Abstract
As well as inducing a protective immune response against reinfection, acute measles is associated with a marked suppression of immune functions against superinfecting agents and recall antigens, and this association is the major cause of the current high morbidity and mortality rate associated with measles virus (MV) infections. Dendritic cells (DCs) are antigen-presenting cells crucially involved in the initiation of primary and secondary immune responses, so we set out to define the interaction of MV with these cells. We found that both mature and precursor human DCs generated from peripheral blood monocytic cells express the major MV protein receptor CD46 and are highly susceptible to infection with both MV vaccine (ED) and wild-type (WTF) strains, albeit with different kinetics. Except for the down-regulation of CD46, the expression pattern of functionally important surface antigens on mature DCs was not markedly altered after MV infection. However, precursor DCs up-regulated HLA-DR, CD83, and CD86 within 24 h of WTF infection and 72 h after ED infection, indicating their functional maturation. In addition, interleukin 12 synthesis was markedly enhanced after both ED and WTF infection in DCs. On the other hand, MV-infected DCs strongly interfered with mitogen-dependent proliferation of freshly isolated peripheral blood lymphocytes in vitro. These data indicate that the differentiation of effector functions of DCs is not impaired but rather is stimulated by MV infection. Yet, mature, activated DCs expressing MV surface antigens do give a negative signal to inhibit lymphocyte proliferation and thus contribute to MV-induced immunosuppression.
Keywords: surface markers, interleukin 12, strain dependence, proliferative inhibition
Acute measles, a well known disease occurring early in childhood, leads to a lifelong protective immune response against reinfection. Immune activation after measles virus (MV) infection or after MV vaccination is characterized by an increased spontaneous lymphoproliferation (1, 2), lymphokine production (3), and increased expression of the interleukin 2 (IL-2) receptor (4), resulting in the generation of an efficient MV-specific cellular and humoral immune response (for review, see ref. 5). Activation of T cell immunity appears to be essential to overcome acute measles (6) whereas the efficient induction of a humoral immune response is a prerequisite for protection against reinfection. Paradoxically, in addition to substantial MV-specific immune activation, a marked suppression of immune reactions to other antigens is observed that lasts for weeks to months after recovery from the acute illness (reviewed in ref. 7). Associated with a pronounced lymphopenia affecting both B and CD4/CD8 T cells (1), MV-induced immunosuppression is characterized in vivo by the disappearance of delayed-type hypersensitivity reactions (8–10), reactivation of latent mycobacterial infections, and an increased susceptibility to opportunistic infections. The latter comprises the major cause of a constantly high MV-associated death rate in infants, particularly in Third World countries (11). As a hallmark of immune dysfunction, peripheral blood lymphocytes (PBLs) isolated from measles patients fail to proliferate in response to a variety of stimuli in tissue culture including mitogens and recall antigens (8, 10), and a cytokine imbalance favoring a TH2 response is generally observed (3). After MV infection in vitro, a marked impairment in mitogen-, allo-, and recall antigen-dependent proliferation of PBLs (12, 13) and differentiation of their effector functions [such as cytotoxic T lymphocyte activity or immunoglobulin synthesis (14–16)] are seen. Although MV reveals a strong tropism for both lymphocytes and monocytes, the number of infected cells in vivo is low (17, 18), and infection of these cells does not lead to extensive syncytia formation. Thus, functional deficiencies are thought to result from indirect mechanisms rather than from virus-induced cell disruption (7). Mechanisms proposed so far include a major arrest of MV-infected lymphocytes in the G1 phase of the cell cycle (19) as well as apoptosis (20, 21). Moreover, a cytokine pattern compatible with a predominant TH2 response has been observed after stimulation of these cells in vitro (5), which has been suggested to be causatively linked to a strongly reduced release of IL-12 from monocytic cells after surface interaction between MV and its major protein receptor CD46 (22). Recently, we have shown that surface contact between MV glycoproteins and a so far undefined receptor (not CD46) renders uninfected human PBLs unresponsive to mitogen stimulation and leads to proliferative arrest of a variety of permanent lymphocytic and monocytic cell lines in vitro (23). Dendritic cells (DCs) are essential antigen-presenting cells (APCs) in immune activation because they are specialized to capture and present antigens to both naive and primed T cells. At different stages of DC development, they display a different functional repertoire of cell surface proteins (24). DCs can promote extensive replication of HIV-1 (25) and transmit HIV-1 to susceptible CD4+ T cells by formation of DC–T cell conjugates (26–28). Infection of DCs also has been linked to their functional impairment (29), so this particular virus–host interaction is thought to play an important role in the pathogenesis of HIV-1 infection (25). So far, the role of DCs in MV-specific immune activation and their potential role in MV pathogenesis have not been addressed. In the present study, we observed that both mature and precursor DCs, isolated from peripheral blood mononuclear cells (PBMCs) by two different protocols, are highly susceptible to infection with both a wild-type and a vaccine strain of MV. We further found that infection of precursor DCs with MV leads to a rapid up-regulation of activation markers indicative of functional maturation of these cells and to an enhanced synthesis of IL-12, thus generating efficient stimulators of both primary and secondary immune responses. Despite this activated phenotype, however, MV-infected DCs suppress mitogen-dependent proliferation of uninfected PBLs in vitro, indicating that they may potentially contribute to immune suppression in acute measles.
MATERIALS AND METHODS
Viruses and Antibodies.
