Abstract
Melanomas tend to become less pigmented in the course of malignant progression. Thus, as proliferation increases, the tumors are decreasingly characterized by the tissue-specific phenotype of normally differentiated melanocytes. To learn whether the decline in melanization is associated with a shift from constitutive to alternative splicing of some pigment gene pre-mRNAs, melanomas were collected from Tyr-SV40E transgenic mice of the standard C57BL/6 strain. The mRNAs of the tyrosinase gene, which has a key role in melanogenesis, were analyzed by quantitative reverse transcriptase–PCR in 34 samples from 16 cutaneous tumors and 9 metastases. The cutaneous tumors included some cases with distinct melanotic and amelanotic zones, which were separately analyzed. All tyrosinase transcripts found in the melanomas were also found in normal skin melanocytes. However, the Δ1b and Δ1d alternatively spliced transcripts, due to deletions within the first exon, were specifically augmented in most of the tumors over their very low levels in skin; the exceptions were some all-amelanotic tumors in which no tyrosinase transcripts were detected. The level of Δ1b rose as high as 11.3% of total tyrosinase mRNAs as compared with 0.6% in skin; Δ1d reached 4.0% as compared with 0.8% in skin. Expression of these splice variants was highest in the melanotic components of zonal primary tumors, relatively lower in their amelanotic components, and still lower in all-amelanotic primary tumors and amelanotic metastases. The increase in Δ1b and Δ1d transcripts may be predicted to increase the levels of unusual peptides, which could have antigenic potential in the tumors, especially in the relatively early phases of malignancy. Analyses of the alternative transcripts of other pigment genes may identify additional candidate antigens, ultimately enabling melanoma cells in all phases of the disease to be represented as a basis for immune intervention.
Keywords: transgenic mouse models, RNA processing, melanocytic genes, melanoma progression, melanoma metastasis
Malignant progression is marked by increased cell growth and proliferation at the expense of cell differentiation (1–4). Gene mutation is more likely to be directly implicated in the accelerated growth than in the declining differentiation of a tumor. The products of “cell-specialization” genes, whose expression is changing, might thus provide more consistent (nonmutated) targets for immunotherapeutic intervention than those of “cell-growth” genes whose composition might be changing. Melanomas offer a favorable opportunity to test this general proposition, since they undergo a characteristic decline in pigmentation (5), which is normally the hallmark of differentiated melanocytes.
The hypothesis investigated here is that the pigmentary decrease in melanomas may reflect shifts from constitutive toward alternative splicing of the pre-mRNAs of some of the tissue-specific pigment genes, thereby decreasing those mRNAs whose proteins have essential functions in melanogenesis. As a possible further consequence, novel peptides with antigenic potential in melanoma might be derived from splice variants that are overrepresented in the melanomas. While genes not involved in pigmentation, such as CD44, may also undergo splicing changes in tumors (6, 7), their expression is not limited to the melanocytic lineages.
Malignant melanomas were obtained in Tyr-SV40E transgenic mice (8–10), whose disease closely parallels the course of human melanoma (11). This mouse model has a number of advantages: (i) all the animals have the same genetic background; (ii) single-gene substitutions can be introduced, for example, at loci affecting pigmentation or the immune response; and (iii) many experimental interventions are feasible. The tumors represent various stages of melanoma progression and metastasis. They were analyzed by quantitative reverse transcriptase (RT)-PCR for changes in the transcript profile of the murine tyrosinase gene, in comparison with the transcripts in melanocytes of normal mouse skin. Unusual peptides predicted from alternative transcripts overrepresented in the melanomas can later be tested for their immunogenicity and their utility in melanoma immunotherapy. This is a “reverse” approach toward treatment relative to the strategies currently being explored elsewhere, in which some aspect of the immune response is the point of departure, such as identification of peptides recognized by cytotoxic T-lymphocytes from melanoma patients or eluted from complexes with major histocompatibility proteins. Some of the peptides discovered in those studies have been found to originate from pigment genes, for example, from the tyrosinase (12–14), tyrosinase-related protein-1/gp75 (15), tyrosinase-related protein-2 (16), Pmel17/gp100/silver (17–20), or MART-1/Melan-A (21, 22) genes.
The tyrosinase gene plays a key role in melanogenesis (23). The mouse gene has been characterized (24, 25); further details concerning its pre-mRNA splicing patterns will be presented elsewhere. We describe here an increase in specific alternative splice variants of tyrosinase in the murine melanomas, especially in the relatively early phases of malignancy.
MATERIALS AND METHODS
Melanomas.
