Abstract
Leukocyte migration from a hemopoietic pool across marrow endothelium requires active pseudopod formation and adhesion. Leukocytes rarely show pseudopod formation while in circulation. At question then is the mechanism that serves to minimize leukocyte pseudopod formation in the circulation. We tested the hypothesis that fluid shear stress acts to prevent pseudopod formation. When individual human leukocytes (neutrophils, monocytes) spreading on glass surfaces in vitro were subjected to fluid shear stress (≈1 dyn/cm2), an instantaneous retraction of pseudopods was observed. Removal of the fluid shear stress in turn led to the return of pseudopod projection and cell spreading. When steady shear stress was prolonged over several minutes, leukocyte swelling occurs together with an enhanced random motion of cytoplasmic granules and a reduction of cytoplasmic stiffness. The response to shear stress could be suppressed by K+ channel blockers and chelation of external Ca2+. In rat mesentery microvessels after occlusion, circulating leukocytes project pseudopods in free suspension or when attached to the endothelium, even though immediately after occlusion only few pseudopods were present. When flow was restored, pseudopods on adhering leukocytes were retracted and then the cells began to roll and detach from the endothelium. In conclusion, plasma shear stress in the circulation serves to reduce pseudopod projection and adhesion of circulating leukocytes and vice versa reduction of shear stress leads to pseudopod projection and spreading of leukocytes on the endothelium.
Keywords: pseudopod, adhesion, ion channels, deformability, shear stress
Under physiological conditions, most circulating leukocytes in the blood stream exhibit low levels of pseudopod projection or expression of membrane molecules for adhesion to endothelium. Projection of pseudopods is associated with F-actin formation and cytoplasm stiffening (1). Leukocytes with pseudopods show enhanced resistance to flow in microvessels (2) to the point of complete capillary arrest (3, 4). In contrast, leukocytes require activation and projection of pseudopods (also referred to as lamellipods or microvilli) to migrate from their hemopoietic compartment of origin across marrow endothelium into the circulation (5). Migration across the peripheral vascular endothelium also requires active pseudopod formation and adhesion. Rheological studies of leukocytes under static conditions have yielded higher values for the cytoplasmic properties than under in vivo conditions, when they are subject to physiological shear stress prior to deformation (6). The evidence thus suggests that in the circulation a mechanism exists for shifting leukocytes from active pseudopod projection to a more passive state without pseudopods.
Endothelial cells (7, 8), erythrocytes (9), and platelets (10) are shear sensitive. Shear stress and shear stress gradients (11) serve to modulate endothelial cell shape as well as orientation of the cytoskeletal actin and tubulin network, membrane ion transport, endocytosis, and gene expression (12–15) and β2 integrin-mediated leukocyte adhesion (16). No investigations, however, have been reported on the response of individual leukocytes to shear stress.
Using a combination of in vitro experiments on isolated human cells and in vivo observations in the rat mesentery, we present evidence that fluid shear stress in the range encountered in the circulation induces a retraction of pseudopods in leukocytes and reduced adhesion to endothelium by way of a shear sensitive membrane ion exchange mechanism. We also provide evidence that reduction of the fluid shear stress in vivo leads to projection of pseudopods on leukocytes in vessels of the rat.
MATERIALS AND METHODS
Since circulating cells are subject to a shear stress during motion in blood vessels, our strategy was to interrupt the circulation and study pseudopod formation in vivo during periods of reduced shear. But since occlusion of the circulation is accompanied by variables other than shear (e.g., a shift in the tissue oxygen level), we also studied the mechanism of shear stress sensitive pseudopod projection under controlled in vitro conditions.
In Vivo Experiments.
Leukocyte pseudopod formation was observed in microvessels of the rat mesentery during local obstruction of flow with a micropipette, and by aspiration of venous blood samples into a fixative before and after a period of flow stasis at near zero shear stresses. This approach made it possible to investigate pseudopod formation in vivo without and with prior exposure to a shear stress encountered during normal blood flow. Anticoagulants were avoided since they may serve to modify pseudopod formation.
Animal Subjects.
All animal procedures were approved by the University of California, San Diego, Animal Subjects Committee. The femoral vein of mature male Wistar rats (300–350 g; Harlan–Sprague–Dawley) was cannulated under general anesthesia (sodium-pentobarbital, Abbott; 50 mg/kg, i.m.). Booster dosages (5 mg/kg) were administered as required. The rats were placed on a heating pad, covered with a blanket, and maintained at 37°C. The ileocecal portion of the mesentery was exteriorized and draped over a pedestal for intravital microscopy. The exposed tissue was superfused with a Krebs–Henseleit bicarbonate-buffered solution saturated with a 95% N2 and 5% CO2 mixture (36.5°C, pH 7.4). The mesentery was covered with a thin polyethylene foil (Saran Wrap, Dow) except for a small region (10 mm × 15 mm) over the observation site to minimize exposure to room temperature and atmospheric oxygen.
Intravital Microscopy.
