Abstract
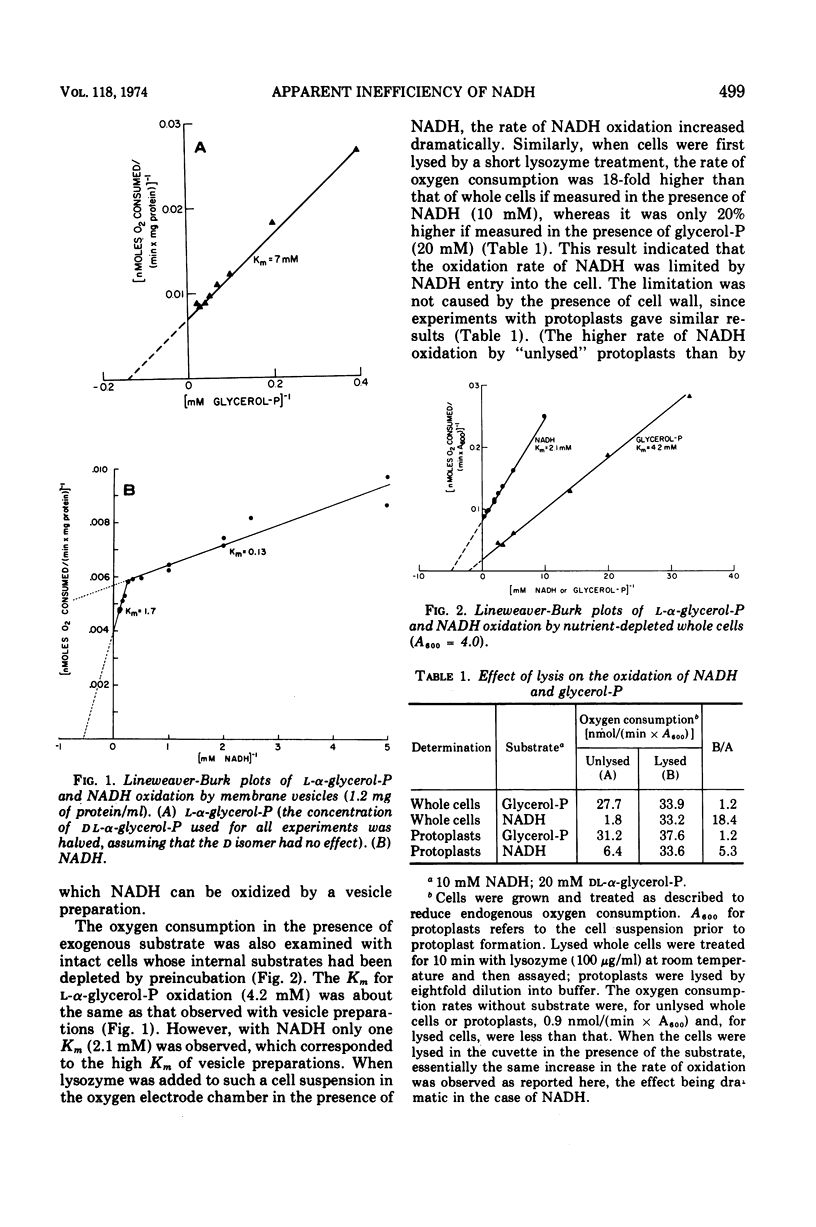
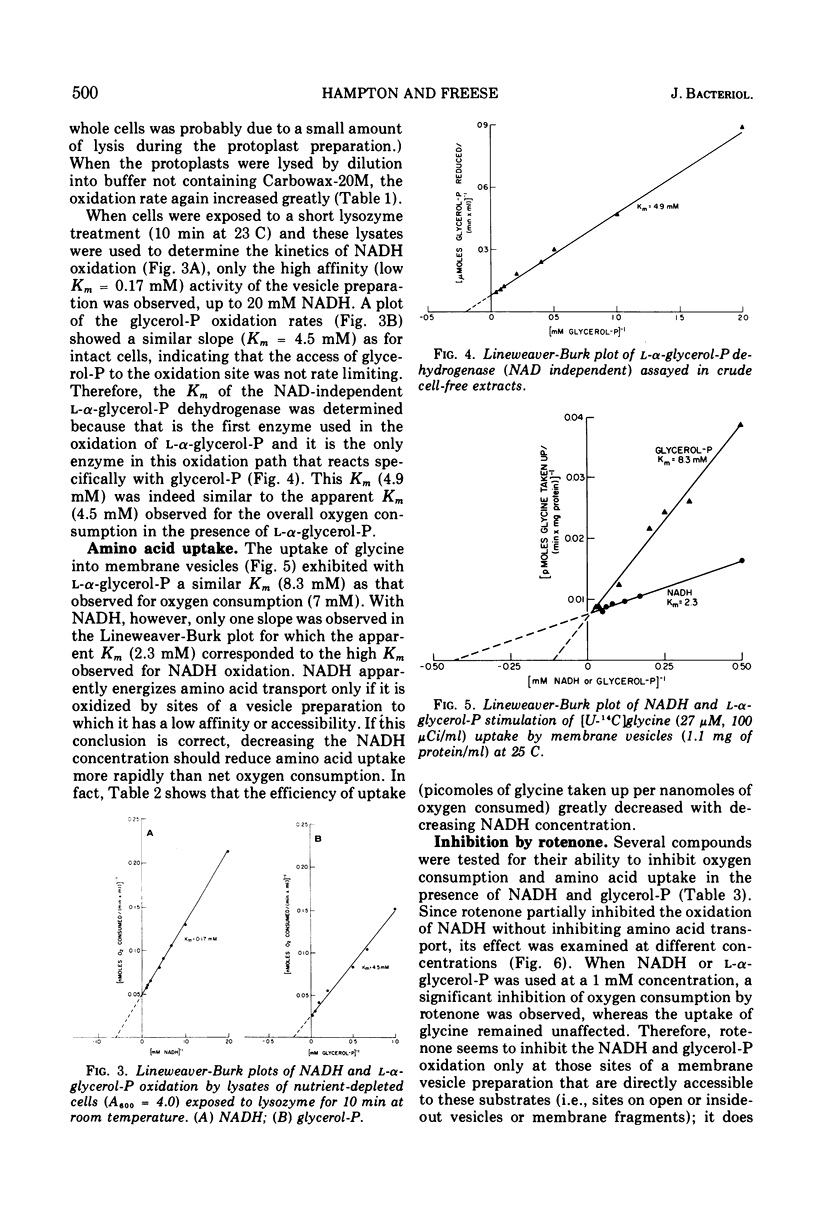
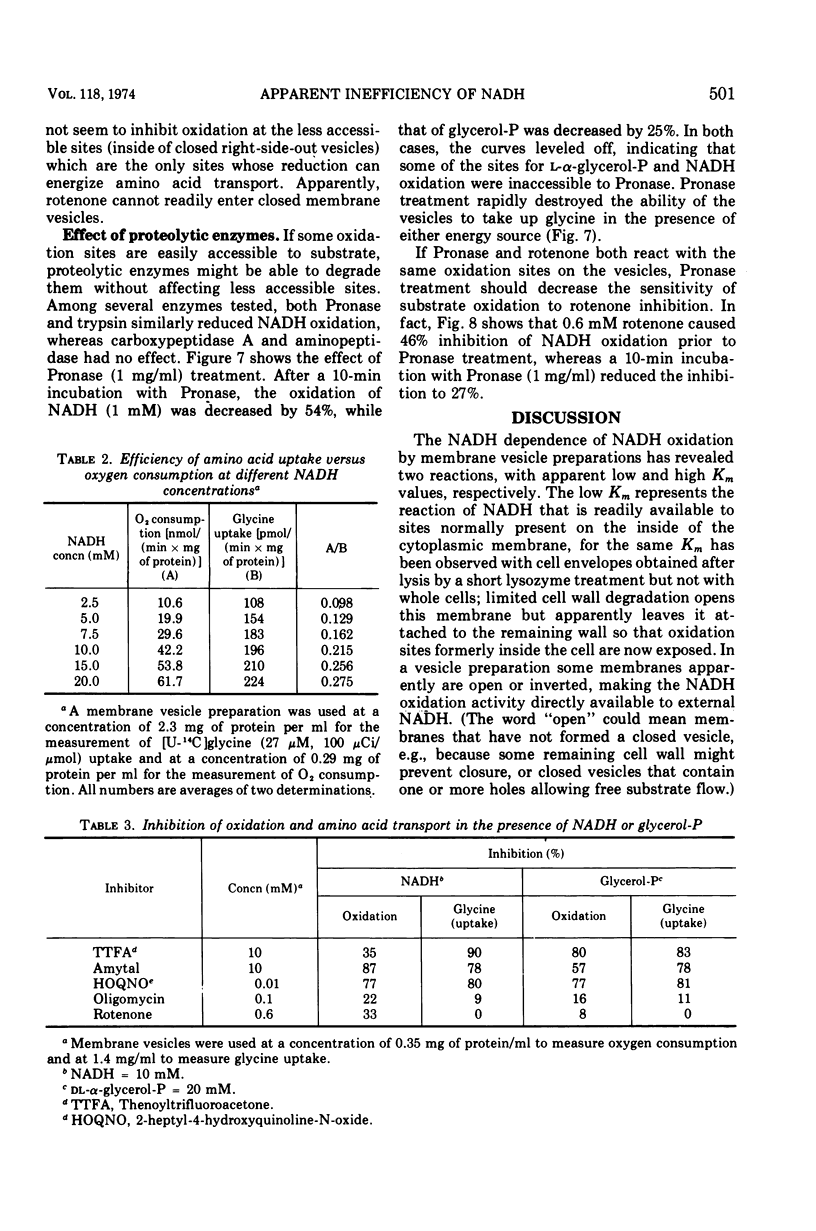
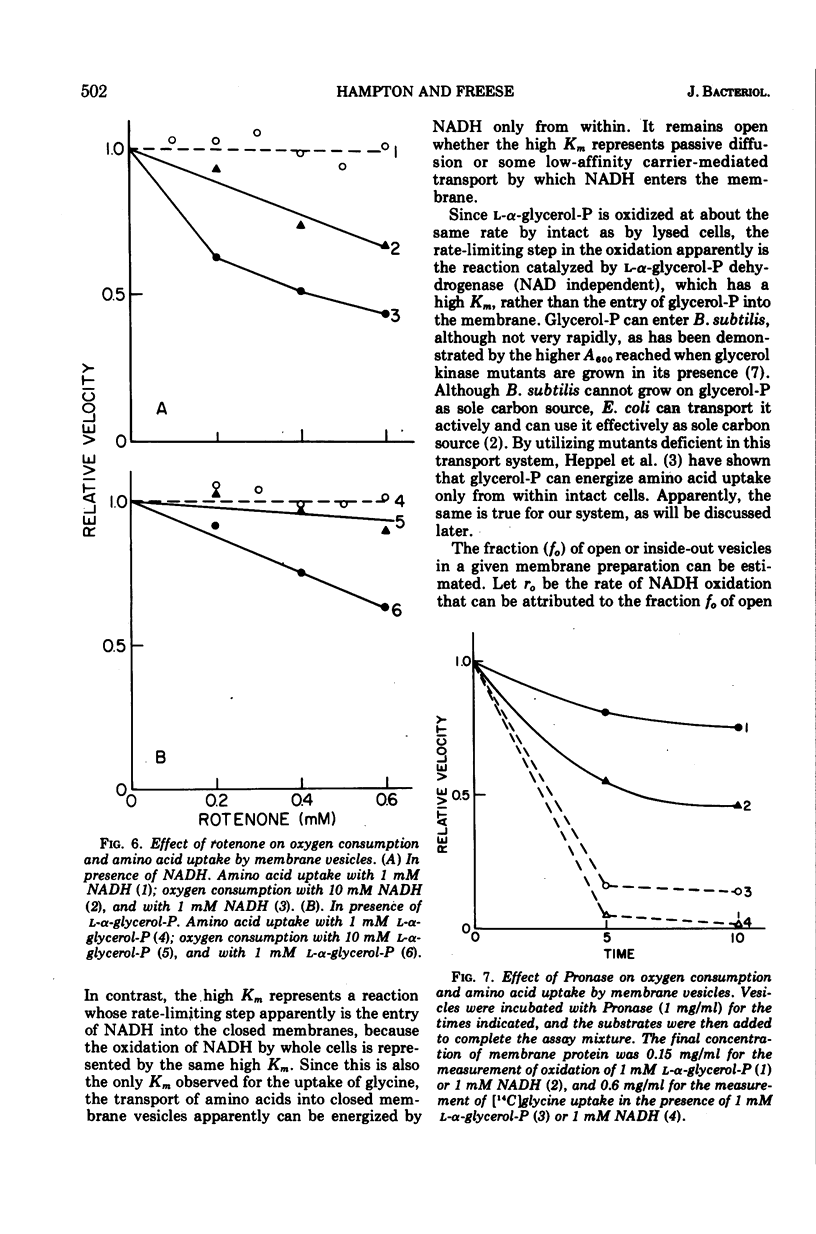
Lineweaver-Burk plots of reduced nicotinamide adenine dinucleotide (NADH) oxidation by membrane preparations from Bacillus subtilis are biphasic, with two Km values for NADH. The higher Km corresponds to the only Km observed for NADH oxidation by whole cells, whereas the lower Km corresponds to that observed with open cell envelopes. Membrane preparations apparently contain a small fraction of open or inverted vesicles which is responsible for the low Km reaction, whereas entry of NADH into the larger portion of closed, normally oriented vesicles is rate limiting and responsible for the high