Cutaneous melanomas were produced in melanoma-susceptible Tyr-SV40E transgenic mice of the C57BL/6 inbred strain (8) by grafting discs of full-thickness body skin from line 8 hemizygous (high-susceptibility) donors to line 12 hemizygous (low-susceptibility) hosts as described (9). Melanomas arose in the grafted skin, under the promotional stimulus of factors associated with wound healing, after an average latency of 34 weeks (range: 21–46 weeks); some of the primary tumors metastasized into organs of the hosts. The cutaneous melanomas averaged 929 mm3 in size at the time they were collected; all had ulcerated and were invasive. Metastases varied over a wide size range. Tumor pieces were snap-frozen in dry ice and stored at −70°C.
Thirty-four melanoma samples were analyzed; 24 were obtained from 16 primary cutaneous tumors and 10 were from 9 metastases. When multiple samples were analyzed from a given tumor they represented areas differing in melanization. Eight of the primary tumors were comprised of a distinctly melanotic and an amelanotic zone; pairs of melanotic and amelanotic samples were taken directly from five of the zonal tumors, and from early-passage transplant lines derived from the other three tumors that were maintained by subcutaneous inoculation in transgenic hosts. Of the eight remaining (nonzonal) primary tumors, one had mottled pigmentation and seven were amelanotic. Four of the analyzed metastases were found in regional lymph nodes, three were in lung, and two were in liver. One lymph node metastasis was melanotic, another was partly melanotic and partly amelanotic, and all the other metastases were amelanotic.
Amplification and Quantitation of Alternative Transcripts.
RNA was isolated from the transgenic tumors, or from dorsal body skin of 8- or 15-day mice of the standard C57BL/6 strain, by the acid guanidinium thiocyanate-phenol-chloroform procedure (26). Tyrosinase cDNAs were generated from total RNA (2.5 μg) using the following specific primer complementary to the 3′ untranslated region of the tyrosinase RNA sequence: 5′-CAACAAATAGGTCGAGTGAGG-3′. Reverse transcription was performed with SuperScript II (GIBCO/BRL) according to the manufacturer’s instructions. The sense and antisense primers for PCR amplification were: 5′-AGGAGAAAATGTTCTTGGCTGTTTTGT-3′ and 5′-CCTAGGATGTTCACAGATGGCTCTG-3′, which respectively overlap the start and stop codons such that constitutive and alternative transcripts were amplified concurrently. To prevent mis-priming, the reaction was carried out with a hot-start procedure (27). After mixing all components in the first heating step, the reaction mixture (final volume 75 μl) had the following composition: 200 ng RNA, 10 mM Tris⋅HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 0.2 mM of each dNTP, 0.25 μM of each primer, and 3.75 units of Taq DNA polymerase (AmpliTaq, Perkin–Elmer). The amplification began with two cycles in which DNA denaturation occurred at 95°C for 3 min, annealing at 62°C for 1 min, and extension at 72°C for 1 min and 30 sec. In subsequent cycles, the denaturation step was carried out at 95°C for 1 min. For each tumor sample, it was necessary to determine the number of PCR cycles that would provide sufficient amplification of all transcripts without exiting from the exponential phase. This was carried out by comparison with a reaction from a skin sample run for 25 cycles, which was found to be within the log phase of the PCR. The cycle numbers ranged from 20–25.
For Southern blot analysis, 10 μl of the PCR products were size-fractionated on a 1.2% agarose gel and transferred to a Nylon membrane (Nytran Plus, Schleicher & Schuell). Hybridizations were performed according to Church and Gilbert (28). The RT-PCR products were analyzed with a probe designated prTyr (5′-GTTCACAGATGGCTCTGATACA-3′), which hybridized to all the transcripts, or with the prEx3 probe (5′-TCTGCTATCCCTGTGAGTGG-3′), which excluded the Δ3 transcripts. The latter was used to reveal the Δ1e and Δ4 transcripts, which were otherwise obscured by Δ3. The analysis of Δ1b and Δ1d groups of transcripts were based on oligonucleotide probes complementary to the splice junction sequences created by deletions within exon 1; these probes were prΔ1b (5′-GTGGATGACCATCATTTGTAG-3′) and prΔ1d (5′-CTGCGGAAACTATCATTTGTA-3′).
For quantitation, a known amount of an RNA internal standard was included in the RT-PCR to assess the levels of tyrosinase mRNAs and to compare data from separate experiments. This internal standard corresponded to the full-length tyrosinase in which a 98-bp deletion had been introduced in exon 1. The level of the standard was set for the RNA of each tumor sample so that the band on the Southern blot was approximately in the mid-range of intensities of the other bands present. Band intensities were measured with a BAS1000 phosphorimager (Fuji).