The mesenteric microcirculation was visualized with an intravital microscope (Leitz) through a 40× water immersion objective (Zeiss, numerical aperture = 0.75) using a Halogen light source with heat filter. Images were recorded with a color CCD television camera (DEI-470, Optonics, Goleta, CA) and stored on a super VHS cassette recorder (model BR-5601MU; JVC, Tokyo) for playback analysis. Capillaries and postcapillary venules with diameters up to about 40 μm were used for analysis of pseudopod formation on leukocytes that were either free in the blood stream or attached to endothelium. Histological staining of the mesentery with toluidine blue showed that most leukocytes adhering to postcapillary endothelium were neutrophils with occasional monocytes (<3%) but no detectable lymphocytes, eosinophils, or basophils (data not shown).
To reduce temporarily the fluid shear stress in individual microvessels down to near zero, one of the outflow venules was occluded for selected periods of time with a micropipette mounted on a micromanipulator. Pseudopod formation was observed during occlusion of the blood flow. Adhesive leukocytes that remained in the observation field were also observed during restoration of flow.
To explore the number of pseudopods on leukocytes that had not adhered to the endothelium, a tourniquet cuff was placed around the rat hind leg. Whole blood was collected before and after occlusion of the circulation to the hind leg. Blood was drawn from a femoral artery catheter directly into 2% (wt/vol) glutaraldehyde to fix and arrest pseudopods within <5 sec, a time too short for projection or retraction of individual pseudopods (time constant 1 min; ref. 1). The fixed leukocytes were stained with crystal violet (0.02% in acetic acid) to identify segmented nuclei and 100 neutrophils were counted under light microscopy with a ×100 oil immersion objective. Projections on neutrophils were labeled as pseudopods when they were at least 1 μm (17).
In Vitro Experiments.
The purpose of the in vitro experiments was to investigate the different cell responses of isolated neutrophils to well-defined fluid stress conditions. The magnitude of the fluid stress was kept sufficiently low (≈1 dyn/cm2) to avoid significant viscoelastic cell deformation (18).
Cell Collection.
Fresh leukocytes from finger prick blood of volunteers were collected in capillary hematocrit tubes (ammonium heparin prefilled; Clay Adams). The red cells were allowed to sediment at room temperature for about 30 min. The supernatant mixture of platelets and leukocytes (1 part) was resuspended in (20 parts) Plasma-Lyte (Baxter Health Care, Mundelein, IL) with 1.5% BSA (A2153; Sigma) (pH 7.4, 310 mOsm). Neutrophils, monocytes, and lymphocytes were identified by their morphology (cell size, cytoplasmic granules, nuclear shape) at ×5,000 magnification (19).
Micropipette Set-Up.
About 0.5 ml of the cell suspension was deposited into a small chamber with a transparent bottom on an inverted microscope (Leitz Diavert) with ×50 objective (NA = 1.4, oil immersion) and ×20 objective (Leitz) calibrated with a micrometer (10 μm divisions, Leica). The microscope light source had a heat filter and all experiments were carried out at room temperature. The microscope eyepiece was connected to a closed circuit television system, with a black-and-white-coupled charge device camera (model JE2362; Javelin, Tokyo), analog background subtraction (model LKH 9000; L. K. Hawke, Research Triangle Park, NC), video timer (model G-77; Odetics, Anaheim, CA), VHS video cassette recorder (model AG 1270; Panasonic), and monitor (model CT-1388; Panasonic).
Micropipettes were fabricated using a micropipette puller (Flaming Brown micropipette puller, model P.80/PC; Sutter Instruments, Novato, CA) (internal diameter, 2–5 μm) and filled with the same plasma-lyte solution as was used for suspension of the leukocytes. The micropipettes were connected to a reservoir with hydrostatic pressure adjustment. Two set-ups were used, a single micropipette for leukocytes adhering to the glass surface, and a dual micropipette arrangement for nonadherent leukocytes.
Adherent leukocytes, which were spread on the glass surface, were identified and a single micropipette was positioned above the cell (Fig. 1A) so that a jet of fluid could be applied over their surface. The distance between micropipette tip and cell surface was determined by the micropipette angle and horizontal distance from the cell centroid (≈10 μm). The jet centerline velocity at the micropipette tip was determined for different reservoir pressures and internal micropipette tip diameters with a microsphere suspension on the basis of the velocity cross-correlation technique (20).
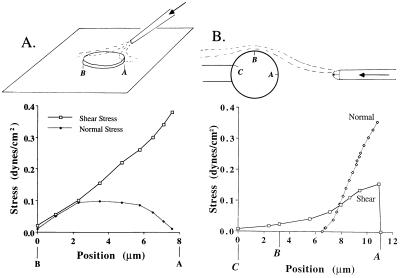
Figure 1.
