Abstract
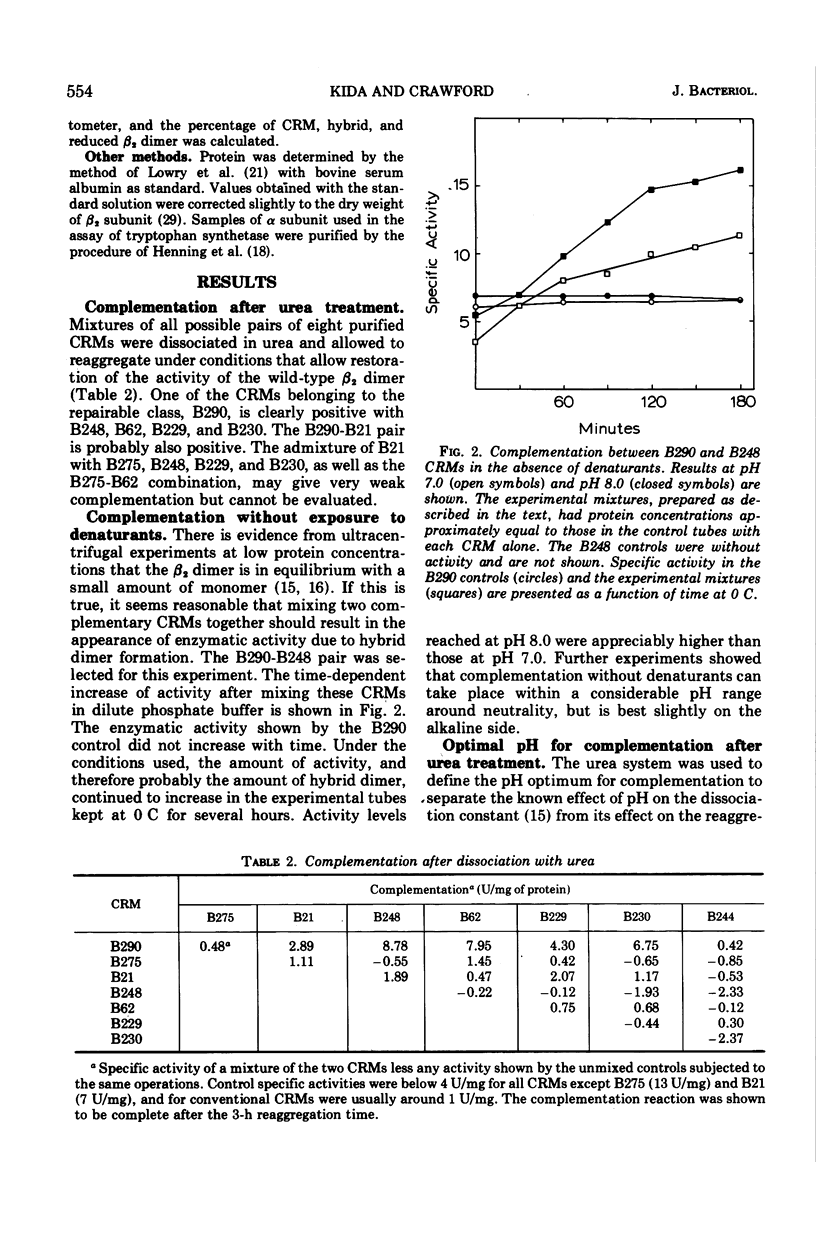
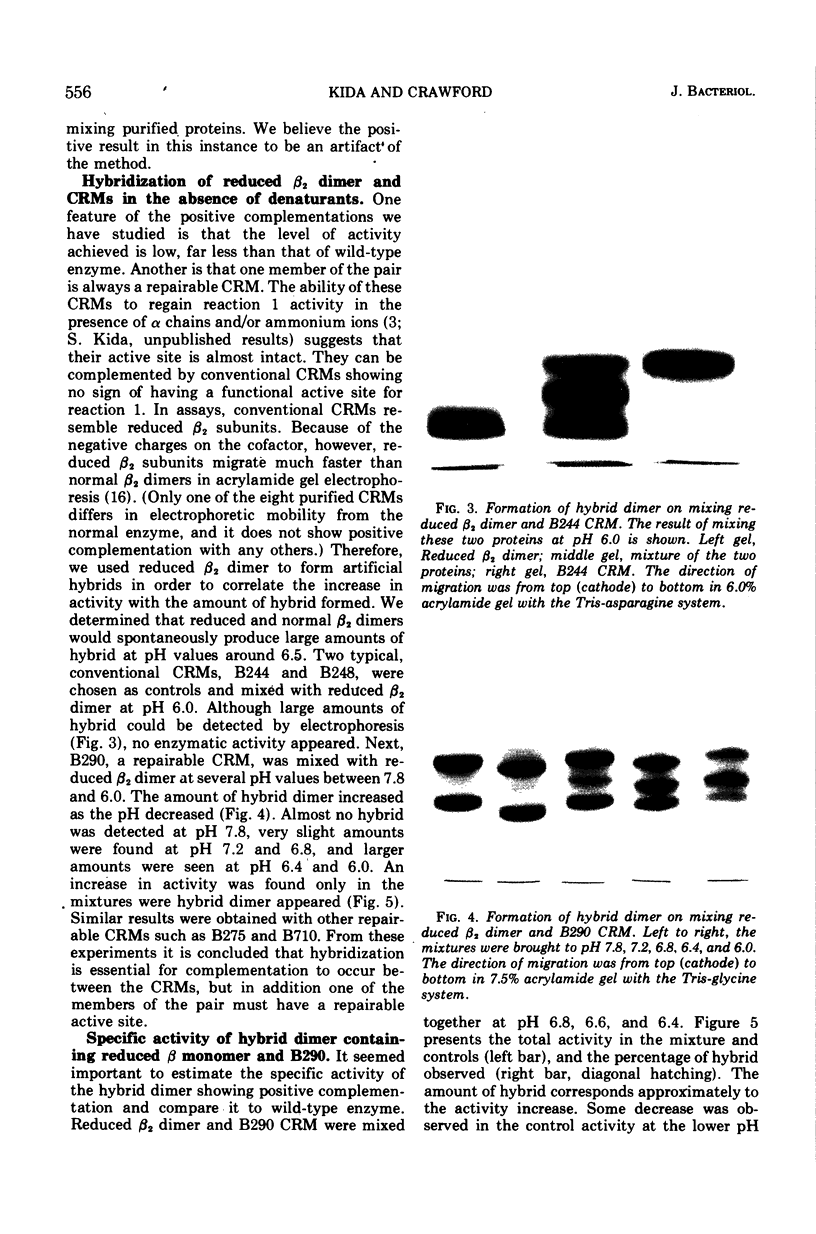
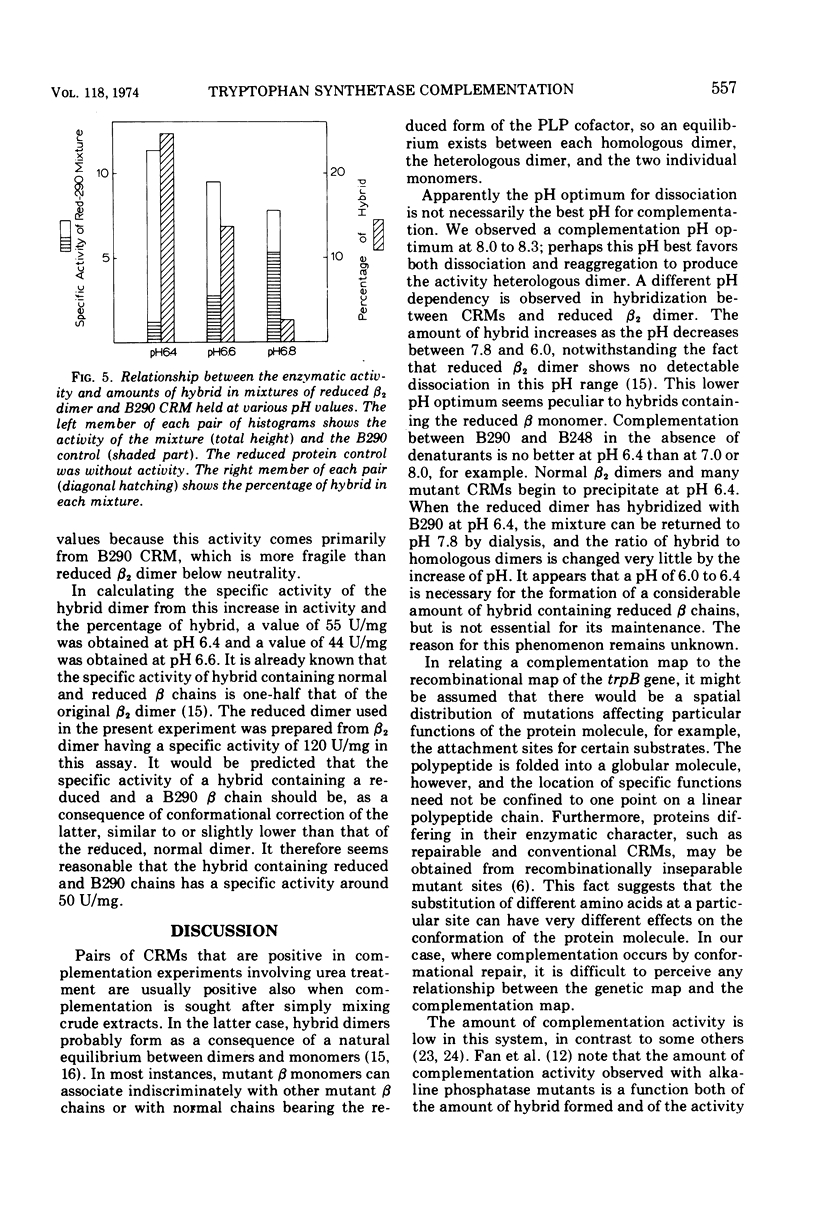
Cross-reacting β2 subunits (CRMs) were purified from eight trpB missense mutants to test for complementation in vitro after urea dissociation and reaggregation. One CRM (B290, demonstrating “repairability,” i.e., the appearance of enzymatic activity on combination with α subunits) was clearly positive with four others, all “non-repairable” CRMs resulting from mutations at three different but neighboring sites. One complementing pair, B290-B248, was studied in more detail and found, upon mixing purified proteins, to give complementation in the absence of denaturants. Complementation activity was low in each case. To study the mechanism of the modest increases in activity, we used a reduced β2 subunit as an artificial CRM to form hybrids where both the amount of activity due to complementation and the amount of hybrid could be measured. (In a reduced β2 subunit, the two pyridoxal phosphate cofactors have been chemically reduced by sodium borohydride and are covalently attached to lysine residues. This abolishes activity in the tryptophan synthetic reaction and causes the protein to migrate much faster than normal in acrylamide gel electrophoresis.) Reduced β2 subunit formed hybrid dimers with the non-repairable CRMs B244 and B248 at pH 6.0, but no enzymatic activity appeared. On the other hand, when reduced β2 subunit was mixed with B290 CRM at pH 6.0 to 6.6, an activity increase was seen that was proportional to the amount of hybrid. We conclude that hybrid formation is essential for complementation and that the mechanism of complementation in this system is the correction of a repairable active site on the