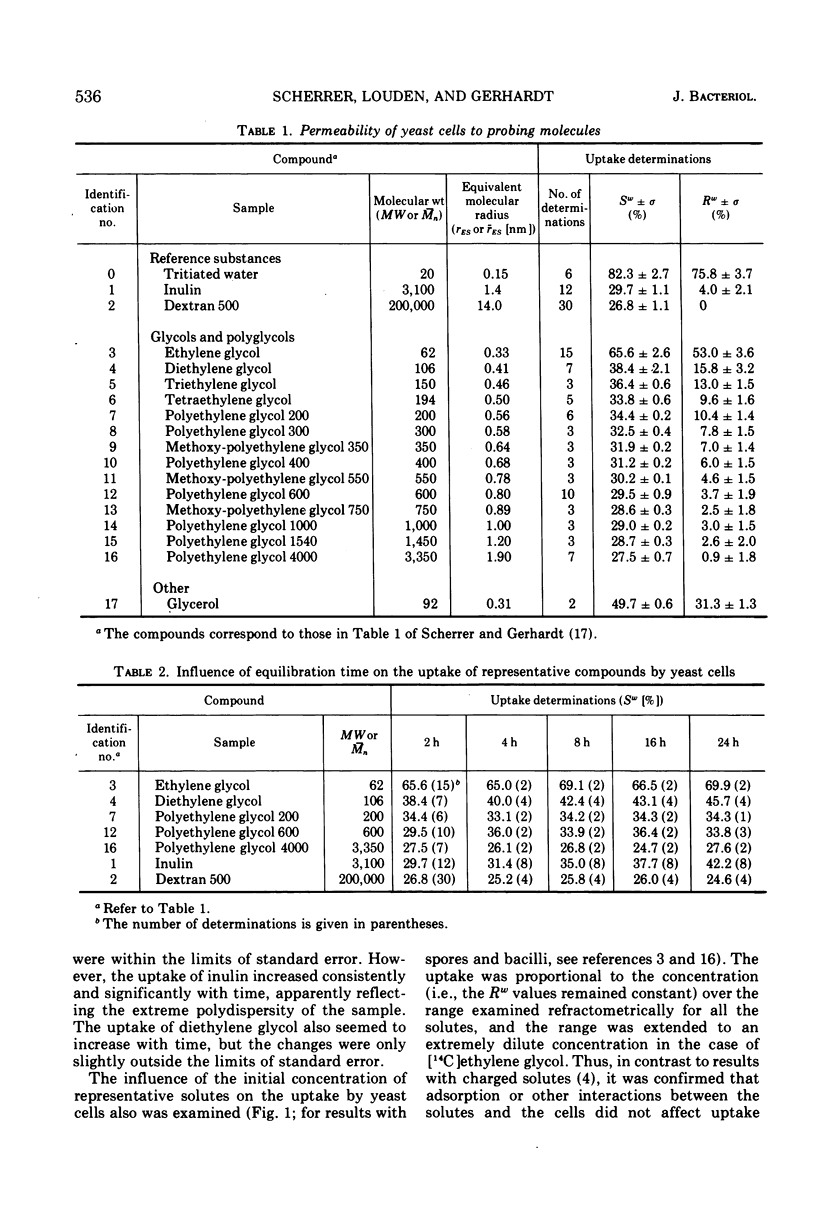
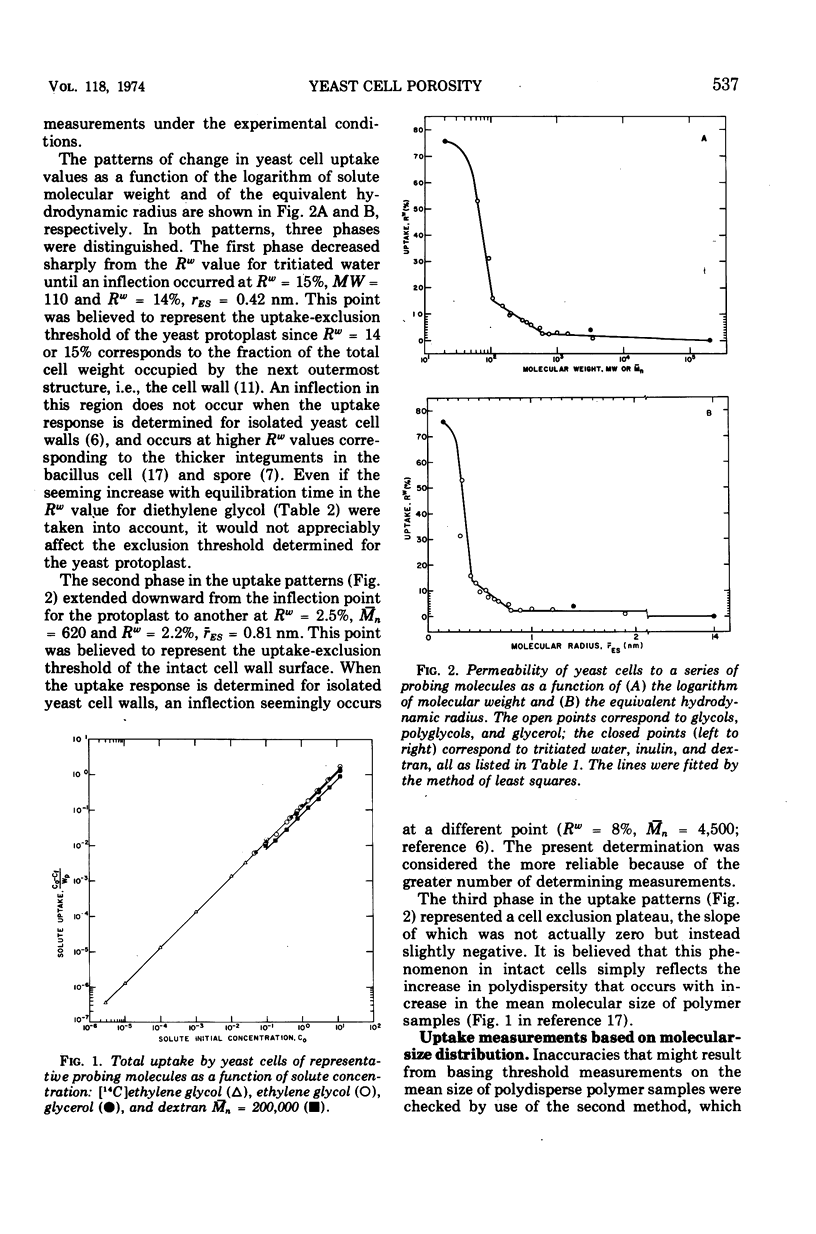
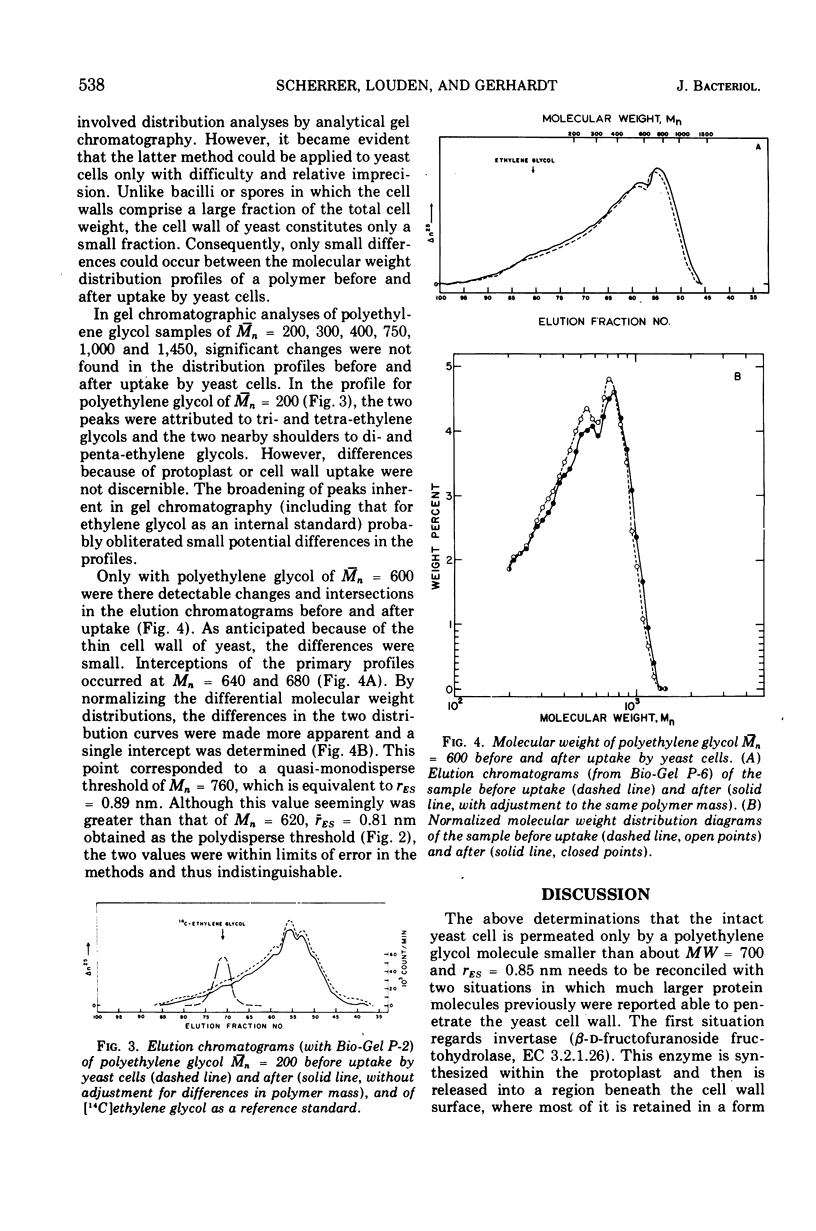
Abstract
The limiting sizes of molecules that can permeate the intact cell wall and protoplast membrane of Saccharomyces cerevisiae were determined from the inflection points in a triphasic pattern of passive equilibrium uptake values obtained with a series of inert probing molecules varying in molecular size. In the phase identified with the yeast protoplast, the uptake-exclusion threshold corresponded to a monodisperse ethylene glycol of molecular weight = 110 and Einstein-Stokes hydrodynamic radius (rES) = 0.42 nm. In the cell wall phase, the threshold corresponded to a polydisperse polyethylene glycol of number-average molecular weight (¯Mn) = 620 and average radius (rES) = 0.81 nm. The third phase corresponded to complete exclusion of larger molecules. The assessment of cell wall porosity was confirmed by use of a second method involving analytical gel chromatographic analyses of the molecular weight distribution for a single polydisperse polyglycol before and after uptake by the cells, which indicated a quasi-monodisperse threshold for the cell wall of Mn = 760 and rES = 0.89 nm. The results were reconciled with two situations in which much larger protein molecules previously have been reported able to penetrate the yeast cell wall.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold W. N. Location of acid phosphatase and -fructofuranosidase within yeast cell envelopes. J Bacteriol. 1972 Dec;112(3):1346–1352. doi: 10.1128/jb.112.3.1346-1352.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLACK S. H., GERHARDT P. Permeability of bacterial spores. I. Characterization of glucose uptake. J Bacteriol. 