be used to predict speech-processor program parameters on an individual basis so that speech-perception performance with the implant can be improved.
Acknowledgments
This study was funded by the NIH, NIDCD, grant R03 DC007017. Human subjects recruitment was supported by grant P30 DC04662. Portions of this work were presented in: “A comparison between ECAP and psychophysical channel interaction,” Proceedings of the 10th Symposium on Cochlear Implants in Children, Dallas, TX, March 15-18, 2005; “Psychophysical and physiologic forward masking patterns in cochlear implants,” Proceedings of the 2005 Conference on Implantable Auditory Prostheses, Pacific Grove, CA, July 30-August 4, 2005; and “Psychophysical versus physiologic forward masking in cochlear implants,” Advanced Bionics Auditory Research Bulletin, 2005. The authors wish to thank Paul Abbas and Wenjun Wang of the University of Iowa; Hongyang Tan, Kelly Barrow, Donna Neff, Walt Jesteadt, Michael Gorga, and Patricia Stelmachowicz of Boys Town National Research Hospital; Leonid Litvak of Advanced Bionics Corporation; and the 18 subjects who participated in this study. We would also like to thank Gail Donaldson, Monita Chatterjee, and an anonymous reviewer for insightful comments on an earlier version of this manuscript.
Research supported by NIH/NIDCD R03 DC007017 and P30 DC04662.
Footnotes
For unmasked thresholds, interval 1 never contained a stimulus. The stimulus randomly occurred in either interval 2 or interval 3 because that was the procedure used for masked thresholds.
References
- Abbas PJ, Brown CJ. Electrically evoked brainstem potentials in cochlear implant patients with multi-electrode stimulation. Hearing Research. 1988;36:153–162. doi: 10.1016/0378-5955(88)90057-3. [DOI] [PubMed] [Google Scholar]
- Abbas PJ, Brown CJ, Shallop JK, Firszt JB, Hughes ML, Hong SH, Staller SJ. Summary of results using the Nucleus CI24M implant to record the electrically evoked compound action potential. Ear & Hearing. 1999;20:45–59. doi: 10.1097/00003446-199902000-00005. [DOI] [PubMed] [Google Scholar]
- Abbas PJ, Hughes ML, Brown CJ, Miller CA, South H. Channel interaction in cochlear implant users evaluated using the electrically evoked compound action potential. Audiology & Neurotology. 2004;9:203–213. doi: 10.1159/000078390. [DOI] [PubMed] [Google Scholar]
- Arndt P, Staller S, Arcaroli J, Hines A, Ebinger K. Technical Report. Englewood, CO: Cochlear Corporation; 1999. Within-subjects comparison of advanced coding strategies in the Nucleus 24 cochlear implant. [Google Scholar]
- Battmer RD, Feldmeier I, Kohlenberg A, Lenarz T. Performance of the new Clarion speech processor 1.2 in quiet and in noise. American Journal of Otology. 1997;18:S144–S146. [PubMed] [Google Scholar]
- Bierer JA, Middlebrooks JC. Auditory cortical images of cochlear-implant stimuli: Dependence on electrode configuration. Journal of Neurophysiology. 2002;87:478–492. doi: 10.1152/jn.00212.2001. [DOI] [PubMed] [Google Scholar]
- Boëx C, de Balthasar C, Kós MI, Pelizzone M. Electrical field interactions in different cochlear implant systems. Journal of the Acoustical Society of America. 2003a;114:2049–2057. doi: 10.1121/1.1610451. [DOI] [PubMed] [Google Scholar]
- Boëx C, Kós MI, Pelizzone M. Forward masking in different cochlear implant systems. Journal of the Acoustical Society of America. 2003b;114:2058–2065. doi: 10.1121/1.1610452. [DOI] [PubMed] [Google Scholar]