RESULTS
Differences in Levels of the Full-Length Tyrosinase Transcript in Melanotic vs. Amelanotic Regions of Zonal Primary Melanomas.
As in human melanomas (5), mouse melanomas tend to evolve from a melanotic toward an amelanotic state (29, 30)—a change generally accompanied by accelerated cell division. Molecular comparisons between the melanotic and amelanotic regions in a given zonal tumor thus afford contemporaneous “snapshots” of stages in progression of the tumor. (It should be noted that “amelanotic” refers to the readily visible phenotype of a tumor region or an entire tumor and does not exclude the possibility that some melanin may be present.)
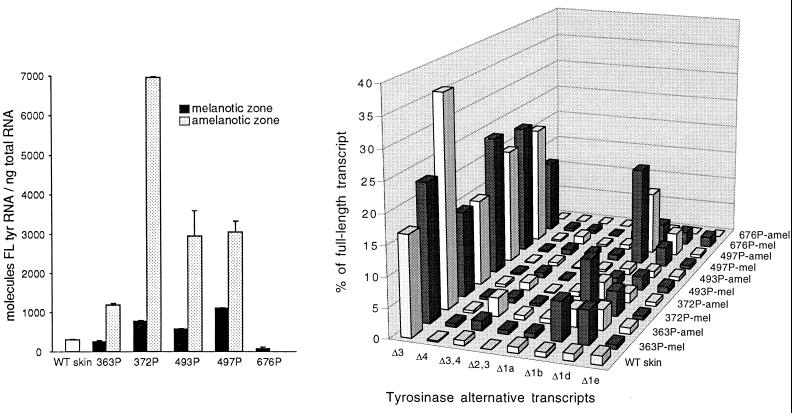
Levels of the full-length (constitutively spliced) tyrosinase transcript in melanotic and amelanotic samples from zonal primary melanomas often exceeded those in normal C57BL/6 skin, in which the gene is specifically expressed in melanocytes. Surprisingly, the levels were three to nine times higher in the amelanotic than in the melanotic components of four of the five original cutaneous tumors (Fig. 1 Left) and in two of the three transplanted tumors (155P and 222P) (data not shown). The question arises whether some melanin biosynthesis was being channeled from the eumelanin (black-brown) into the phaeomelanin (yellow-red) pathway; the latter would yield a very pale coloration consistent with the appearance of tumors classified as amelanotic. Previous analyses of the transplanted tumors revealed the presence of tyrosinase protein in the amelanotic (as well as the melanotic) sample of 155P, and of tyrosinase enzyme activity in both components of 222P (30). Therefore, despite the visible phenotype, tyrosinase message was in fact translated in the amelanotic regions of at least these tumors. In contrast, there was little or no tyrosinase transcription in the amelanotic zone of tumors 676P and 159P.
Figure 1.
Tyrosinase mRNA expression in cutaneous zonal melanomas and wild-type (WT) C57BL/6 mouse skin. Samples were analyzed from the melanotic and amelanotic zones of each numbered primary (P) melanoma. Transcripts were quantitated after amplification by RT-PCR, electrophoresis, and hybridization with the prTyr probe. (Left) Absolute levels of the full-length tyrosinase mRNA relative to total RNA. Results are means and SD for two to five separate experiments. (Right) Levels of individual alternative mRNAs relative to the full-length mRNA in the same tumors. The most abundant alternative isoforms in the melanotic and amelanotic zones of each primary melanoma are represented by a dark and a light bar, respectively. (The amelanotic component of 676P lacked tyrosinase expression.)
Northern blots prepared from the same panel of tumors showed little variation in 18S RNA levels. Actin, the 16S ribosomal protein gene, and the T-antigen gene were distinctly more highly expressed in the amelanotic zones—along with tyrosinase in tyrosinase-positive cases; glyceraldehyde-3-phosphate dehydrogenase was only slightly elevated in these zones (data not shown). The results are consistent with greater mitotic activity and with malignant progression in the amelanotic tumor components.
Differences in Levels of the Full-Length Tyrosinase Transcript in Amelanotic Primary Melanomas and Their Amelanotic Metastases.
