Abstract
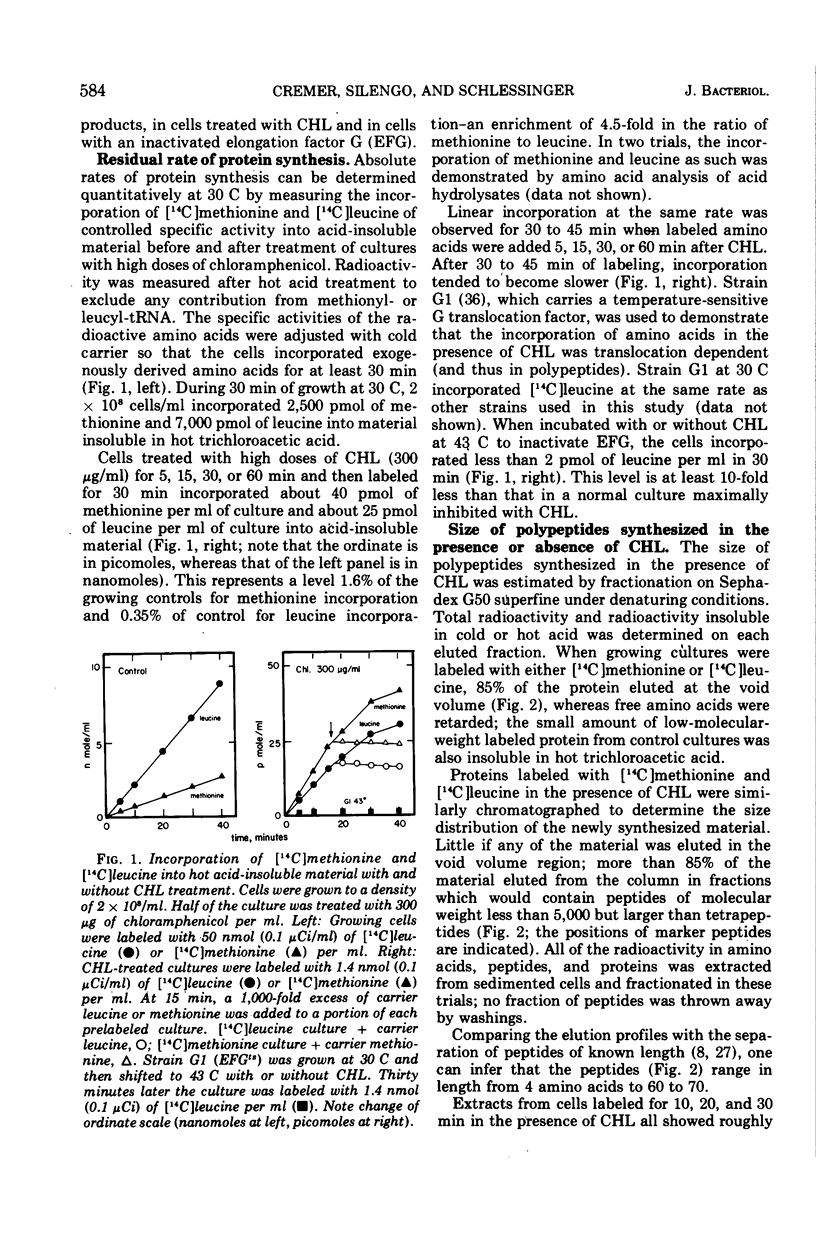