- Brown CJ, Abbas PJ, Borland J, Bertschy M. Electrically evoked whole nerve action potentials in Ineraid cochlear implant users: Responses to different stimulating electrode configurations and comparison to psychophysical responses. Journal of Speech and Hearing Research. 1996;39:453–467. doi: 10.1044/jshr.3903.453. [DOI] [PubMed] [Google Scholar]
- Brown CJ, Abbas PJ, Gantz BJ. Preliminary experience with Neural Response Telemetry in the Nucleus CI24M cochlear implant. American Journal of Otology. 1998;19:320–327. [PubMed] [Google Scholar]
- Brown CJ, Hughes ML, Luk B, Abbas PJ, Wolaver AA, Gervais JP. The relationship between EAP and EABR thresholds and levels used to program the Nucleus CI24M speech processor: Data from adults. Ear & Hearing. 2000;21:151–163. doi: 10.1097/00003446-200004000-00009. [DOI] [PubMed] [Google Scholar]
- Chatterjee M. Temporal mechanisms underlying recovery from forward masking in multielectrode-implant listeners. Journal of the Acoustical Society of America. 1999;105:1853–1863. doi: 10.1121/1.426722. [DOI] [PubMed] [Google Scholar]
- Chatterjee M, Galvin JJ, Fu QJ, Shannon RV. Effects of stimulation mode, level and location on forward-masked excitation patterns in cochlear implant patients. Journal of the Association for Research in Otolaryngology. 2006;7:15–25. doi: 10.1007/s10162-005-0019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee M, Shannon RV. Forward masked excitation patterns in multielectrode electrical stimulation. Journal of the Acoustical Society of America. 1998;103:2565–2572. doi: 10.1121/1.422777. [DOI] [PubMed] [Google Scholar]
- Cohen LT, Richardson LM, Saunders E, Cowan RSC. Spatial spread of neural excitation in cochlear implant recipients: comparison of improved ECAP method and psychophysical forward masking. Hearing Research. 2003;179:72–87. doi: 10.1016/s0378-5955(03)00096-0. [DOI] [PubMed] [Google Scholar]
- Cohen LT, Saunders E, Clark GM. Psychophysics of a prototype peri-modiolar cochlear implant electrode array. Hearing Research. 2001;155:63–81. doi: 10.1016/s0378-5955(01)00248-9. [DOI] [PubMed] [Google Scholar]
- Cohen LT, Saunders E, Richardson LM. Spatial spread of neural excitation: comparison of compound action potential and forward-masking data in cochlear implant recipients. International Journal of Audiology. 2004;43:346–355. doi: 10.1080/14992020400050044. [DOI] [PubMed] [Google Scholar]
- Cohen LT, Xu J, Xu SA, Clark GM. Improved and simplified methods for specifying positions of the electrode bands of a cochlear implant array. The American Journal of Otology. 1996;17:859–865. [PubMed] [Google Scholar]
- Dillier N, Lai WK, Almqvist B, Frohne C, Muller-Deile J, Stecker M, von Wallenberg E. Measurement of the electrically evoked compound action potential via a neural response telemetry system. Annals of Otology, Rhinology, & Laryngology. 2002;111:407–414. doi: 10.1177/000348940211100505. [DOI] [PubMed] [Google Scholar]
- Dingemanse JG, Frijns JHM, Briaire JJ. Psychophysical assessment of spatial spread of excitation in electrical hearing with single and dual electrode contact maskers. Ear & Hearing. 2006;27:645–657. doi: 10.1097/01.aud.0000246683.29611.1b. [DOI] [PubMed] [Google Scholar]
- Donaldson GS, Nelson DA. Place-pitch sensitivity and its relation to consonant recognition by cochlear implant listeners using the MPEAK and SPEAK speech processing strategies. Journal of the Acoustical Society of America. 2000;107:1645–1658. doi: 10.1121/1.428449. [DOI] [PubMed] [Google Scholar]