MV vaccine strain Edmonston-B (ED) was grown and propagated in Vero cells (African green monkey kidney). The MV wild-type strain WTF (30) was propagated in BJAB cells (a human lymphoblastoid cell B cell line). Titers were 1 × 107 pfu/ml for ED and 2 × 106 pfu/ml for WTF.
Isolation and Culture of Peripheral Blood Dendritic Cells.
PBMCs were isolated by Ficoll/Paque (Pharmacia) density gradient centrifugation of heparinized blood obtained from healthy adult donors. Isolation of blood dendritic cells was performed by immunomagnetic separation using a blood dendritic cell isolation kit from Miltenyi Biotec (Bergisch Gladbach, Germany). DCs were cultured in RPMI 1640 medium, 10% fetal calf serum with 800 units/ml gramulocyte–macrophage colony-stimulating factor, and 1000 units/ml IL-4 and were analyzed within 3 days. Establishment of DC cultures in the presence of macrophage-conditioned medium (MCM-DC) has been described in detail elsewhere (31).
Immunocytochemistry.
Double staining was performed following standard procedures using unfixed cells. Unconjugated primary and labeled secondary antibodies (goat anti-mouse fluorescein isothiocyanate or phycoerythrin; Dianova, Hamburg, Germany) were diluted in FACS buffer (PBS/0.4% BSA/0.02% NaN3), which also was used for the intervening washing steps. After application of the third antibody, which was directly conjugated, stainings were analyzed by FACScanning (FACS, Becton Dickinson). Primary antibodies directed against cellular activation markers were obtained from Coulter, the CD83-specific antibody was kindly provided by T. F. Tedder. The anti-CD46 antibody (13/42) and the MV-specific antibodies were generated in our own laboratory.
In Vitro Proliferation Assay.
Presenter cells (PCs) were generated by infecting DCs isolated by immunomagnetic sorting (IMS-DCs) after an overnight culture in the presence of phytohemagglutinin (PHA) (2.5 μg/ml) with MV-ED, or MV-WTF, or mock infection, for 48 h. Expression of viral antigens was determined before UV irradiation of the PCs (0.25 J/cm2 in a biolinker). Human PBLs were depleted of monocytic cells by plastic adherence and were used as responder cells (RCs). They were seeded in the presence of PHA (5 μg/ml) into a 96-cluster plate with a density of 1 × 105 in a volume of 100 μl/well. After addition of the PCs (at the concentration indicated in a volume of 100 μl/well), the PC/RC mixture was incubated for 48 or 72 h and then labeled for 16 h with [3H]thymidine [0.5 μCi/ml (1 Ci = 37 GBq)]. Assays were performed in triplicate and harvested, and the incorporation rates of the label were determined using a β-plate reader.
IL-12 Assay.
DCs were seeded in a density of 1 × 106 cells/ml and stimulated with 100 μg/ml lipopolysaccharide (LPS) or 10 μg/ml fixed Staphylococcus aureus Cowan I strain (SACS; Pansorbin, Calbiochem). Supernatants were harvested 44 h postinfection, and IL-12 was determined using a commercially available ELISA kit (Quantikine Immunoassay, R & D Systems).
RESULTS
MV Infection of Mature DCs.
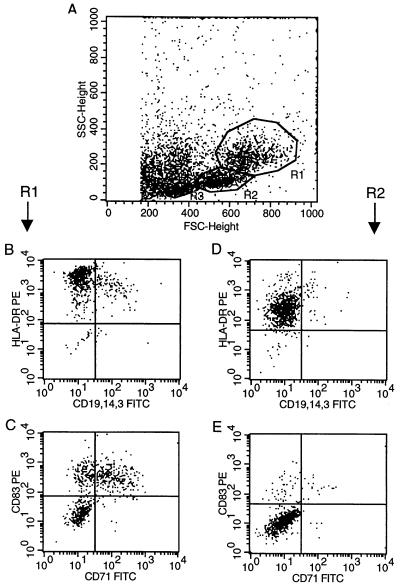
Peripheral blood-derived DCs cultures were generated by two different methods. One preparation was obtained using the lymphocyte-depleted fraction of human PBMCs cultured in the presence of granulocyte–macrophage colony-stimulating factor and IL-4 for 8 days. MCM was added to these cultures after day 6 to induce full maturation of DCs (MCM-DCs) (31). Phenotypic analysis using immunofluorescent staining revealed that, starting on day 8, between 30 and 80% of the cells expressed high levels of HLA-DR and CD83, the surface marker for mature DCs (32, 33) (not shown). The second protocol used to obtain DCs was based on IMS of PBMCs. In this procedure, PBMCs were first depleted of CD3+, CD11b+, and CD16+ cells and were then positively selected for the CD4+ fraction. This was further cultured in the presence of IL-4 and granulocyte–macrophage colony-stimulating factor (IMS-DCs). The phenotype of the final population, which comprised ≈0.5 to 1% of the unfractionated PBMCs, was characterized by FACS analysis after an overnight culture. The morphological dot blot reproducibly revealed three distinct cell populations differing in size and granularity (shown in Fig. 1A). Cells gated in region 1 (R1) were considered to represent mature DCs based on their size, high HLA-DR expression (Fig. 1B), and expression of CD83 (Fig. 1C). Precursor DCs (pre-DCs) gated in region 2 (R2) were of smaller size, failed to express CD83 and CD71 (Fig. 1E), and were positive for HLA-DR to intermediate levels (Fig. 1D). Cells in region 3 (R3) represented contaminating lymphocytes, predominantly CD19+ B cells (not shown). In addition to the HLA-DR, CD83, and CD71, both mature DC populations (MCM-DCs and IMS-DCs) stained positive for the major MV receptor CD46 (Fig. 2B and data not shown).