B290 β chain by a conformational change occuring when hybrid dimer is formed. This type of complementation must be restricted to a small class of CRMs having a conformationally deformed active site. From the amount of hybrid present and the increase in activity, a specific activity of 50 U/mg was calculated for the hybrid containing reduced and B290 β chains. This value is slightly less than but close to the activity of the hybrid formed between reduced and normal β chains, shown earlier to have half the specific activity of the normal dimer.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apte B. N., Zipser D. In vivo splicing of protein: one continuous polypeptide from two independently functioning operons. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2969–2973. doi: 10.1073/pnas.70.10.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CODDINGTON A., FINCHAM J. R. PROOF OF HYBRID ENZYME FORMATION IN A CASE OF INTER-ALLELIC COMPLEMENTATION IN NEUROSPORA CRASSA. J Mol Biol. 1965 May;12:152–161. doi: 10.1016/s0022-2836(65)80289-3. [DOI] [PubMed] [Google Scholar]
- CRAWFORD I. P., JOHNSON L. M. Mutants of Escherichia coli defective in the B protein of tryptophan synthetase. I. Selection and classification. Genetics. 1963 May;48:725–736. doi: 10.1093/genetics/48.5.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRICK F. H., ORGEL L. E. THE THEORY OF INTER-ALLELIC COMPLEMENTATION. J Mol Biol. 1964 Jan;8:161–165. doi: 10.1016/s0022-2836(64)80156-x. [DOI] [PubMed] [Google Scholar]
- Crawford I. P., Ito J., Hatanaka M. Genetic and biochemical studies of enzymatically active subunits of E. coli tryptophan synthetase. Ann N Y Acad Sci. 1968 Jun 14;151(1):171–179. doi: 10.1111/j.1749-6632.1968.tb11887.x. [DOI] [PubMed] [Google Scholar]