- Dunn CC, Tyler RS, Witt SA, Gantz BJ. Effects of converting bilateral cochlear implant subjects to a strategy with increased rate and number of channels. Annals of Otology, Rhinology, & Laryngology. 2006;115:425–432. doi: 10.1177/000348940611500605. [DOI] [PubMed] [Google Scholar]
- Eisen MD, Franck KH. Electrode interaction in pediatric cochlear implant subjects. Journal of the Association for Research in Otolaryngology. 2005;6:160–170. doi: 10.1007/s10162-005-5057-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley CC, Wilson BS, White MW. Models of neural responsiveness to electrical stimulation. In: Miller JM, Spelman FA, editors. Cochlear Implants: Models of the Electrically Stimulated Ear. New York: Springer-Verlag; 1990. pp. 55–96. [Google Scholar]
- Firszt JB, Chambers RD, Kraus N. Neurophysiology of cochlear implant users II: Comparison among speech perception, dynamic range, and physiological measures. Ear & Hearing. 2002;23:516–531. doi: 10.1097/00003446-200212000-00003. [DOI] [PubMed] [Google Scholar]
- Frijns JHM, Briaire JJ, de Laat JAPM, Grote JJ. Initial evaluation of the Clarion CII cochlear implant: Speech perception and neural response imaging. Ear & Hearing. 2002;23:184–197. doi: 10.1097/00003446-200206000-00003. [DOI] [PubMed] [Google Scholar]
- Frijns JHM, Briaire JJ, Grote JJ. The importance of human cochlear anatomy for the results of modiolus-hugging multichannel cochlear implants. Otology & Neurotology. 2001;22:340–349. doi: 10.1097/00129492-200105000-00012. [DOI] [PubMed] [Google Scholar]
- Frijns JHM, de Snoo SL, Schoonhoven R. Potential distributions and neural excitation patterns in a rotationally symmetric model of the electrically stimulated cochlea. Hearing Research. 1995;87:170–186. doi: 10.1016/0378-5955(95)00090-q. [DOI] [PubMed] [Google Scholar]
- Frijns JHM, de Snoo SL, ten Kate JH. Spatial selectivity in a rotationally symmetric model of the electrically stimulated cochlea. Hearing Research. 1996;95:33–48. doi: 10.1016/0378-5955(96)00004-4. [DOI] [PubMed] [Google Scholar]
- Henry BA, McKay CM, McDermott HJ, Clark GM. The relationship between speech perception and electrode discrimination in cochlear implantees. Journal of the Acoustical Society of America. 2000;108:1269–1280. doi: 10.1121/1.1287711. [DOI] [PubMed] [Google Scholar]
- Huang TC, Reitzen SD, Marrinan MS, Waltzman SB, Roland JT. Modiolar coiling, electrical thresholds, and speech perception after cochlear implantation using the Nucleus Contour Advance electrode with the Advance Off Stylet technique. Otology & Neurotology. 2006;27:159–166. doi: 10.1097/01.mao.0000187047.58544.d0. [DOI] [PubMed] [Google Scholar]
- Hughes ML, Abbas PJ. The relation between electrophysiological channel interaction and electrode pitch ranking in cochlear implant recipients. Journal of the Acoustical Society of America. 2006a;119:1527–1537. doi: 10.1121/1.2163273. [DOI] [PubMed] [Google Scholar]
- Hughes ML, Abbas PJ. Electrophysiologic channel interaction, electrode pitch ranking, and behavioral threshold in straight versus perimodiolar cochlear implant electrode arrays. Journal of the Acoustical Society of America. 2006b;119:1538–1547. doi: 10.1121/1.2164969. [DOI] [PubMed] [Google Scholar]
- Kral A, Hartmann R, Mortazavi D, Klinke R. Spatial resolution of cochlear implants: the electrical field and excitation of auditory afferents. Hearing Research. 1998;121:11–28. doi: 10.1016/s0378-5955(98)00061-6. [DOI] [PubMed] [Google Scholar]