Figure 1.
Dot blot analysis of IMS-DCs isolated from peripheral blood. After a 24-h culture in the presence of granulocyte–macrophage colony-stimulating factor and IL-4, cell morphology (A) and protein expression (B–E) for mature DCs [region 1 (R1); B and C] and precursor DCs [region 2 (R2); D and E] were analyzed. For double immunofluorescence staining, mAb against HLA-DR and a mixture of CD3-, CD14-, and CD19-specific antibodies (B and D) or antibodies against CD83 and CD71 (C and E) were used.
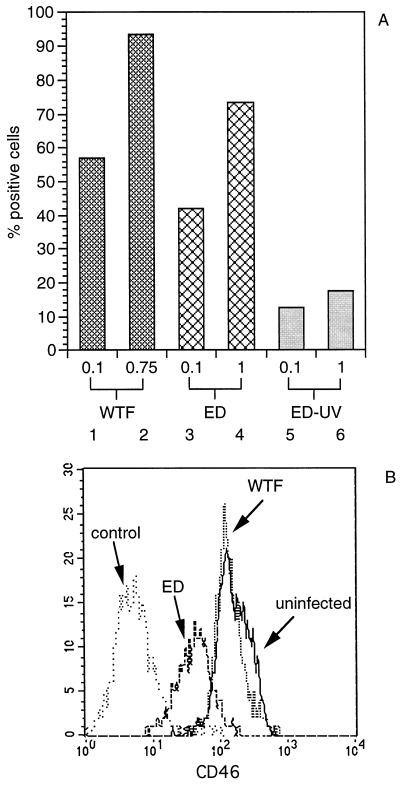
Figure 2.
Expression of MV surface proteins F and H and CD46 on infected MCM-DCs. (A) Cells were infected after 8 days in culture with MV-WTF (lanes 1 and 2, moi 0.1 and 0.75) or MV-ED (lanes 3 and 4, moi 0.1 and 1) or were incubated with UV-irradiated MV (ED-UV, lanes 5 and 6, corresponding to mois of 0.1 and 1) for 24 h, stained using a mixture of mAb against MV surface proteins F and H and subsequently analyzed by FACScanning. (B) CD46 expression on uninfected and MV-infected cells was measured by indirect immunofluorescence with a monoclonal anti-CD46 antibody (13/42). Cells were infected with MV-WTF or MV-ED at an moi of 0.1 as indicated for 24 h. An anti-CD19 antibody was used as negative control.
Both MCM-DCs and IMS-DCs revealed an extremely high susceptibility to infection with MV using the vaccine ED strain and a MV WTF at two different input multiplicities (moi) (as shown for MCM-DCs in Fig. 2). A representative experiment (shown in Fig. 2A) revealed that, after a 24-h infection with WTF, as many as 60% of the cells stained positive for MV surface antigens using an moi of 0.1, and infection levels were close to 100% using an moi of 0.75 (Fig. 2A, lanes 1 and 2). Of interest, the susceptibility of the culture to infection with MV-ED was lower at both mois tested both in MCM-DCs (Fig. 2A, lanes 3 and 4) and in IMS-DCs (not shown and Fig. 4A). After UV irradiation before infection, MV retained only a residual activity to replicate in DCs (Fig. 2A, lanes 5 and 6). The high expression levels of MV surface proteins observed after infection with live virus are largely abolished by UV inactivation, so their accumulation was due to active MV replication and did not reflect binding of the inoculum to the cell surface. Similar results were obtained using IMS-DCs (data not shown).
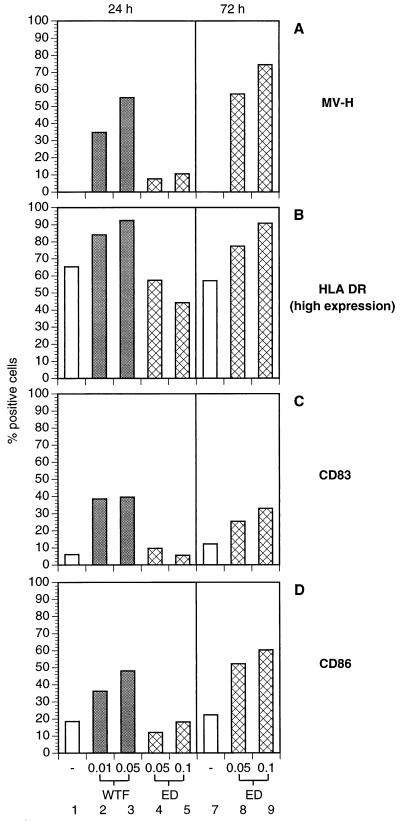
Figure 4.
Surface protein expression of MV-infected pre-DCs. IMS-DCs were left uninfected (A–D, each lanes 1 and 7) or were infected for 24 h (A–D; WTF: lanes 2 and 3, moi 0.01 and 0.05; ED: lanes 4 and 5, moi 0.05 and 0.1) or 72 h (A–D; ED: each lanes 8 and 9, moi 0.05 and 0.1). Surface expression of MV-H, HLA-DR, CD86, and CD83 on pre-DCs gated in R2 (Fig. 1, R2) was determined using specific antibodies. Because of the rapid infection kinetics, WTF-infected cells could not be analyzed after 72 h.