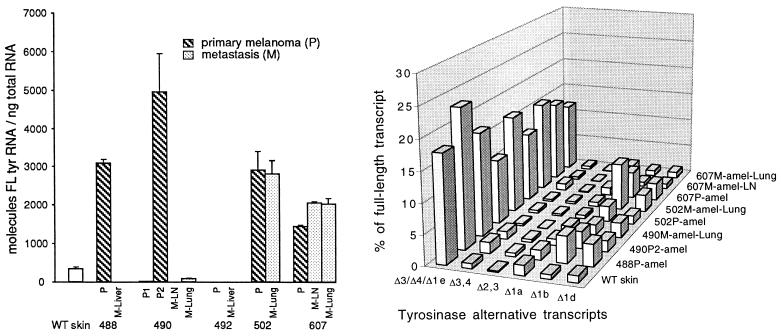
Levels of tyrosinase full-length mRNAs were extremely varied among six amelanotic (nonzonal) primary melanomas (including two, 490P1 and 490P2, which were found in the same skin graft) and seven amelanotic metastases present in the same animals (Fig. 2 Left). Similar variability was detected among the following: an amelanotic and a mottled primary melanoma whose metastases were not analyzed and three samples of lymph node metastases (two melanotic and one amelanotic), whose largely amelanotic primary tumor was not analyzed (data not shown).
Figure 2.
Tyrosinase mRNA expression in amelanotic cutaneous melanomas and their amelanotic metastases, and in wild-type (WT) C57BL/6 skin. The numbered primary (P) melanomas, shown with their metastases (M) in lymph node (LN), lung, and liver, were analyzed as in Fig. 1. (Left) Absolute levels of the full-length tyrosinase mRNA relative to total RNA. Results are means and SD for two to five separate experiments. (Right) Levels of alternative mRNAs relative to the full-length mRNA in the same tumors. The most abundant alternative transcripts are represented by the bars, with the Δ3, Δ4, and Δ1e transcripts combined here. [The following tumors with minimal tyrosinase expression or none, as shown in the histogram (Left), are omitted in the diagram (Right): 488M-Liver, 490P1, 490M-LN, 492P, and 492M-Liver.]
Transcript levels in some metastases differed greatly from those of the corresponding cutaneous tumors, e.g., in 488M as compared with 488P. Two primary tumors (490P1 and 492P) and four metastases (two in liver, one in lung, and one in lymph node) expressed only minimal tyrosinase full-length mRNA, or none. The lymph node metastasis in case 490 may have originated from the P1 primary tumor, from a very minor population of low-tyrosinase cells in the P2 primary tumor, or from high-tyrosinase cells in P2, which then lost tyrosinase transcription or RNA processing in their new site. Remetastasis from existing metastases, combined with progression, may have further complicated the picture.
Increases of Δ1b and Δ1d Alternative Splice Variants of Tyrosinase in Zonal Primary Melanomas.
The normal repertoire of alternatively spliced mRNAs of murine tyrosinase was first characterized; at least 19 splice variants were identified, and their abundances determined, in wild-type C57BL/6 mouse skin (S.R.K., N.L.F., and B.M., unpublished data). Deletion of exon 3 (Δ3) was the most frequent type of alternative splicing. The level of the Δ3 transcript in skin in this study (51.4 ± 4.3 molecules per ng of total cellular RNA) was 16.7% that of the full-length transcript (which was 306.8 ± 3.5 molecules per ng of RNA). Next in abundance was Δ1e, one of several splice variants resulting from a deletion within exon 1; this was present at 4.5 ± 0.6 molecules per ng of RNA or 1.5% of the full-length mRNA level.
In comparison with normal skin, virtually all samples from the zonal primary melanomas showed a specific increase of the Δ1b and Δ1d alternative splice variants, the exceptions being those amelanotic samples (676P-amel and 159P-amel) in which no transcripts of tyrosinase were detected (Fig. 1 Left and Table 1). The increase in the tumors was generally greater for Δ1b than for Δ1d. The level of Δ1b rose as high as 11.3% of total tyrosinase mRNAs as compared with only 0.6% in skin; Δ1d rose up to 4.0% as compared with 0.8% in skin. Each of these splice variants was more highly expressed in the melanotic than in the amelanotic zone of the tumor, except for Δ1d in tumor 497P. Transcript profiles for the most abundant splice variants are shown as percentages of the full-length transcript in the “cityscape” in Fig. 1 (Right), for the same tumors as analyzed in the histogram in Fig. 1 (Left). The Δ1b and Δ1d mRNAs represent deletions within exon 1 that are due, respectively, to usage of a splice donor site at nucleotides 291 and 369, in conjunction with shared usage of the splice acceptor site at the 5′ end of exon 2 (nucleotide 881 in the cDNA sequence). Increased levels of these splice variants may result from a melanoma-specific change augmenting the choice of their common splice acceptor site, inasmuch as levels of the Δ1a and Δ1e transcripts, which share only one or the other splice donor site, did not increase in the tumors.