- Kwon BJ, van den Honert C. Effect of electrode configuration on psychophysical forward masking in cochlear implant listeners. Journal of the Acoustical Society of America. 2006;119:2994–3002. doi: 10.1121/1.2184128. [DOI] [PubMed] [Google Scholar]
- Levitt H. Transformed up-down methods in psychoacoustics. Journal of the Acoustical Society of America. 1971;49:467. [PubMed] [Google Scholar]
- Liang DH, Lusted HS, White RL. The nerve-electrode interface of the cochlear implant: Current spread. IEEE Transactions on Biomedical Engineering. 1999;46(1):35–43. doi: 10.1109/10.736751. [DOI] [PubMed] [Google Scholar]
- Lim HH, Tong YC, Clark GM. Forward masking patterns produced by intracochlear electrical stimulation of one and two electrode pairs in the human cochlea. Journal of the Acoustical Society of America. 1989;86:971–980. doi: 10.1121/1.398732. [DOI] [PubMed] [Google Scholar]
- Mens LH, Berenstein CK. Speech perception with mono- and quadrupolar electrode configurations: A crossover study. Otology & Neurotology. 2005;26:957–964. doi: 10.1097/01.mao.0000185060.74339.9d. [DOI] [PubMed] [Google Scholar]
- Middlebrooks JC. Effects of cochlear-implant pulse rate and inter-channel timing on channel interactions and thresholds. Journal of the Acoustical Society of America. 2004;116:452–468. doi: 10.1121/1.1760795. [DOI] [PubMed] [Google Scholar]
- Nelson DA, Donaldson GS. Psychophysical recovery from single-pulse forward masking in electric hearing. Journal of the Acoustical Society of America. 2001;109:2921–2933. doi: 10.1121/1.1371762. [DOI] [PubMed] [Google Scholar]
- Nelson DA, Donaldson GS. Psychophysical recovery from pulse-train forward masking in electric hearing. Journal of the Acoustical Society of America. 2002;112:2932–2947. doi: 10.1121/1.1514935. [DOI] [PubMed] [Google Scholar]
- Nelson DA, Van Tassell DJ, Schroder AC, Soli S, Levine S. Electrode ranking of ‘place pitch’ and speech recognition in electrical hearing. Journal of the Acoustical Society of America. 1995;98:1987–1999. doi: 10.1121/1.413317. [DOI] [PubMed] [Google Scholar]
- Osberger MJ, Fisher L. New directions in speech processing: patient performance with simultaneous analog stimulation. Annals of Otology Rhinology and Laryngology. 2000;185(Suppl):70–73. doi: 10.1177/0003489400109s1230. [DOI] [PubMed] [Google Scholar]
- Parkinson AJ, Arcaroli J, Staller SJ, Arndt PL, Cosgriff A, Ebinger K. The Nucleus 24 Contour cochlear implant system: Adult clinical trial results. Ear & Hearing. 2002;23:41S–48S. doi: 10.1097/00003446-200202001-00005. [DOI] [PubMed] [Google Scholar]
- Peterson GE, Lehiste I. Revised CNC lists for auditory tests. Journal of Speech and Hearing Disorders. 1962;27:62–70. doi: 10.1044/jshd.2701.62. [DOI] [PubMed] [Google Scholar]
- Pfingst BE, Franck KH, Xu L, Bauer EM, Zwolan TA. Effects of electrode configuration and place of stimulation on speech perception with cochlear prostheses. Journal of the Association for Research in Otolaryngology. 2001;2:87–103. doi: 10.1007/s101620010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfingst BE, Zwolan TA, Holloway LA. Effects of stimulus configuration on psychophysical operating levels and on speech recognition with cochlear implants. Hearing Research. 1997;112:247–260. doi: 10.1016/s0378-5955(97)00122-6. [DOI] [PubMed] [Google Scholar]
- Rebscher SJ, Snyder RL, Leake PA. The effect of electrode configuration and duration of deafness on threshold and selectivity of responses to intracochlear electrical stimulation. Journal of the Acoustical Society of America. 2001;109(5):2035–2048. doi: 10.1121/1.1365115. [DOI] [PubMed] [Google Scholar]