Km reaction. In contrast, the oxidation of l-α-glycerol-phosphate (glycerol-P) by membrane preparations shows only one Km that corresponds to that of glycerol-P oxidation by whole cells or lysates. Since glycerol-P dehydrogenase (NAD independent) has the same Km, this enzyme reaction rather than entry of glycerol-P into vesicles represents the rate-limiting step for glycerol-phosphate oxidation. The Km for amino acid uptake by vesicles in the presence of NADH corresponds to the high Km for NADH oxidation, indicating that NADH energizes transport only if it enters closed, normally oriented vesicles. Studies with rotenone and proteolytic enzymes support this interpretation. The apparent efficiency of NADH in energizing uptake seems to be lower than that of glycerol-P because, under the experimental conditions usually employed, open or inverted vesicles that do not participate in amino acid uptake are responsible for the major portion of NADH oxidation. When the results are corrected for this effect, the efficiency of NADH is essentially the same as that of l-α-glycerol-P.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes E. M., Jr, Kaback H. R. Mechanisms of active transport in isolated membrane vesicles. I. The site of energy coupling between D-lactic dehydrogenase and beta-galactoside transport in Escherichia coli membrane vesicles. J Biol Chem. 1971 Sep 10;246(17):5518–5522. [PubMed] [Google Scholar]
- HAYASHI S., KOCH J. P., LIN E. C. ACTIVE TRANSPORT OF L-ALPHA-GLYCEROPHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1964 Sep;239:3098–3105. [PubMed] [Google Scholar]
- Konings W. N., Freese E. Amino acid transport in membrane vesicles of Bacillus subtilis. J Biol Chem. 1972 Apr 25;247(8):2408–2418. [PubMed] [Google Scholar]
- LIN E. C., KOCH J. P., CHUSED T. M., JORGENSEN S. E. Utilization of L-alpha-glycerophosphate by Escherichia coli without hydrolysis. Proc Natl Acad Sci U S A. 1962 Dec 15;48:2145–2150. doi: 10.1073/pnas.48.12.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- O Y. K., Freese E. B., Freese E. Abnormal septation and inhibition of sporulation by accumulation of L- -glycerophosphate in Bacillus subtilis mutants. J Bacteriol. 1973 Feb;113(2):1034–1045. doi: 10.1128/jb.113.2.1034-1045.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]