Table 1.
Alternative transcripts as percentages of total tyrosinase transcripts in melanotic vs. amelanotic zones of primary (P) zonal melanomas
| Sample* | Principal alternative mRNAs† | Δ3 | Δ1b | Δ1d |
|---|---|---|---|---|
| WT skin | 15.5 | 11.7 | 0.6 | 0.8 |
| 363P-mel | 29.4 | 16.3 | 4.6 | 4.0 |
| -amel | 33.7 | 23.8 | 2.0 | 2.3 |
| 372P-mel | 24.4 | 10.9 | 6.8 | 3.2 |
| -amel | 18.8 | 11.8 | 2.4 | 1.3 |
| 493P-mel | 23.8 | 18.0 | 1.2 | 1.7 |
| -amel | 19.8 | 15.9 | 0.8 | 0.9 |
| 497P-mel | 32.9 | 15.0 | 11.3 | 2.2 |
| -amel | 28.9 | 14.6 | 7.6 | 2.8 |
| 676P-mel | 18.8 | 10.1 | 2.5 | 1.8 |
| -amel | — | — | — | — |
| 155P-mel | 20.8 | 12.3 | 3.0 | 2.0 |
| -amel | 19.4 | 14.8 | 0.7 | 1.4 |
| 159P-mel | 16.7 | 12.8 | 1.0 | 1.0 |
| -amel | — | — | — | — |
| 222P-mel | 25.5 | 17.6 | 2.1 | 1.6 |
| -amel | 33.7 | 27.8 | 0.7 | 1.3 |
WT, wild type.
155P, 159P, and 222P were transplanted from cutaneous zonal melanomas.
In this group are most of the relatively abundant alternative transcripts detected by the prTyr probe, including Δ3, Δ1b, and Δ1d, and excluding transcripts of low abundance (<0.1 molecule per ng of RNA). —, Lack of detectable transcripts.
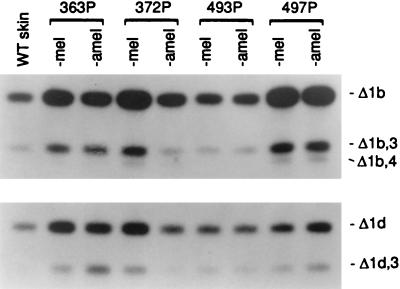
To determine the levels of alternative splice variants in relation to levels of the full-length mRNA, the PCR cycle number for each RT-PCR sample was chosen so as to load equal amounts of the full-length mRNA for Southern blot analysis. Specific hybridization probes were then used and the bands were quantitated. In the examples in Fig. 3, the Southern blots were hybridized with probes designated prΔ1b or prΔ1d to reveal the Δ1b and Δ1d transcripts and their codeletions with other exons. All the transcripts in the Δ1b group would have the same open reading frame and would therefore yield the same target peptide; the same is true for the various transcripts in the Δ1d group. Analyses with other specific probes revealed increases in the Δ3 splice variant in two-thirds of the tumor samples, and a slight rise in Δ3,4 transcripts, as shown in Fig. 1 (Right). The latter may signify that an exon prone to deletion, as in the case of exon 3, can enhance deletion of an adjacent exon, resulting in some double-deletion transcripts of low abundance.
Figure 3.
Analysis of Δ1b and Δ1d sets of tyrosinase alternative transcripts in four of the zonal primary melanomas shown in Fig. 1 Right. To determine alternative transcript levels relative to the full-length transcript, the PCR cycle number for each sample was chosen so that an equal quantity of full-length tyrosinase mRNA was loaded. Southern blots were hybridized with probe prΔ1b (Upper) or prΔ1d (Lower), which are, respectively, specific for the set characterized by the Δ1b or Δ1d deletion in exon 1.
Increases in specific alternative splicing modes in the tumors occurred at the expense of constitutive splicing of the tyrosinase pre-mRNA. The combined principal alternative mRNAs now constituted up to 33.7% of the total tyrosinase transcripts in the tumors as compared with 15.5% in skin (Table 1). The predicted Δ1b and Δ1d proteins would lack one or both of the copper-binding sites required for tyrosinase enzyme activity (31). They would also lack the transmembrane domain and cytoplasmic tail (32); hence they might fail to traffic to the melanosomes—the organelles in which melanin is produced.
Relative Decline in Δ1b and Δ1d Alternative Splice Variants of Tyrosinase in Amelanotic Primary Melanomas and Their Amelanotic Metastases.