Schematic of the in vitro experiments for (A) adhesive cells with a single ejecting micropipette, and (B) for nonadhesive cells with a dual micropipette setup. The bottom panels show the shear stress along a midline on the cell surface computed numerically as outlined in Materials and Methods. (A) The micropipette is inclined at 30° to the surface and the tip of the pipette is 5 μm from the center of the cell top surface. The cell diameter is assumed 8 μm, its thickness 2 μm, the internal pipette tip diameter 2 μm, and the centerline velocity of the fluid jet out of the pipette tip 0.74 mm/sec. (B) The pipette (tip diameter, 5 μm) on the left serves to hold the cell with a small aspiration pressure in the focal plane. The micropipette on the right serves to apply a fluid jet with centerline velocity of 1 cm/sec at an internal pipette diameter of 3.5 μm. The distance ΔL between the cell surface and the pipette tip was assumed to be ΔL = 7 μm and cell diameter 7 μm. The normal stress reaches low but non-zero levels at the radial position of ≈6.5 μm; its peak is at position A. In both cases, the stress distribution over the surface of the cell is a linear function of the center line velocity of the fluid jet from the ejecting micropipette, but nonlinear function of cell diameter and distance ΔL. Reduction of ΔL in case B from 7 μm to 5.6 μm (80%) causes a doubling of the shear stress to ≈0.83 dyn/cm2 on the cell surface.
Nonadherent leukocytes were gently aspirated into a holding micropipette and kept in focus (Fig. 1B Left). The opposite (ejecting) pipette was used to apply a fluid jet onto the cell surface. The magnitude of the fluid stress on the leukocyte was controlled by the distance between the cell surface and pipette tip (about 10 μm) as well as the velocity of the fluid jet out of the ejecting pipette (internal tip diameters, 2 and 3 μm).
Cell Volume.
The diameter of leukocytes while in their spherical state without pseudopods was estimated from digitized in vitro images using an intensity line scan after digitization of the images on a laboratory computer (Image 1.44 on Macintosh II Ci, Apple II+). The cell image was recorded at ×5,000 magnification with the microscope focused on the cell midplane at minimal thickness of the interference rings along its perimeter. The cell diameter was determined from the light intensity profile using the transition from the dark outer interference ring to its neighboring inner interference ring at the position of the cell surface. This point could readily be detected even in swollen leukocytes. The cell volume was computed from the diameter assuming a sphere.
Random Granule Motion.
Continuous application of a surface fluid stress leads to an increase in the random motion of neutrophil granules. To determine an average granule velocity, a video window (model 204A; IPM, Le Mesa, CA) was placed over the cytoplasmic granules at approximately two-thirds the radial distance from the cell center. The video window (0.3 μm × 0.3 μm) serves to generate an analog signal that is proportional to the average light intensity in the window and is shifted by the motion of individual granules moving within the window. The analog output was differentiated with respect to time and stored digitally on a laboratory computer (Mac-Lab, Macintosh SE 30, Apple II+). The average granule velocity was computed over ≈10 sec and estimated by placing the video window on the centerline of the micropipette tip while microspheres were ejected (diameter 0.4 μm) at known velocity (see above).
Leukocyte Deformability.
The deformability of leukocytes during application of the fluid stress was determined by means of the cell aspiration technique (18). Briefly, the holding pipette (Fig. 1B Left) was used to aspirate the cell locally under selected aspiration pressures, while the ejecting pipette (Fig. 1B Right) provided a fluid flow over the cell. The time course of leukocyte deformation into the aspiration pipette was determined during video replay.
Leukocyte Surface Stress Analysis.
Since the Reynolds number of the flow field around the leukocytes in both setups is less than 10−2, and since the diluted plasma solution is incompressible and has constant viscosity (μ = 1.2 cP; 1 P = 0.1 Pa⋅sec), the flow around the leukocyte can be determined by Stokes approximation of the equation of motion (21)
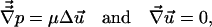 |
where p is the pressure and u⃗ is the velocity vector. To estimate the magnitude of the normal stress and shear stress on the leukocyte surface, the velocity field in both micropipette setups was computed with a finite element computation using commercial software (fidap; San Diego Supercomputer Center) subject to the following boundary conditions. The fluid on the glass surface, micropipette, and cell surface satisfied zero slip condition. The velocity profile out of the pipette tip is parabolic according to Poiseuille’s equation, and the pressure in the fluid field far away from the cell is constant (zero). The leukocyte was assumed to have a flat disc shape in the case of adherent cells and to be a sphere in the case of the dual micropipette setup while details of the cell surface shapes were neglected. The computation allowed to estimate the magnitude of the normal and shear stress on the surface of the cell for the two flow configurations (Fig. 1).
Statistics.
Results are presented as mean ± SD. Comparisons between groups was carried out by an unpaired Mann–Whitney U test and ANOVA. P < 0.05 was considered to be significant.
RESULTS
In Vivo Observations.
The majority of leukocytes in the active circulation had a spherical shape and showed no pseudopods detectable with light microscopy. Cessation of blood flow in a single rat mesentery microvessel led to projection of pseudopods (Fig. 2 A–D). Pseudopod projection continued to be exhibited as long as there was no flow in the vessel. Upon restoration of flow, leukocytes that were attached and spread out retracted their pseudopods within ≈1 min (Fig. 2D), then started to roll on the endothelium and eventually were carried out of the observation field. Leukocyte rolling was preceded by pseudopod retraction irrespective of the size or location of the vessel. Projection of pseudopods was encountered on leukocytes that were attached to the endothelium (Fig. 2) as well as on those suspended freely in the vessel lumen among red cells (Fig. 2 E–H). Individual leukocytes did project pseudopods at different rates (Fig. 3), and in some cases projections were not observed for 20 min or longer.