- Crawford I. P., Sikes S., Belser N. O., Martinez L. Mutants of Escherichia coli defective in the B protein of tryptophan synthetase. 3. Intragenic clustering. Genetics. 1970 Jun;65(2):201–211. doi: 10.1093/genetics/65.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford I. P., Sikes S. Mutants of Escherichia coli defective in the B protein of tryptophan synthetase. IV. Recombination map. Genetics. 1970 Dec;66(4):607–616. doi: 10.1093/genetics/66.4.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford I. P., Yanofsky C. ON THE SEPARATION OF THE TRYPTOPHAN SYNTHETASE OF ESCHERICHIA COLI INTO TWO PROTEIN COMPONENTS. Proc Natl Acad Sci U S A. 1958 Dec 15;44(12):1161–1170. doi: 10.1073/pnas.44.12.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creighton T. E., Yanofsky C. Association of the alpha and beta-2 subunits of the tryptophan synthetase of Escherichia coli. J Biol Chem. 1966 Feb 25;241(4):980–990. [PubMed] [Google Scholar]
- FAHEY J. L., MCKELVEY E. M. QUANTITATIVE DETERMINATION OF SERUM IMMUNOGLOBULINS IN ANTIBODY-AGAR PLATES. J Immunol. 1965 Jan;94:84–90. [PubMed] [Google Scholar]
- Fan D. P., Schlesinger M. J., Torriani A., Barrett K. J., Levinthal C. Isolation and characterization of complementation products of Escherichia coli alkaline phosphatase. J Mol Biol. 1966 Jan;15(1):32–48. doi: 10.1016/s0022-2836(66)80207-3. [DOI] [PubMed] [Google Scholar]
- HATANAKA M., WHITE E. A., HORIBATA K., CRAWFORD I. P. A study of the catalytic properties of Escherichia coli tryptophan synthetase, a two-component enzyme. Arch Biochem Biophys. 1962 Jun;97:596–606. doi: 10.1016/0003-9861(62)90129-7. [DOI] [PubMed] [Google Scholar]