While levels of the Δ1b and Δ1d alternative transcripts in comparison with the full-length transcript were also higher in these amelanotic tumors than in skin (Fig. 2 Right), the increases were less marked than in the zonal primary melanomas. In only five cases did the combined major alternative transcripts constitute higher percentages of total tyrosinase transcripts than in skin (Table 2). Furthermore, a larger number of cases lacked any detectable tyrosinase transcripts. The Δ3, Δ4, and Δ1e transcripts, which comigrate, were separated through the use of restriction enzymes (data not shown); they did not vary significantly from each other and were plotted together in this cityscape and were combined in Table 2.
Table 2.
Alternative transcripts as percentages of total tyrosinase transcripts in amelanotic primary (P) melanomas and their amelanotic metastases (M)
| Sample | Principal alternative mRNAs* | Δ3/Δ4/Δ1e | Δ1b | Δ1d |
|---|---|---|---|---|
| WT skin | 15.5 | 11.7 | 0.6 | 0.8 |
| 488P | 26.9 | 16.9 | 3.2 | 2.7 |
| M-liver | — | — | — | — |
| 490P1 | — | — | — | — |
| P2 | 20.8 | 13.7 | 2.2 | 1.4 |
| M-LN | — | — | — | — |
| M-Lung | 14.6 | 9.2 | 1.4 | 2.1 |
| 492P | — | — | — | — |
| M-Liver | — | — | — | — |
| 502P | 18.7 | 13.3 | 2.1 | 1.2 |
| M-Lung | 20.2 | 9.3 | 6.3 | 2.3 |
| 607P | 21.5 | 12.1 | 3.5 | 2.3 |
| M-LN | 13.9 | 10.1 | 0.8 | 0.9 |
| M-Lung | 15.0 | 11.7 | 0.6 | 0.7 |
WT, wild type; LN, lymph node.
In this group are most of the relatively abundant alternative transcripts detected by the prTyr probe, including the Δ3/Δ4/Δ1e comigrating transcripts, Δ1b, and Δ1d, and excluding transcripts of low abundance (<0.1 molecule per ng of RNA). —, Lack of detectable transcripts.
DISCUSSION
All the tyrosinase mRNAs found in transgenic mouse melanomas were previously found in normal mouse skin (S.R.K., N.L.F., and B.M., unpublished data). None are mutant isoforms; only their relative abundances have changed. Therefore, any immunotherapy protocol directed against peptides derived from overrepresented splice variants would rely on products of an apparently nonmutated gene present in all the tumor cells, rather than on products of a mutant gene subject to the caprices of ongoing mutation.
The increased expression of specific tyrosinase mRNA isoforms in the melanomas is a concomitant, rather than a cause, of malignant progression and it follows an orderly course. The uniform genetic background of all the transgenic melanomas allows this course to be traced. In relatively early stages of malignancy, when the cutaneous tumors are entirely or partly melanotic, there is in the melanotic regions an increase in levels of Δ1b and, to a lesser extent, of Δ1d transcripts in comparison with their levels in normal skin melanocytes (Figs. 1 and 3, Table 1). Whereas the melanotic tumor cells may themselves be invasive and metastasis-competent, cells in the amelanotic phases are increasingly mitotically active and metastatic (data not shown). When amelanotic zones evolve in the primary tumors, the levels of Δ1b and Δ1d mRNAs in those zones are still above the levels in skin but have begun to decline. The decline continues as the primary tumors become more uniformly amelanotic and is ongoing in their metastases (Fig. 2, Table 2). Coincident with these changes, small but increasing numbers of tumor samples fail to express any tyrosinase transcripts. The cells of such tumors or parts of tumors would be impervious to treatment relying on tyrosinase-derived peptides. Thus, a window of opportunity for treatment with peptide–antigen vaccines based on Δ1b and Δ1d products may be present chiefly in the early period of malignancy. To test this possibility, the peptides can be synthesized and subjected to various assays, such as binding to Class I major histocompatibility proteins of the C57BL/6 types (33), and recognition by cytotoxic T-lymphocytes, and then administered in adjuvant vaccines at different stages of melanoma progression.
These results have several implications for other aspects of melanoma treatment and for detection of metastases. If there were sufficient expression and presentation of the target peptides in normal melanocytes, an autoimmune response (34) in treated individuals might cause some blanched skin patches (vitiligo) due to melanocyte lysis (21, 35, 36). However, it is likely that the retinal pigment epithelium—upon which the health of the neural retina depends—would be spared. The latter conclusion is drawn from the fact that tyrosinase transcription is very low in postnatal mouse eyes (N.L.F. and B.M., unpublished data). Although RT-PCR analyses of pigment–gene mRNAs may also be useful to detect micrometastases, it now becomes evident that tyrosinase alone would be inadequate to reveal all melanoma cells in peripheral blood samples or in lymph node biopsies (Fig. 2, Table 2).