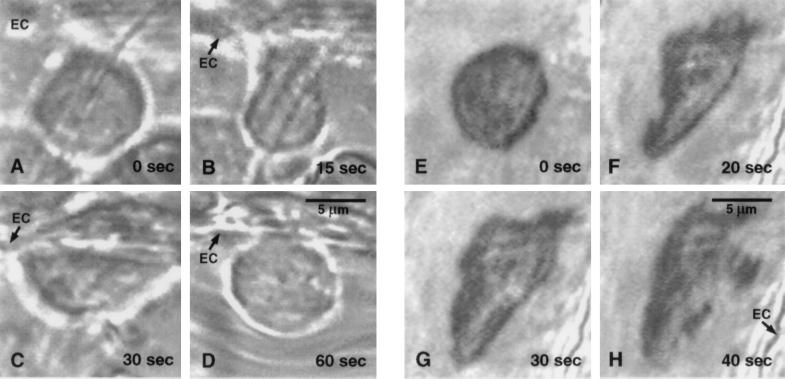
Figure 2.
Selected frames during the time course of pseudopod formation of a neutrophil in a rat mesentery venule. (A–D) Adhering neutrophil. (A) The leukocyte shape is spherical without pseudopods immediately after occlusion of the vessel with a micropipette; (B and C) leukocyte spreading on the endothelium (EC) by active pseudopod formation during stasis, (D) retraction of pseudopods upon restoration of flow. (E–H) Freely suspended neutrophil. (E) The cell is initially spherical. (F) Pseudopod projection shortly after flow stoppage, and continuation throughout stasis (G and H). The cell is carried away from the observation field upon return of flow.
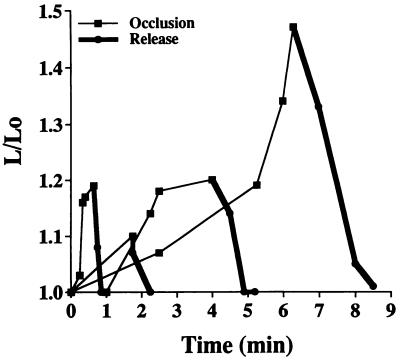
Figure 3.
Time course of pseudopod formation for selected neutrophils and monocytes in rat mesentery venules (20–40 μm in diameter) during occlusion (thin lines) and after return of flow (thick lines). L refers to the maximum length across the cell and Lo refers to the diameter of the cell in its spherical state without pseudopods. All leukocytes showed projecting pseudopods to spread on the endothelium (as shown in Fig. 2) and were rolling out of the observation field after retraction of the pseudopod (L/Lo = 1).
Pseudopod Projection after Phlebotomy.
In blood samples collected via the femoral artery, the percentage of neutrophils that exhibited pseudopods before cessation of blood flow was 6.3 ± 3.1% (mean ± SD, n = 4). After occlusion of blood flow and lowering of shear stress to near zero values for a period of 15 min, the fraction of neutrophils with pseudopods rose to 24.3 ± 5.3% (P < 0.01).
In Vitro Observations.
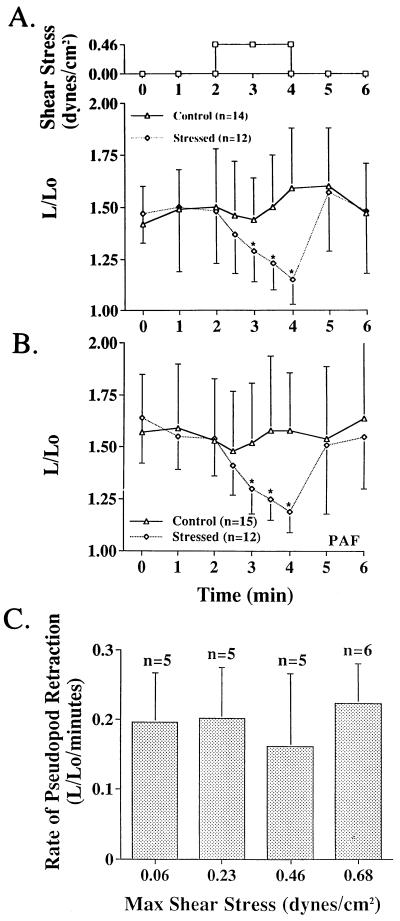
Application of a fluid stress (≈0.4 dyn/cm2 peak shear stress, Fig. 1A) to an adherent neutrophil caused immediate retraction of surface extensions (Fig. 4). Continuous application of fluid stress led to a complete rounding of the cell and detachment from the glass surface. The cell continued to retract its pseudopods while shear stress was applied (Fig. 4C). After allowing the shear stress to return to zero, pseudopods were again projected (Fig. 4D). This response to fluid shear was found for every cell tested and was reproducible (Fig. 5A). Retraction of pseudopods on adhesive cells was observed even in the presence of an activator for neutrophils. The rate of pseudopod retraction in the presence of 10−8 M platelet-activating factor (Fig. 5B) was comparable to the rate of retraction observed in plasma without activator (Fig. 5A). Enhancement of the estimated peak fluid stress on the cell surface (≈0.06–0.68 dyn/cm2) by raising the fluid jet velocity out of the micropipette did not affect the rate of pseudopod retraction (Fig. 5C).