To define whether MV infection is associated with functional alterations of MCM-DCs, surface markers such as HLA-DR, CD83, CD80 (B7–1), CD86 (B7–2), and CD46 were analyzed in uninfected, MV-ED- and WTF-infected cultures after 24 h. Except for down-regulation of CD46 after MV-ED infection (Fig. 2B), no further alterations of the surface marker expression were reproducibly detected in the infected MCM-DCs either in the ungated population or in the infected cell gate (data not shown).
Precursor DCs (Pre-DCs) Differentiate Rapidly After MV Infection.
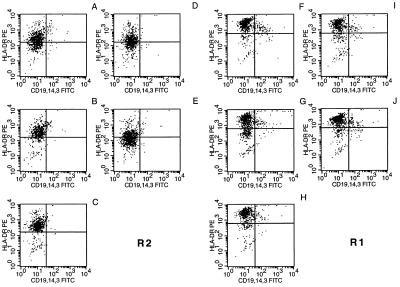
Inhibition of nondifferentiated, but not of differentiated, lymphocyte effector functions has been described after MV infection in tissue culture (16). This observation, and the unaltered expression of activation markers on MCM-DCs after MV infection, prompted us to investigate the effect of MV infection on pre-DCs. DCs were isolated by IMS and infected with low mois of both MV-ED and WTF (moi 0.05). In addition, a higher dose of MV-ED (moi 0.1) and a lower dose of WTF (moi 0.01) were used to compensate for the higher susceptibility of DCs to WTF-infection (Fig. 2A). The same gates as shown in Fig. 1 were used for analyzing the infected and uninfected IMS-DCs. Similar to MCM-DCs, mature IMS-DCs gated in R1 expressed high levels of HLA-DR (Fig. 3F) as did MV-infected IMS-DCs gated in R1 (Fig. 3 G–J) with no significant infection-dependent differences being observed. Similarly, the intermediate expression levels of this antigen were not significantly altered in the pre-DC population gated in R2 after infection with MV-ED (Fig. 3D, moi 0.05; Fig. 3E, moi 0.1; and Fig. 4B, lanes 4 and 5) compared with the uninfected control (Fig. 3A). Infection with WTF, however, led to a rapid up-regulation of HLA-DR expression indicating differentiation of the pre-DC population (Fig. 3 B and C and Fig. 4B, lanes 2 and 3). The up-regulation of HLA-DR in pre-DCs by WTF correlated with a markedly higher expression of MV antigens in these cultures (Fig. 4A, lanes 2 and 3) compared with MV-ED (Fig. 4A, lanes 4 and 5). It was only 48 h later that comparable levels of MV antigen expression were observed in MV-ED-infected pre-DCs (Fig. 4A, lanes 8 and 9), when infection-dependent disintegration was complete for WTF-infected pre-DCs (data not shown). Similar to HLA-DR, both CD83 and CD86 were up-regulated in correlation with the expression of MV antigens in the infected pre-DCs (for WTF after 24 h and for MV-ED after 72 h; Fig. 4 C and D). Thus, infection of pre-DCs with WTF efficiently and rapidly triggered maturation of these cells as defined by the up-regulation of activation markers whereas the MV-ED strain was much less effective.
Figure 3.
Expression of HLA-DR on the surface of IMS-DCs after MV infection. IMS-DCs were double-stained for HLA-DR and CD19/CD14/CD3 expression 24 h postinfection with MV WTF moi 0.01 (B and G) and moi 0.05 (C and H) or MV ED moi 0.05 (D and I) and moi 0.1 (E and J) or were left uninfected (A and F). Mature DCs (R1; F–J) and pre-DC (R2; A–E) were gated and analyzed in analogy to Fig. 1.
MV Infection Stimulates IL-12 Release from DCs.
IL-12 is critical for the induction of cell-mediated immune responses (34). In addition to monocyte/macrophages, DCs have been identified as major producers of this cytokine and thus contribute to the development of TH1 responses (35). IMS-DCs were infected with MV-WTF (moi 0.01) or MV-ED (moi 0.5) or were left uninfected for 44 h, and synthesis of IL-12 was induced by treatment with LPS (1 μg/ml) or SACS (10 μg/ml) 2.5 h postinfection. IL-12 was detected in supernatants of uninfected IMS-DCs stimulated with LPS alone at up to 12.74 pg/ml whereas SACS induction induced only low levels of this cytokine (Table 1, Exp. 1). However, in the presence of both individual inducers, MV infection significantly stimulated IL-12 release. LPS-induced cultures infected with MV-WTF released 49 pg/ml, and >80 pg/ml was measured in supernatants of MV-ED-infected DCs. The differences in IL-12 secretion of the infected cultures correlated with the percentage of cells staining positive for MV antigens; >90% of the MV-ED-infected DCs was positive, and only 50% of those infected with MV-WTF was positive (Table 1, Exp. 1). Although IL-12 release was generally lower after SACS induction, both MV-ED and MV-WTF infection augmented IL-12 synthesis. To confirm the infection-dependent stimulation of IL-12 release, the experiment was repeated using DCs isolated from a different donor. Although the absolute levels of IL-12 revealed individual differences, augmentation of IL-12 after LPS stimulation clearly correlated with the number of infected cells in the cultures (Table 1, Exp. 2).
Table 1.