Target peptides, in addition to those from tyrosinase, would be needed for a treatment cocktail capable of encompassing all stages of melanoma cells; otherwise, tyrosinase-based treatment might favor survival of the most malignant cells. In another report, we noted that the mouse tyrosinase-related protein-2 gene, alone among four candidate pigment genes (tyrosinase, tyrosinase-related protein-1, tyrosinase-related protein-2, and silver), generated its protein in all 10 mouse melanoma samples tested, and might therefore usefully contribute to such a cocktail (30).
The expectation that splice variants of pigment genes could be a source of melanoma antigens was recently reinforced by the detection of a cytotoxic T-lymphocyte clone from a melanoma patient that recognized a peptide apparently originating from a splice variant of the human tyrosinase-related protein-1 gene (37). With the use of quantitative RT-PCR, as in this study, it becomes feasible to describe the exact transcript profiles of multi-exon pigment genes and to screen melanomas from genetically identical mice for increases in specific splice variants. The results may then expedite searches of human melanomas, whose genetic backgrounds are more varied, for similar changes due to the homologous genes.
Acknowledgments
S.R.K. is the recipient of a fellowship from the Fox Chase Cancer Center Board of Associates. W.K.S. is a Visiting Scientist from the University of Pennsylvania. This work was supported by an American Cancer Society Special Research Grant for Metastatic Melanoma (NP-917) to B.M. and by U.S. Public Health Services Grants CA-06927 and RR-05539 to the Fox Chase Cancer Center.
ABBREVIATION
- RT
reverse transcriptase
References
- 1.Mintz B. Harvey Lect. 1978;71:193–246. [PubMed] [Google Scholar]
- 2.Mintz B, Fleischman R A. Adv Cancer Res. 1981;34:211–278. doi: 10.1016/s0065-230x(08)60243-2. [DOI] [PubMed] [Google Scholar]
- 3.Houghton A N, Real F X, Davis L J, Cordon-Cardo C, Old L J. J Exp Med. 1987;164:812–829. doi: 10.1084/jem.165.3.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Houghton A N. J Exp Med. 1994;180:1–4. doi: 10.1084/jem.180.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGovern V J. Melanoma: Histological Diagnosis and Prognosis. New York: Raven; 1983. [Google Scholar]
- 6.Cooper D L, Dougherty G J. Nat Med. 1995;1:635–637. doi: 10.1038/nm0795-635. [DOI] [PubMed] [Google Scholar]
- 7.Manten-Horst E, Danen E H J, Smit L, Snoek M, Le Poole I C, Van Muijen G N P, Pals S T, Ruiter D J. Int J Cancer. 1995;64:182–188. doi: 10.1002/ijc.2910640307. [DOI] [PubMed] [Google Scholar]
- 8.Bradl M, Klein-Szanto A, Porter S, Mintz B. Proc Natl Acad Sci USA. 1991;88:164–168. doi: 10.1073/pnas.88.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mintz B, Silvers W K. Proc Natl Acad Sci USA. 1993;90:8817–8821. doi: 10.1073/pnas.90.19.8817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mintz B, Silvers W K, Klein-Szanto A J P. Proc Natl Acad Sci USA. 1993;90:8822–8826. doi: 10.1073/pnas.90.19.8822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark W H, Jr, Elder D E, Guerry D, IV, Epstein M N, Greene M H, Van Horn M. Hum Pathol. 1984;15:1147–1165. doi: 10.1016/s0046-8177(84)80310-x. [DOI] [PubMed] [Google Scholar]
- 12.Brichard V, Van Pel A, Wölfel T, Wölfel C, De Plaen E, Lethé B, Coulie P, Boon T. J Exp Med. 1993;178:489–495. doi: 10.1084/jem.178.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wölfel T, Van Pel A, Brichard V, Schneider J, Seliger B, Meyer zum Büschenfelde K-H, Boon T. Eur J Immunol. 1994;24:759–764. doi: 10.1002/eji.1830240340. [DOI] [PubMed] [Google Scholar]