- Ryan AF, Miller JM, Wang ZX, Woolf NK. Spatial distribution of neural activity evoked by electrical stimulation of the cochlea. Hearing Research. 1990;50:57–70. doi: 10.1016/0378-5955(90)90033-l. [DOI] [PubMed] [Google Scholar]
- Saunders E, Cohen L, Aschendorff A, Shapiro W, Knight M, Stecker M, Richter B, Waltzman S, Tykocinski M, Roland T, Laszig R, Cowan R. Threshold, comfortable level and impedance changes as a function of electrode-modiolar distance. Ear & Hearing. 2002;23:28S–40S. doi: 10.1097/00003446-200202001-00004. [DOI] [PubMed] [Google Scholar]
- Seligman P, McDermott H. Architecture of the Spectra 22 speech processor. Annals of Otology Rhinology and Laryngology. 1995;166(Suppl):139–141. [PubMed] [Google Scholar]
- Shannon RV. Multichannel electrical stimulation of the auditory nerve in man. I. Basic psychophysics. Hearing Research. 1983a;11:157–189. doi: 10.1016/0378-5955(83)90077-1. [DOI] [PubMed] [Google Scholar]
- Shannon RV. Multichannel electrical stimulation of the auditory nerve in man. II. Channel interaction. Hearing Research. 1983b;12:1–16. doi: 10.1016/0378-5955(83)90115-6. [DOI] [PubMed] [Google Scholar]
- Shpak T, Berlin M, Luntz M. Objective measurements of auditory nerve recovery function in Nucleus CI 24 implantees in relation to subjective preference of stimulation rate. Act Otolaryngol. 2004;124:582–586. doi: 10.1080/00016480310000755-1. [DOI] [PubMed] [Google Scholar]
- Skinner MW, Clark GM, Whitford LA, Seligman PM, Staller SJ, et al. Evaluation of a new spectral peak (SPEAK) coding strategy for the Nucleus 22 channel cochlear implant system. American Journal of Otology. 1994;15:15–27. [PubMed] [Google Scholar]
- Snyder RL, Bierer JA, Middlebrooks JC. 5th Quarterly Progress Report. 2001. Protective effects of patterned electrical stimulation on the deafened auditory system. NPP Contract N01-DC-0-2108. [Google Scholar]
- Throckmorton CS, Collins LM. Investigation of the effects of temporal and spatial interactions on speech-recognition skills in cochlear-implant subjects. Journal of the Acoustical Society of America. 1999;105:861–873. doi: 10.1121/1.426275. [DOI] [PubMed] [Google Scholar]
- van den Honert C, Stypulkowski PH. Single fiber mapping of spatial excitation patterns in the electrically stimulated auditory nerve. Hearing Research. 1987;29:195–206. doi: 10.1016/0378-5955(87)90167-5. [DOI] [PubMed] [Google Scholar]
- White MW, Merzenich MM, Gardi JN. Multichannel cochlear implants: Channel interactions and processor design. Archives of Otolaryngology Head & Neck Surgery. 1984;110:493–501. doi: 10.1001/archotol.1984.00800340005002. [DOI] [PubMed] [Google Scholar]
- Wilson BS, Finley CC, Lawson DT, Wolford RD, Eddington DK, Rabinowitz WM. Better speech recognition with cochlear implants. Nature. 1991;352:236–238. doi: 10.1038/352236a0. [DOI] [PubMed] [Google Scholar]
- Xu J, Xu SA, Cohen LT, Clark GM. Cochlear View: Postoperative radiography for cochlear implantation. American Journal of Otology. 2000;21:49–56. [PubMed] [Google Scholar]
- Zwolan TA, Collins LM, Wakefield GH. Electrode discrimination and speech perception in postlingually deafened adult cochlear implant subjects. Journal of the Acoustical Society of America. 1997;102:3673–3685. doi: 10.1121/1.420401. [DOI] [PubMed] [Google Scholar]
- Zwolan TA, Kileny PR, Ashbaugh C, Telian SA. Patient performance with the Cochlear Corporation “20 + 2” implant: Bipolar versus monopolar activation. American Journal of Otology. 1996;17:717–723. [PubMed] [Google Scholar]