MV infection of IMS-DCs stimulates IL-12 release
| Exp. | LPS | SACS | Infected cells* |
|---|---|---|---|
| 1 | |||
| Uninfected | 12.74† | 7.5 | — |
| WTF (moi 0.01) | 49.54 | 15.3 | 53 |
| ED (moi 0.5) | 85.00 | 18.9 | 93 |
| 2 | |||
| Uninfected | NDet | ND | — |
| WTF (moi 0.05) | 37.2 | ND | 46.8 |
| ED (moi 0.1) | 10.6 | ND | 24.2 |
IMS-DCs seeded at a density of 106 cells/ml were infected with MV-WTF or MV-ED and stimulated after 2.5 h with LPS (1 μg/ml) or SACS (10 μg/ml), and supernatants were harvested after 44 h. IL-12 concentration was determined using an ELISA kit. ND, not done; NDet, not detectable; Exp., experiment.
Percentage of cells staining for MV surface proteins.
IL-12 concentrations in pg/ml.
MV-Infected DCs Suppress Mitogen-Dependent Proliferation of Both Autologous and Allogeneic PBLs.
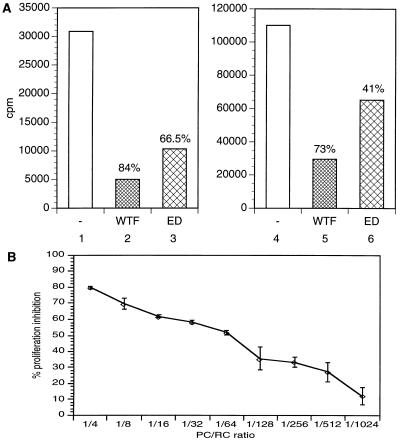
MV infection was found to induce functional maturation of pre-DCs (Figs. 3 and 4), and down-regulation of functionally important surface molecules from mature DCs was not observed after MV-infection, so MV-infected DC cultures should be highly potent in inducing a proliferative response in PBLs. IMS-DCs were infected with either WTF or MV-ED (moi of 0.1) to obtain a maximal expression of MV proteins. After 48 h, infected (or uninfected) DCs were UV-irradiated (to inactivate infectious MV), washed, and added as PCs to autologous (Fig. 5A, lanes 1 to 3) or allogeneic (Fig. 5A, lanes 4 to 7) RCs at a ratio of 1:4 (PC/RC) in the presence of PHA. Intact PCs could not be detected later than 24 h after cocultivation (not shown). After 88 h of incubation (including 16 h of [3H]-thymidine labeling), mitogen-dependent proliferation of PBLs was significantly reduced after cocultivation with MV-infected DCs (Fig. 5A, lanes 2, 3, 5, and 6) compared with uninfected DCs (Fig. 5A, lanes 1 and 4). Proliferative inhibition observed after cocultivation of both autologous and allogeneic RCs with WTF-infected IMS-DCs was stronger than that observed using MV-ED-infected IMS-DCs (Fig. 5A, lanes 2 and 5 and lanes 3 and 6, respectively), most likely as a result of the higher expression levels of MV surface proteins in these WTF-infected cultures (data not shown). MV-infected IMS-DCs also inhibited mitogen-dependent proliferation of autologous PBLs when UV irradiation before cocultivation was omitted (not shown). An independent experiment was performed to show that the inhibitory activity of MV-ED-infected, UV-irradiated DCs could be titrated. PC/RC ratios from 1:4 to 1:1024 were used, and the cells were cocultivated for 48 h before a 16-h labeling period. Furthermore, washing of the DCs before cocultivation was omitted. In this particular experiment, proliferative inhibition of the RCs using a 1:4 PC/RC ratio reached close to 80% (Fig. 5B) (with a frequency of DCs staining positive for MV antigens of more than 95%), and an 10% inhibition was still detectable in the final dilution (1:1024).
Figure 5.
Inhibition of proliferation induced by MV-infected IMS-DCs. (A) IMS-DCs were infected with MV-WTF (lanes 2 and 5) or ED (lanes 3 and 6) at an moi of 0.1 for 48 h or were left uninfected (lanes 1 and 4). After UV irradiation, IMS-DCs (both infected and uninfected) were washed and cocultivated with a 4-fold excess of PHA-stimulated autologous (lanes 1 to 3) or allogeneic (lanes 4 to 6) PBLs for an additional 3 days. Proliferation rates were defined after a 16-h labeling period and are indicated as counts per minute (cpm). Inhibition of the proliferative response of PBLs by cocultivation with WTF- (lanes 2 and 5) or ED-infected (lanes 3 and 6) IMS-DCs compared with cocultivation with uninfected IMS-DCs (lanes 1 and 4) is indicated by %. (B) IMS-DCs were infected with MV-ED (moi 1) for 48 h (or left uninfected), UV-irradiated, and cocultivated with PBLs in the presence of PHA in the PC/RC ratios indicated for further 48 h, followed by a 16-h labeling.