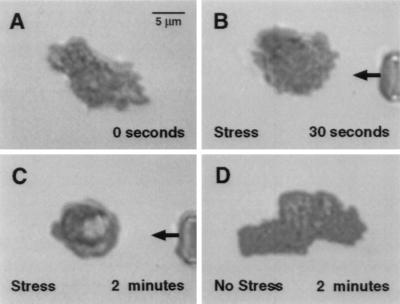
Figure 4.
Time course of cell spreading and pseudopod formation of a human neutrophil on an a glass slide (A) before, (B) 30 sec, and (C) 120 sec after application of a fluid jet by a micropipette (arrow) (Fig. 1A). The cell continues to retract its pseudopods during application of the fluid jet, but spreads immediately after removal of the pipette and return of fluid shear stress to near zero levels (D).
Figure 5.
(A) Time course of human neutrophil spreading before and after application of a fluid shear stress for a period of 2 min by means of the single micropipette setup (Fig. 1A). L represents the maximum length cross the cell and Lo the diameter of the undeformed spherical cell. Control is without application of shear stress. (B) Time course of human neutrophil spreading in the presence of platelet-activating factor before and after application of shear stress for 2 min by means of the single micropipette setup (Fig. 1A). (C) Rate of pseudopod retraction measured as the slope to the curve L/Lo in the first minute after application of shear stress (A) for different levels of shear stress. There are no significant differences between the retraction rates. ∗, P < 0.05 compared with control.
Pseudopods were also retracted when individual neutrophils with pseudopods were subjected to fluid flow using the dual micropipette setup for cells without attachment to a surface. The rate of pseudopod retraction in such unattached cells was faster than in cells that are attached to glass.
Leukocyte Volume and Granule Motion.
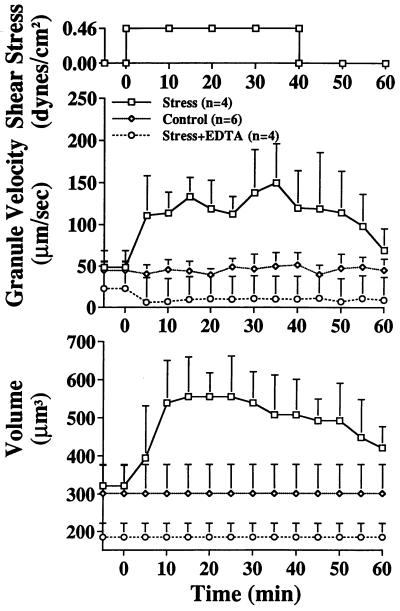
Continued application of fluid stress after retraction of pseudopods or application of fluid stress to a leukocyte that had no pseudopods led to a striking change in the cell interior. Several minutes after application of constant fluid shear stress (Fig. 1B), the random motion granule velocity in neutrophil cytoplasm became significantly enhanced (Fig. 6 Upper). The levels of granule velocity reached ≈three-fold higher than without shear stress, suggesting that the cell interior had been transformed into a less viscous cytoplasm. In control buffer, the enhancement of the granule velocity was accompanied by cell swelling without pseudopod formation (Fig. 6, Lower), to a point of ≈60% enlargement of the cell volume (Fig. 7). Upon withdrawal of the stress the cell volume recovered, coincident with a decrease in granule velocity.
Figure 6.
Random velocity of human neutrophil granules and cell volume before and after application of fluid shear stress (Upper) with the dual micropipette setup (Fig. 1B). The neutrophils are nonadherent and have a spherical shape without pseudopod projection. Control is without application of a shear stress. During application there is a significant swelling of the cell and a significant enhancement of Brownian granule motion. This response is blocked after chelation of Ca2+ with EDTA (40 mM).
Figure 7.
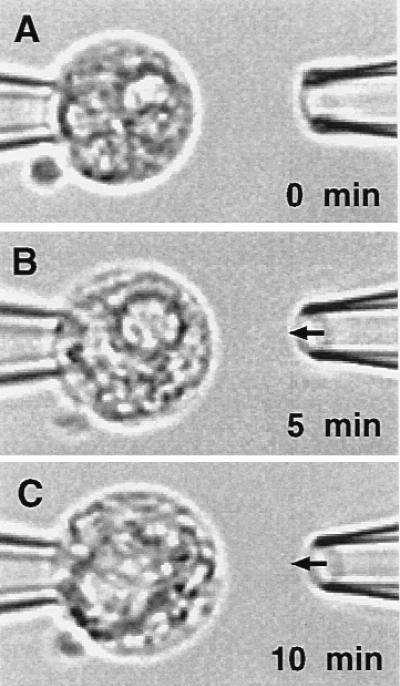
Light micrograph of a human neutrophil (A) before, (B) 5 min, and (C) 10 min after application of a fluid jet to its cell surface with dual micropipette setup (Fig. 1B). There is swelling of the cell and a review of the video tapes shows a vigorous random motion of the granules in C during shear, in contrast to a slow motion of the granules in unsheared cells (A).
Leukocyte Deformability.