1961 Nov;82:743–749. doi: 10.1128/jb.82.5.743-749.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONWAY E. J., DOWNEY M. An outer metabolic region of the yeast cell. Biochem J. 1950 Sep;47(3):347–355. doi: 10.1042/bj0470347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERHARDT P., BLACK S. H. Permeability of bacterial spores. II. Molecular variables affecting solute permeation. J Bacteriol. 1961 Nov;82:750–760. doi: 10.1128/jb.82.5.750-760.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERHARDT P., JUDGE J. A. POROSITY OF ISOLATED CELL WALLS OF SACCHAROMYCES CEREVISIAE AND BACILLUS MEGATERIUM. J Bacteriol. 1964 Apr;87:945–951. doi: 10.1128/jb.87.4.945-951.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidby D. K., Davies R. Invertase and disulphide bridges in the yeast wall. J Gen Microbiol. 1970 Jun;61(3):327–333. doi: 10.1099/00221287-61-3-327. [DOI] [PubMed] [Google Scholar]
- Lampen J. O. External enzymes of yeast: their nature and formation. Antonie Van Leeuwenhoek. 1968;34(1):1–18. doi: 10.1007/BF02046409. [DOI] [PubMed] [Google Scholar]
- NORTHCOTE D. H., HORNE R. W. The chemical composition and structure of the yeast cell wall. Biochem J. 1952 May;51(2):232–236. doi: 10.1042/bj0510232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottolenghi P. The uptake of bovine serum albumin by a strain of Saccharomyces and its physiopathological consequences. C R Trav Lab Carlsberg. 1967;36(6):95–111. [PubMed] [Google Scholar]
- PHELPS C. F. THE PHYSICAL PROPERTIES OF INULIN SOLUTIONS. Biochem J. 1965 Apr;95:41–47. doi: 10.1042/bj0950041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PREISS J. W. The localization of invertase in the yeast cell with low voltage electrons. Arch Biochem Biophys. 1958 May;75(1):186–195. doi: 10.1016/0003-9861(58)90409-0. [DOI] [PubMed] [Google Scholar]