- 14.Brichard V G, Herman J, Van Pel A, Wildmann C, Gaugler B, Wölfel T, Boon T, Lethé B. Eur J Immunol. 1996;26:224–230. doi: 10.1002/eji.1830260135. [DOI] [PubMed] [Google Scholar]
- 15.Wang R-F, Robbins P F, Kawakami Y, Kang X-Q, Rosenberg S A. J Exp Med. 1995;181:799–804. doi: 10.1084/jem.181.2.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang R-F, Appella E, Kawakami Y, Kang X, Rosenberg S A. J Exp Med. 1996;184:2207–2216. doi: 10.1084/jem.184.6.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cox A L, Skipper J, Chen Y, Henderson R A, Darrow T L, Shabanowitz J, Engelhard V H, Hunt D F, Slingluff C L., Jr Science. 1994;264:716–719. doi: 10.1126/science.7513441. [DOI] [PubMed] [Google Scholar]
- 18.Bakker A B H, Schreurs M W J, de Boer A J, Kawakami Y, Rosenberg S A, Adema G J, Figdor C G. J Exp Med. 1994;179:1005–1009. doi: 10.1084/jem.179.3.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bakker A B H, Schreurs M W J, Tafazzul G, de Boer A J, Kawakami Y, Adema G J, Figdor C G. Int J Cancer. 1995;62:97–102. doi: 10.1002/ijc.2910620118. [DOI] [PubMed] [Google Scholar]
- 20.Zarour H, De Smet C, Lehmann F, Marchand M, Lethé B, Romero P, Boon T, Renauld J-C. J Invest Dermatol. 1996;107:63–67. doi: 10.1111/1523-1747.ep12298177. [DOI] [PubMed] [Google Scholar]
- 21.Kawakami Y, Eliyahu S, Delgado C H, Robbins P F, Rivoltini L, Topalian S L, Rosenberg S A. Proc Natl Acad Sci USA. 1994;91:3515–3519. doi: 10.1073/pnas.91.9.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castelli C, Storkus W J, Maeurer M J, Martin D M, Huang E C, Pramanik B N, Nagabhushan T L, Parmiani G, Lotze M T. J Exp Med. 1995;181:363–368. doi: 10.1084/jem.181.1.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hearing V J, Jimenez M. Int J Biochem. 1987;19:1141–1147. doi: 10.1016/0020-711x(87)90095-4. [DOI] [PubMed] [Google Scholar]
- 24.Ruppert S, Müller G, Kwon B, Schütz G. EMBO J. 1988;7:2715–2722. doi: 10.1002/j.1460-2075.1988.tb03125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Porter S, Mintz B. Gene. 1991;97:277–282. doi: 10.1016/0378-1119(91)90063-h. [DOI] [PubMed] [Google Scholar]
- 26.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 27.Chou Q, Russell M, Birch D E, Raymond J, Bloch W. Nucleic Acids Res. 1992;20:1717–1723. doi: 10.1093/nar/20.7.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Church G M, Gilbert W. Proc Natl Acad Sci USA. 1984;81:1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Vries T J, Kitson J L, Silvers W K, Mintz B. Cancer Res. 1995;55:4681–4687. [PubMed] [Google Scholar]
- 30.Orlow S J, Hearing V J, Sakai C, Urabe K, Zhou B-K, Silvers W K, Mintz B. Proc Natl Acad Sci USA. 1995;92:10152–10156. doi: 10.1073/pnas.92.22.10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oetting W S, King R A. J Invest Dermatol. 1994;103:131S–136S. doi: 10.1111/1523-1747.ep12399447. [DOI] [PubMed] [Google Scholar]
- 32.Beermann F, Orlow S J, Boissy R E, Schmidt A, Boissy Y L, Lamoreux M L. Exp Eye Res. 1995;61:599–607. doi: 10.1016/s0014-4835(05)80053-3. [DOI] [PubMed] [Google Scholar]
- 33.Falk K, Rötzschke O, Stevanovic S, Jung G, Rammensee H G. Nature (London) 1991;351:290–296. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- 34.Tsomides T J, Eisen H N. Proc Natl Acad Sci USA. 1994;91:3487–3489. doi: 10.1073/pnas.91.9.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robbins P F, El-Gamil M, Kawakami Y, Rosenberg S A. Cancer Res. 1994;54:3124–3126. [PubMed] [Google Scholar]
- 36.Song Y-H, Connor E, Li Y, Zorovich B, Balducci P, Maclaren N. Lancet. 1994;344:1049–1052. doi: 10.1016/s0140-6736(94)91709-4. [DOI] [PubMed] [Google Scholar]
- 37.Wang R-F, Parkhurst M R, Kawakami Y, Robbins P F, Rosenberg S A. J Exp Med. 1996;183:1131–1140. doi: 10.1084/jem.183.3.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]