DISCUSSION
Peripheral blood-derived DCs are APCs that play an important role in stimulating primary immune responses. Antigen capture in the periphery by immature DCs leads to both activation and differentiation of these cells into mature DCs that have a reduced ability to capture and process new proteins for presentation but have a high ability to stimulate resting CD4+ and CD8+ T cells to grow and differentiate (24). Both substantial immune activation and disruption of immune functions are observed during acute measles, so we set out to characterize basic features of the interaction of MV with this particular cell type. Two different approaches have been used to establish DC cultures, one of which (MCM-DCs) yielded a highly pure population of mature DCs (31); the other yielded a mixture of both mature and precursor DCs (IMS-DCs) that still contained ≈10–20% contaminating cells, predominantly B cells (Fig. 1A). Mature DCs isolated by both procedures readily expressed specific surface markers such as CD83 and HLA-DR at high levels and, at lower levels, the costimulatory molecules CD80 and CD86. Pre-DCs isolated by IMS expressed, as typical for immature DCs, CD4, intermediate levels of HLA-DR, and background levels of CD83 (32, 33). In contrast to what has been described by Zhou and Tedder (32), both MCM-DCs and IMS-DCs stained positive for CD46, the major protein receptor for MV-ED (36, 37) (Fig. 2B). The reason for this discrepancy is not clear; in both studies, the expression of this protein was analyzed on mature DCs and, in our hands, was found also on pre-DCs. Because human cells not expressing CD46 cannot be infected with the MV-ED strain (36, 37), the high susceptibility of DCs to infection with this virus provides a further strong argument for the presence of CD46 on these cells. Except for the MV-ED-mediated down-regulation of CD46 that has been described earlier (30, 38–40), no significant infection-dependent alterations of the expression of activation markers such as HLA-DR, CD83, CD80, CD86, or CD71 were observed in mature DCs obtained either under MCM or IMS conditions. This could be attributed to the already high expression levels of these antigens before infection. This assumption is supported by previous findings describing that MV infection of lymphocytes generally does not affect effector functions already differentiated but only those still to be acquired after infection (16). Thus, we focused our interest on the interaction of both MV strains with pre-DCs. Using the same mois, MV-WTF infection proceeded significantly faster in pre-DCs than that with MV-ED (Fig. 4A, lanes 3 and 4). Only after 72 h were comparable expression levels for MV F and H observed in ED-infected pre-DCs (Fig. 4A, lane 8). It is well established that lymphotropic MV strains, such as MV-WTF, that have never been adapted to grow in nonlymphoid cells only replicate efficiently in cells of a lymphoid lineage (41). Whether this strict tropism extends to the monocytic lineage or to DCs is unknown at present. Recent studies suggest that MV wild-type strains may preferentially bind to and use an alternative receptor for cell entry that is not identical to CD46 (42, 43). If this potential alternative receptor is expressed not only on lymphocytes but also on DCs, this could well account for the higher susceptibility of these cells to MV-WTF infection. Pre-DCs underwent a rapid infection-dependent maturation as characterized by the pattern of surface activation markers (Figs. 3 and 4) while the extent of up-regulation correlated strictly with the expression levels of MV antigens. Whether up-regulation of activation markers on DCs is induced by mediators released after MV infection or results merely from a surface contact between MV and its receptor protein(s) has still to be addressed. Successful activation of DCs by MV infection also is revealed by their capacity to synthesize and release IL-12 because mature DCs form one of the major IL-12-secreting cell populations (34). Again, the amount of IL-12 released strictly correlated with the expression levels of MV structural proteins (Table 1). In contrast to our findings, IL-12 synthesis was suppressed markedly in monocytes and macrophages after interaction with MV or application of anti-CD46 antibodies or dimerized C3b (in the absence of MV), indicating that triggering of CD46 induced down-regulation of this cytokine (22). The most likely explanation for this discrepancy is that <3% of monocytes/macrophages were productively infected in this study whereas DCs proved to be highly susceptible to MV infection and up to 90% stained positive for MV antigens (Table 1). Although crosslinking of CD46 either by MV particles or antibodies may down-regulate IL-12 synthesis by monocyte/macrophages, this effect may be counteracted by a positive regulatory signal after productive MV infection in DCs. Thus, MV infection in pre-DCs is associated with a rapid, efficient maturation as characterized by the induction of activation markers and the release of IL-12. Despite this highly activated phenotype, MV-infected IMS-DCs confer a negative signal to naive, uninfected PBLs to prevent proliferation in response to mitogens (Fig. 5). Thus, they behave like MV-infected PBLs, MV-infected monocytes, or 293 cells expressing the MV glycoproteins (23) that have been shown to induce a state of proliferative unresponsiveness in PBLs to mitogenic stimulation in vitro. Inhibition of mitogen-dependent proliferation of PBLs after cocultivation with MV-infected DCs was not due to the removal of IL-12 by the washing step included before mixing because it also was observed when the washing step was omitted and IL-12 was transferred (Fig. 5B). Of interest, proliferation of PBLs in response to other stimuli, including a mixed lymphocyte reaction, also was affected by this contact-mediated inhibition (23). Similarly, Langerhans cells from AIDS patients and macrophages from both AIDS and HIV+ non-AIDS patients stimulated allogeneic T cells poorly when compared with control APCs (29). Decreased recall antigen and mitogen-induced T cell responsiveness was observed in HIV+ patients using either autologous Langerhans cells or autologous macrophages as APC/accessory cells. However, it could be shown that APC from HIV+ patients were impaired only in their ability to generate a primary immune response (i.e., allogen-induced T cell stimulation) whereas they retained the ability to generate a secondary immune response (i.e., recall antigen-induced syngeneic T cell stimulation). This could be shown by using autologous responder T cells isolated from HIV− and HIV+ twins. Our data clearly indicate that MV infection of the APC alone is necessary and sufficient to induce unresponsiveness because uninfected DCs do not induce this effect (Fig. 5, lanes 1 and 4). Although both autologous or allogeneic responder PBLs were sensitive to the effect, our experimental conditions do not, however, allow a distinction between stimulation of primary and secondary immune responses (Fig. 5). Infection of blood-derived DCs could well play an important role in the induction of MV-induced immunosuppression. It is conceivable that pre-DCs infected with MV in the periphery during the first or second viremic phase would undergo a rapid maturation, inducing them to home to local lymph nodes and to stimulate both primary and secondary immune responses. When expressing MV antigens, however, these cells would be highly efficient in inducing proliferative unresponsiveness in T cells in response to recall antigens and opportunistic infections, when the efficient priming of immune reactions against MV was already complete. In this context, the higher susceptibility of DCs to infection with MV-WTF should be reemphasized; this was associated both with a higher capacity to induce differentiation of pre-DCs into mature DCs and a stronger ability to suppress activation of PBLs in vitro. These findings might well be paralleled in vivo by the higher efficiency of the natural MV infection to induce both immune activation and immunosuppression compared with vaccination.