- HENNING U., HELINSKI D. R., CHAO F. C., YANOFSKY C. The A protein of the tryptophan synthetase of Escherichia coli. Purification, crystallization, and composition studies. J Biol Chem. 1962 May;237:1523–1530. [PubMed] [Google Scholar]
- Hathaway G. M., Crawford I. P. Studies on the association of beta-chain monomers of Escherichia coli tryptophan synthetase. Biochemistry. 1970 Apr 14;9(8):1801–1808. doi: 10.1021/bi00810a020. [DOI] [PubMed] [Google Scholar]
- Hathaway G. M., Kida S., Crawford I. P. Subunit structure of the B component of Escherichia coli tryptophan synthetase. Biochemistry. 1969 Mar;8(3):989–997. doi: 10.1021/bi00831a032. [DOI] [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- LOPER J. C. Enzyme complementation in mixed extracts of mutants from the Salmonella histidine B locus. Proc Natl Acad Sci U S A. 1961 Sep 15;47:1440–1450. doi: 10.1073/pnas.47.9.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Miles E. W., Hatanaka M., Crawford I. P. A new thiol-dependent transamination reaction catalyzed by the B protein of Escherichia coli tryptophan synthetase. Biochemistry. 1968 Aug;7(8):2742–2753. doi: 10.1021/bi00848a008. [DOI] [PubMed] [Google Scholar]
- SCHLESINGER M. J., LEVINTHAL C. Hybrid protein formation of E. coli alkaline phosphatase leading to in vitro complementation. J Mol Biol. 1963 Jul;7:1–12. doi: 10.1016/s0022-2836(63)80014-5. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Wilson D. A., Crawford I. P. Purification and properties of the B component of Escherichia coli tryptophan synthetase. J Biol Chem. 1965 Dec;240(12):4801–4808. [PubMed] [Google Scholar]
- Woodward D. O. ENZYME COMPLEMENTATION IN VITRO BETWEEN ADENYLOSUCCINASELESS MUTANTS OF NEUROSPORA CRASSA. Proc Natl Acad Sci U S A. 1959 Jun;45(6):846–850. doi: 10.1073/pnas.45.6.846. [DOI] [PMC free article] [PubMed] [Google Scholar]