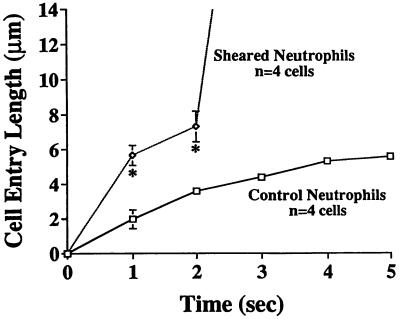
During cell swelling the cytoplasmic stiffness was significantly reduced. Application of a negative aspiration pressure within the holding micropipette (Fig. 1A) in the absence of a fluid shear stress led to a slow creeping cell deformation into the pipette (Fig. 8). When a fluid shear stress by fluid flow from the ejecting pipette was applied for 5 min, there was a rapid neutrophil deformation and full entry into the pipette with the same aspiration pressure.
Figure 8.
Cell surface entry length (mean ± SD) of human neutrophils into the tip of the holding pipette after aspiration (Fig. 1B, left pipette, internal tip diameter ≈3.5 μm) with a negative pressure of 5,000 dyn/cm2. Sheared neutrophils were exposed to a peak shear stress of 0.15 dyn/cm2 for a period of ≈2 min prior to the application of the aspiration pressure, while the unsheared cells were left in a quiescent medium before aspiration. The sheared cells were completely drawn into the mouth of the micropipette after aspiration, a phenomenon not observed in control cells. ∗, P < 0.05
Shear Induced Membrane Ion Transport.
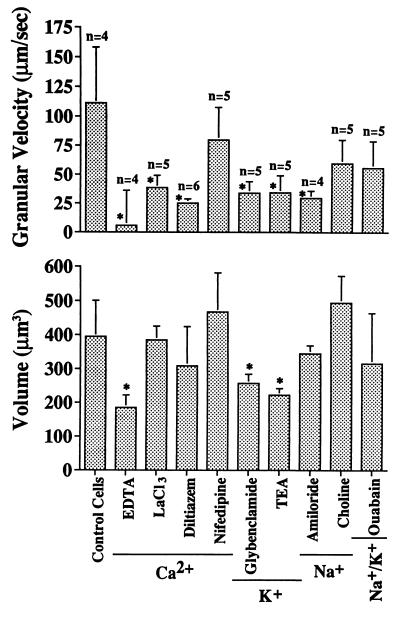
To explore a possible contribution of ion channels to the shear induced leukocyte response, Ca2+ was eliminated from the buffer and residues were chelated by EDTA (25 mM). Under these conditions very few leukocytes adhered to the glass surface or projected pseudopods. Therefore the effect of shear stress was studied with the dual pipette setup. Without external Ca2+, the enhancement of granule motion as well as cell swelling were not seen (Fig. 6). The rise of the granule velocity was blocked when calcium chloride was replaced by lanthanum chloride (20 μM) and with the Ca2+ channel blockers diltiazem (250 μM), but there was no significant effect on cell volume (Fig. 9). The Ca2+ channel blocker nifedipine (50 μM) had no effect on either granule velocity or cell volume during shear. The shear stress response was blocked with the K+ ion channel blockers glybenclamide (20 mM) and tetraethyl ammonium (100 mM). The Na+ channel blocker amiloride (10 μM) served to attenuate the change in granule velocity during shear without a significant effect on cell volume while replacement of Na+ with choline had no effect on either of the shear responses by the neutrophils. The Na+/K+ ATPase dependent membrane channel inhibitor ouabain had no significant influence on the shear response (Fig. 9).
Figure 9.
Granular random velocity and cell volume of human neutrophils upon exposure to 0.15 dyn/cm2 for 5 min with the dual micropipette setup (Fig. 1B). For concentrations of the blockers see Materials and Methods. ∗, P < 0.05 compared with control.
DISCUSSION
These experiments demonstrate that circulating leukocytes respond to fluid stresses on their membrane by retraction of cytoplasmic projections, a process that involves the breakdown of F-actin (22). The order of magnitude of the shear stress (1 dyn/cm2) that was required to elicit this response in vitro is comparable and often even lower than levels in the circulation. Leukocytes in the circulation are subject to a shear stress by the plasma that is further enhanced by red cell and endothelial interactions. High shear stresses may be encountered on the wall of arteries and arterioles as well as in the plasma lubrication layer between the leukocyte and endothelial membrane of single file capillaries (23). As leukocytes migrate into the venous sinuses of bone marrow circulation, they are subjected to fluid shear stress by the blood stream, which may serve to downregulate the cells as they enter the circulation. Interruption of blood flow and reduction of the shear stress to near zero values in vivo leads to projection of pseudopods on leukocytes (Fig. 2).
On the other hand, in postcapillary venules where the circulating leukocytes are displaced by the erythrocytes from the center stream toward the endothelium (24, 25), spreading with pseudopod formation may occur in spite of normal flow. In the presence of inflammatory mediators, the effects of shear stress may be overridden by integrin mediated membrane adhesion (26). Thus the ability of shear stress to inhibit pseudopod formation may be counteracted by stimulatory agents, even though in vitro the activation by a single stimulator can be inhibited by shear stress (Fig. 5B).