Acknowledgments
We thank Anneliese Schimpl, Jürgen Schneider-Schaulies, and Stefan Niewiesk for helpful discussions; Dr. T. F. Tedder for generously providing the anti-CD83 antibody; Jörg Schlender for his help in the proliferation assays; and Ian Johnston for critically reading the manuscript. The work was supported by the Deutsche Forschungsgemeinschaft, the Bundesministerium für Forschung und Technologie, the Robert Pfleger Stiftung and the World Health Organization.
ABBREVIATIONS
- MV
measles virus
- IL
interleukin
- PBLs
peripheral blood lymphocytes
- DC
dendritic cell
- APC
antigen-presenting cell
- PBMCs
peripheral blood mononuclear cells
- ED
Edmonston
- MCM-DCs
DCs grown in the presence of macrophage-conditioned medium
- PCs
presenter cells
- IMS-DC
DC isolated by immunomagnetic sorting
- pfu
plaque forming units
- PHA
phytohemagglutinin
- RCs
responder cells
- LPS
lipopolysaccharide
- SACS
Staphylococcus aureus Cowan I strain
- pre-DCs
precursor DCs
- moi
multiplicity of infection
References
- 1.Arneborn P, Biberfeld G. Infect Immun. 1983;39:29–37. doi: 10.1128/iai.39.1.29-37.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Besser G M, Davis J, Duncan C, Kirk B, Kuper S W. Br J Haematol. 1967;13:189–193. doi: 10.1111/j.1365-2141.1967.tb08730.x. [DOI] [PubMed] [Google Scholar]
- 3.Griffin D E, Ward B J, Jauregui E, Johnston R T, Vaisberg A. N Engl J Med. 1989;320:1667–1672. doi: 10.1056/NEJM198906223202506. [DOI] [PubMed] [Google Scholar]
- 4.Griffin D E, Moench T R, Johnson R T, Lindo de Soriano I, Vaisberg A. Clin Immunol Immunopathol. 1986;40:305–312. doi: 10.1016/0090-1229(86)90035-8. [DOI] [PubMed] [Google Scholar]
- 5.Griffin D E. In: Current Topics of Microbiology and Immunolgy: Measles Virus. ter Meulen V, Billeter M A, editors. Berlin: Springer; 1995. pp. 117–134. [Google Scholar]
- 6.Nahmias A J, Griffith D, Salsbury C, Yoshida K. J Am Med Assoc. 1967;201:729–734. [PubMed] [Google Scholar]
- 7.Borrow P, Oldstone M B A. In: Current Topics of Microbiology and Immunolgy: Measles Virus. ter Meulen V, Billeter M A, editors. Berlin: Springer; 1995. pp. 85–100. [DOI] [PubMed] [Google Scholar]
- 8.Smithwick E M, Berkovich S. In: Cellular Recognition. Smith R T, Godd R A, editors. New York: Appleton Centruey Crafts; 1969. p. 131. [Google Scholar]
- 9.Zweiman B. J Immunol. 1971;106:1154–1158. [PubMed] [Google Scholar]
- 10.Tamashiro V G, Perez H H. Pediatr Infect Dis. 1987;6:451–454. doi: 10.1097/00006454-198705000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Clements C J, Cutts F T. In: Current Topics of Microbiology and Immunolgy: Measles Virus. ter Meulen V, Billeter M A, editors. Berlin: Springer; 1995. pp. 13–33. [Google Scholar]
- 12.Yanagi Y, Cubitt B A, Oldstone M B A. Virology. 1992;187:280–289. doi: 10.1016/0042-6822(92)90316-h. [DOI] [PubMed] [Google Scholar]
- 13.McChesney M B, Oldstone M B A. Adv Immunol. 1989;45:335–380. doi: 10.1016/s0065-2776(08)60696-3. [DOI] [PubMed] [Google Scholar]
- 14.McChesney M B, Fujinami R S, Lampert P W, Oldstone M B A. J Exp Med. 1986;163:1331–1336. [PMC free article] [PubMed] [Google Scholar]
- 15.Casali P, Rice G P, Oldstone M B A. J Exp Med. 1984;159:1322–1337. doi: 10.1084/jem.159.5.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galama J M D, Ubels-Postma J, Vos A, Lucas C J. Cell Immunol. 1980;50:405–415. doi: 10.1016/0008-8749(80)90294-4. [DOI] [PubMed] [Google Scholar]
- 17.Schneider-Schaulies S, Kreth H W, Hofmann G, Billeter M, ter Meulen V. Virology. 1991;182:703–711. doi: 10.1016/0042-6822(91)90611-e. [DOI] [PubMed] [Google Scholar]
- 18.Nakayama T, Mori T, Yamaguchi S, Sonoda S, Asamura R, Takeuchi Y, Urano T. Virus Res. 1995;35:1–16. doi: 10.1016/0168-1702(94)00074-m. [DOI] [PubMed] [Google Scholar]
- 19.McChesney M B, Altman A, Oldstone M B A. J Immunol. 1988;140:1269–1273. [PubMed] [Google Scholar]