- SCHERRER R., GERHARDT P. MOLECULAR SIEVING BY CELL MEMBRANES OF BACILLUS MEGATERIUM. Nature. 1964 Nov 14;204:649–650. doi: 10.1038/204649a0. [DOI] [PubMed] [Google Scholar]
- SCHLENK F., DAINKO J. L. ACTION OF RIBONUCLEASE PREPARATIONS ON VIABLE YEAST CELLS AND SPHEROPLASTS. J Bacteriol. 1965 Feb;89:428–436. doi: 10.1128/jb.89.2.428-436.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer R., Cabrera Beaman T., Gerhardt P. Macromolecular sieving by the dormant spore of Bacillus cereus. J Bacteriol. 1971 Nov;108(2):868–873. doi: 10.1128/jb.108.2.868-873.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer R., Gerhardt P. Molecular sieving by the Bacillus megaterium cell wall and protoplast. J Bacteriol. 1971 Sep;107(3):718–735. doi: 10.1128/jb.107.3.718-735.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svihla G., Dainko J. L., Schlenk F. Ultraviolet micrography of penetration of exogenous cytochrome c into the yeast cell. J Bacteriol. 1969 Oct;100(1):498–504. doi: 10.1128/jb.100.1.498-504.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevithick J. R., Metzenberg R. L. Genetic alteration of pore size and other properties of the Neurospora cell wall. J Bacteriol. 1966 Oct;92(4):1016–1020. doi: 10.1128/jb.92.4.1016-1020.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yphantis D. A., Dainko J. L., Schlenk F. Effect of some proteins on the yeast cell membrane. J Bacteriol. 1967 Nov;94(5):1509–1515. doi: 10.1128/jb.94.5.1509-1515.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]