The acute response of leukocytes is mostly due to shear stress and less to normal stress. Exposure of leukocytes in vitro to hydrostatic pressures (by elevation of the fluid level in the cell chamber) of 100–500 dyn/cm2 had no measurable influence on the shear stress response. This is in line with the observation that during micropipette aspiration experiments leukocytes do not project or retract pseudopods in response to pressures (normal stress) of 1,000 dyn/cm2 and above (1).
Due to the particulate nature of blood, the shear stress in vivo is stochastic. The constant shear stress applied in the in vitro studies may exceed or underestimate values encountered in vivo. The high degree of cell swelling that can be achieved in vitro is usually not observed in vivo, an indication that the exposure to shear stress in the circulation is time variant where instances of high shear close to the endothelium may be followed by instances of lower shear at the vessel center. Leukocyte suspensions exposed to higher shear stress are subject to cell swelling as well as lysis (27) and in T lymphocytes a depression of the proliferative response (28).
The retraction of pseudopods mediated by shear stress serves to minimize leukocyte entrapment in capillaries. Activated circulating leukocytes cause significant flow reduction in capillaries (2) to the point of complete stasis and microvascular entrapment (4, 29). In contrast, the projection of pseudopods becomes enhanced after cessation of blood flow and a reduction of shear stress on leukocytes. The tendency of leukocytes to spread under conditions of low flow may be significant for their adhesion to endothelium in ischemia and at local sites of low shear stress (e.g., at vascular bifurcations). Such a reaction to low shear enables circulating leukocytes to spread on and migrate across the endothelium.
Swelling of leukocytes during continuous application of shear stress is synchronized with enhancement of random granule velocity and reduction of cell stiffness. These phenomena suggest that shear stress may mediate the entry of water into the cell and a possible reduction of F-actin. Shear stress keeps the cell deformable in the circulation and thus serves to facilitate leukocyte passage through the microcirculation.
The response of leukocytes can be induced by stress levels insufficient to deform the cell as studied at a light microscopy resolution. The response is rapid (within seconds) without time for protein synthesis. These results suggest that some signal transmission across the cell membrane is involved, in line with the observations for a requirement of Ca2+ and K+ channels (Fig. 9). The fact that elimination of Ca2+ ions from the external medium serves to abolish the response, suggests that one of the early events in the shear stress response is a Ca2+ influx via diltiazem sensitive channels. The early cell swelling following the application of shear stress may be in line with the Ca2+ influx as well as possibly a reduction of Na+ pump activity under shear stress.
The fact that neutrophils retract their pseudopods while crawling on a glass surface (Fig. 4), suggests that shear stress may influence not only the cytoskeleton but also the expression of adhesion molecules, i.e. integrins and selectins. Neutrophil adhesion to glass requires β2 integrin (30). The fact that retraction occurs within seconds and is reversible suggests that the action of the adhesion proteins may be coupled to actin so that stable adhesion may require an F-actin matrix, which if broken down will lead to lack of β2 integrin anchoring (31).
There are some similarities in the shear response between leukocytes and endothelial cells. The response of endothelial cells to shear stress depends on time. Application of shear stress for hours leads to endothelium alignment in the flow direction and cytoplasmic F-actin bundle formation (32, 33) with a thickening of the cell matrix (34), while short-term turbulent stress fluctuations disrupt the actin matrix and enhance cell turnover (35). The endothelial response is also sensitive to K+ channel activity (36) as in the case of leukocytes (Fig. 9).
Acknowledgments
We thank Arlyn Madriaga and Philip Baddour for assistance with the fluid stress computations. This work was supported by National Science Foundation Grant IBN-9512778 and U.S. Public Health Service Grants HL 10881 and HL 43024.