- 20.Esolen L E, Ward B J, Moench T R, Griffin D E. J Virol. 1995;69:3955–3958. doi: 10.1128/jvi.69.6.3955-3958.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Auwärter P G, Kaneshima H, McCune J M, Wiegand G, Griffin D E. J Virol. 1996;70:3734–3740. doi: 10.1128/jvi.70.6.3734-3740.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karp C L, Wysocka M, Wahl L M, Ahearn J M, Cuomo P J, Sherry B, Trinchieri G, Griffin D E. Science. 1996;273:228–231. doi: 10.1126/science.273.5272.228. [DOI] [PubMed] [Google Scholar]
- 23.Schlender J, Schnorr J J, Spielhofer P, Cathomen T, Cattaneo R, Billeter M A, ter Meulen V, Schneider-Schaulies S. Proc Natl Acad Sci USA. 1996;93:13194–13199. doi: 10.1073/pnas.93.23.13194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steinman R M. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 25.Cameron P, Pope M, Granelli-Piperno A, Steinman R M. J Leukocyte Biol. 1996;59:158–171. doi: 10.1002/jlb.59.2.158. [DOI] [PubMed] [Google Scholar]
- 26.Cameron P U, Freudenthal P S, Barker J M, Gezelter S, Inaba K, Steinman R M. Science. 1992;257:383–386. doi: 10.1126/science.1352913. [DOI] [PubMed] [Google Scholar]
- 27.Cameron P U, Pope M, Gezelter S, Steinman R M. AIDS Res Hum Retroviruses. 1994;10:61–69. doi: 10.1089/aid.1994.10.61. [DOI] [PubMed] [Google Scholar]
- 28.Weissman D, Li Y, Orenstein J M, Fauci A S. J Immunol. 1995;155:4111–4117. [PubMed] [Google Scholar]
- 29.Blauvelt A, Clerici M, Lucey D R, Steinberg S M, Yarchaon R, Walker R, Shearer G M, Katz S I. J Immunol. 1995;154:3506–3515. [PubMed] [Google Scholar]
- 30.Schneider-Schaulies J, Schnorr J J, Brinckmann U, Baczko K, Schneider-Schaulies S, ter Meulen V. Proc Natl Acad Sci USA. 1995;92:3943–3947. doi: 10.1073/pnas.92.9.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romani N, Reider D, Heuer M, Ebner S, Kämpgen E, Eibl B, Niederwieser D, Schuler G. J Immunol Methods. 1996;196:137–151. doi: 10.1016/0022-1759(96)00078-6. [DOI] [PubMed] [Google Scholar]
- 32.Zhou L, Tedder T F. J Immunol. 1995;154:3821–3835. [PubMed] [Google Scholar]
- 33.Zhou L, Tedder T F. Proc Natl Acad Sci USA. 1996;93:2588–2592. doi: 10.1073/pnas.93.6.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trinchieri G. Annu Rev Immunol. 1995;13:521–265. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 35.Heufler C, Koch F, Stanzl U, Topar G, Wysocka M, Trinchieri G, Enk A, Steinman R M, Romani N, Schuler G. Eur J Immunol. 1996;26:659–668. doi: 10.1002/eji.1830260323. [DOI] [PubMed] [Google Scholar]
- 36.Doerig R E, Marcil A, Chopra A, Richardson C D. Cell. 1993;75:295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- 37.Naniche D, Varior-Krishnan G, Cervoni F, Wild T F, Rossi B, Rabourdin-Combe C, Gerlier D. J Virol. 1993;67:6025–6032. doi: 10.1128/jvi.67.10.6025-6032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schneider-Schaulies J, Dunster L M, Kobune F, Rima B K, ter Meulen V. J Virol. 1995;69:7257–7259. doi: 10.1128/jvi.69.11.7257-7259.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schneider-Schaulies J, Schnorr J J, Schlender J, Dunster L M, Schneider-Schaulies S, ter Meulen V. J Virol. 1996;70:255–263. doi: 10.1128/jvi.70.1.255-263.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krantic S, Giminez C, Rabourdin-Combe C. J Gen Virol. 1995;76:2793–2800. doi: 10.1099/0022-1317-76-11-2793. [DOI] [PubMed] [Google Scholar]
- 41.Kobune F, Sakata H, Sugiura A. J Virol. 1990;64:700–705. doi: 10.1128/jvi.64.2.700-705.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lecouturier V, Fayolle J, Caballero M, Carabana J, Celma M L, Fernandez-Munoz R, Wild T F, Buckland R. J Virol. 1996;70:4200–4204. doi: 10.1128/jvi.70.7.4200-4204.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bartz R, Brinckmann U G, Dunster L M, Rima B K, ter Meulen V. Virology. 1996;224:334–337. doi: 10.1006/viro.1996.0538. [DOI] [PubMed] [Google Scholar]