References
- 1.Schmid-Schönbein G W, Skalak R, Sung K-L P, Chien S. In: White Blood Cells, Morphology and Rheology as Related to Function. Bagge U, Born G V R, Gaehtgens P, editors. The Hague, The Netherlands: Nijhoff; 1982. pp. 21–31. [Google Scholar]
- 2.Sutton D W, Schmid-Schönbein G W. Am J Physiol. 1992;262:H1646–H1650. doi: 10.1152/ajpheart.1992.262.6.H1646. [DOI] [PubMed] [Google Scholar]
- 3.Worthen G S, Schwab B, Elson E L, Downey G P. Science. 1989;245:183–186. doi: 10.1126/science.2749255. [DOI] [PubMed] [Google Scholar]
- 4.Ritter L S, Wilson D S, Williams S K, Copeland J G, McDonagh P F. Microcirculation. 1995;2:315–327. doi: 10.3109/10739689509148276. [DOI] [PubMed] [Google Scholar]
- 5.Chamberlain J K, Lichtman M A. Blood. 1978;52:959–968. [PubMed] [Google Scholar]
- 6.Lipowsky H H, Riedel D, Shi G S. Biorheology. 1991;28:53–64. doi: 10.3233/bir-1991-281-206. [DOI] [PubMed] [Google Scholar]
- 7.Davies P. Physiol Rev. 1995;75:519–560. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nerem R M, Girard P R, Helmlinger G, Thoumine O, Wiesner T F, Ziegler T. In: Cell Mechanics and Cellular Engineering. Mow V C, Guilak F, Tran-Son-Tay R, Hochmuth R M, editors. New York: Springer; 1994. pp. 55–69. [Google Scholar]
- 9.Johnson R M. Biophys J. 1994;67:1876–1881. doi: 10.1016/S0006-3495(94)80669-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konstantopoulos K, Wu K K, Uden M M, Banez E I, Shattil S J, Hellums J D. Biorheology. 1995;32:73–93. doi: 10.3233/bir-1995-32106. [DOI] [PubMed] [Google Scholar]
- 11.DePaola N, Gimbrone M A, Davis P F, Dewey C F J. Arterioscler Thromb. 1992;12:1254–1257. doi: 10.1161/01.atv.12.11.1254. [DOI] [PubMed] [Google Scholar]
- 12.Frangos J A, Eskin S G, McIntire L V, Ives C L. Science. 1985;227:1477–1479. doi: 10.1126/science.3883488. [DOI] [PubMed] [Google Scholar]
- 13.Kuchan M J, Hanjoong J, Frangos J A. Am J Physiol. 1994;267:C753–C758. doi: 10.1152/ajpcell.1994.267.3.C753. [DOI] [PubMed] [Google Scholar]
- 14.Resnick N, Collins T, Atkinson W, Bonthron D T, Dewey C F J, Gimbrone M A. Proc Natl Acad Sci USA. 1993;90:4591–4595. doi: 10.1073/pnas.90.10.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shyy Y-J, Hsieh H-J, Usami S, Chien S. Proc Natl Acad Sci USA. 1994;91:4678–4682. doi: 10.1073/pnas.91.11.4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawrence M B, Smith C W, Eskin S G, McIntire L V. Blood. 1990;75:227–237. [PubMed] [Google Scholar]
- 17.Wong P M, Schmid-Schönbein G W. Cell Biophys. 1992;18:203–215. doi: 10.1007/BF02989814. [DOI] [PubMed] [Google Scholar]
- 18.Schmid-Schönbein G W, Sung K-L P, Tözeren H, Chien S, Skalak R. Biophys J. 1981;36:243–256. doi: 10.1016/S0006-3495(81)84726-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmid-Schönbein G W, Shih Y Y, Chien S. Blood. 1980;56:866–875. [PubMed] [Google Scholar]
- 20.Tompkins W R, Monti R, Intaglietta M. Rev Sci Instrum. 1974;45:647–650. [Google Scholar]
- 21.Fung Y C. Biodynamics: Circulation. New York: Springer; 1984. [Google Scholar]
- 22.Sullivan J A, Mandell G L. Cell Motil. 1983;3:31–46. doi: 10.1002/cm.970030104. [DOI] [PubMed] [Google Scholar]
- 23.Tözeren A, Skalak R. J Fluid Mech. 1978;87:1–16. [Google Scholar]
- 24.Goldsmith H L, Spain S. Microvasc Res. 1984;27:204–222. doi: 10.1016/0026-2862(84)90054-2. [DOI] [PubMed] [Google Scholar]
- 25.Schmid-Schönbein G W, Usami S, Skalak R, Chien S. Microvasc Res. 1980;19:45–70. doi: 10.1016/0026-2862(80)90083-7. [DOI] [PubMed] [Google Scholar]
- 26.Ohashi K L, Tung D K-L, Wilson J M, Zweifach B W, Schmid-Schönbein G W. Microcirculation. 1996;3:199–210. doi: 10.3109/10739689609148289. [DOI] [PubMed] [Google Scholar]
- 27.Dewitz T S, Martin R R, McIntire L V. J Lab Clin Med. 1977;90:728–736. [PubMed] [Google Scholar]
- 28.Chittur K K, McIntire L V, Rich R R. Biotechnol Progr. 1988;4:89–96. [Google Scholar]
- 29.Harris A G, Skalak T C. Am J Physiol. 1993;264:H909–H916. doi: 10.1152/ajpheart.1993.264.3.H909. [DOI] [PubMed] [Google Scholar]
- 30.Grau A J, Berger E, Sung K-L P, Schmid-Schönbein G W. Stroke (Dallas) 1992;22:33–39. doi: 10.1161/01.str.23.1.33. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz M A, Schaller M D, Ginsberg M H. Annu Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- 32.Levesque M J, Nerem R M. J Biomech Eng. 1985;107:341–347. doi: 10.1115/1.3138567. [DOI] [PubMed] [Google Scholar]
- 33.Franke R-P, Gräfe M, Schnittler H, Seiffge D, Mittermayer C, Drenkhahn D. Nature (London) 1984;307:648–649. doi: 10.1038/307648a0. [DOI] [PubMed] [Google Scholar]
- 34.Satcher R L. Ph.D. thesis. Cambridge: Massachusetts Institute of Technology; 1993. [Google Scholar]
- 35.Davies P F, Remuzzi A, Gordon E J, Dewey C F, Gimbrone M A. Proc Natl Acad Sci USA. 1986;83:2114–2117. doi: 10.1073/pnas.83.7.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olesen S P, Clapham D E, Davies P F. Nature (London) 1988;331:168–170. doi: 10.1038/331168a0. [DOI] [PubMed] [Google Scholar]