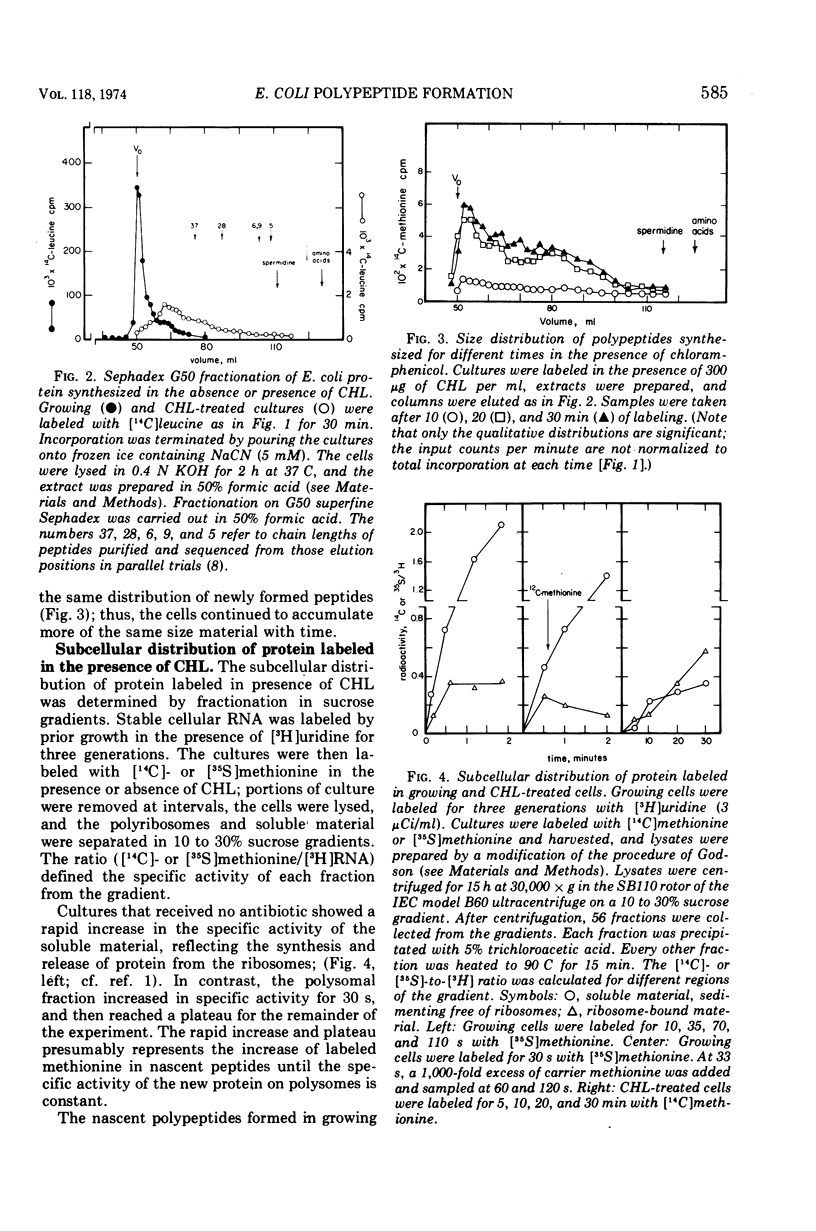
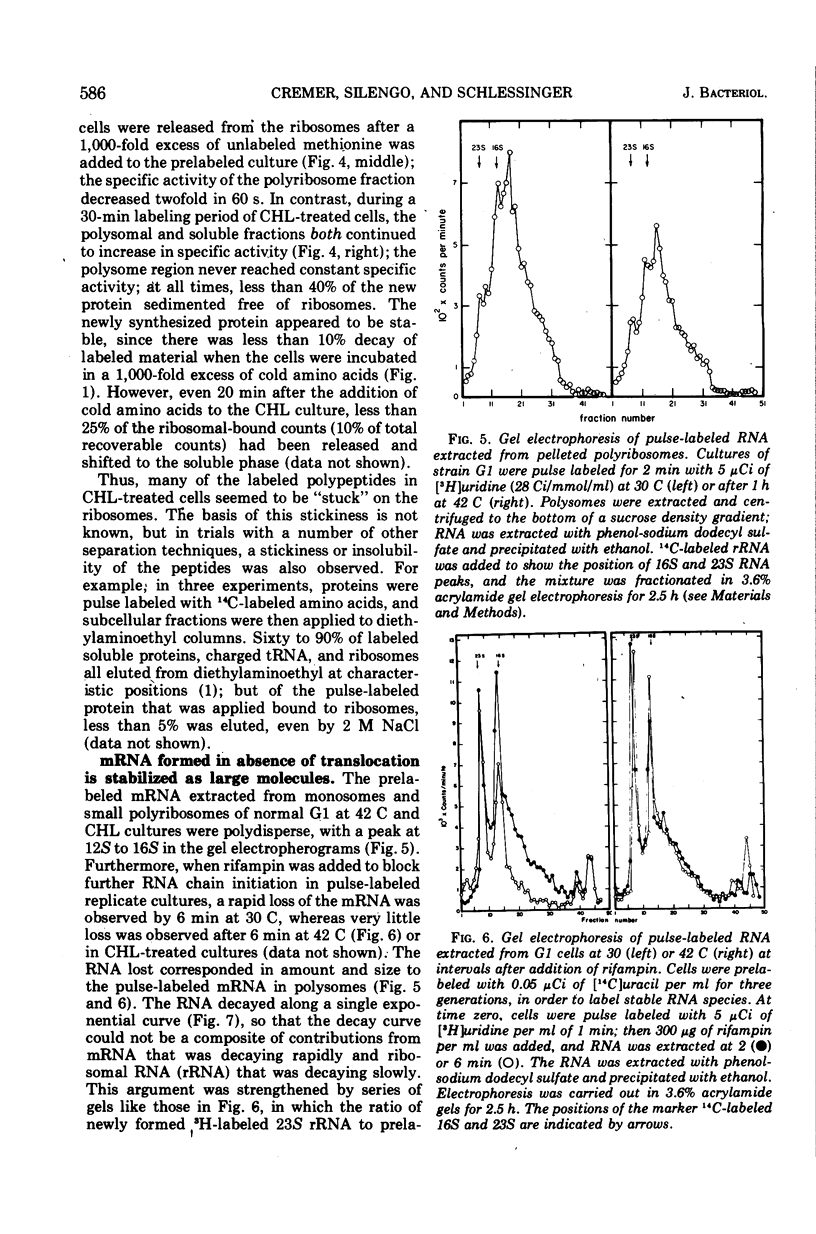
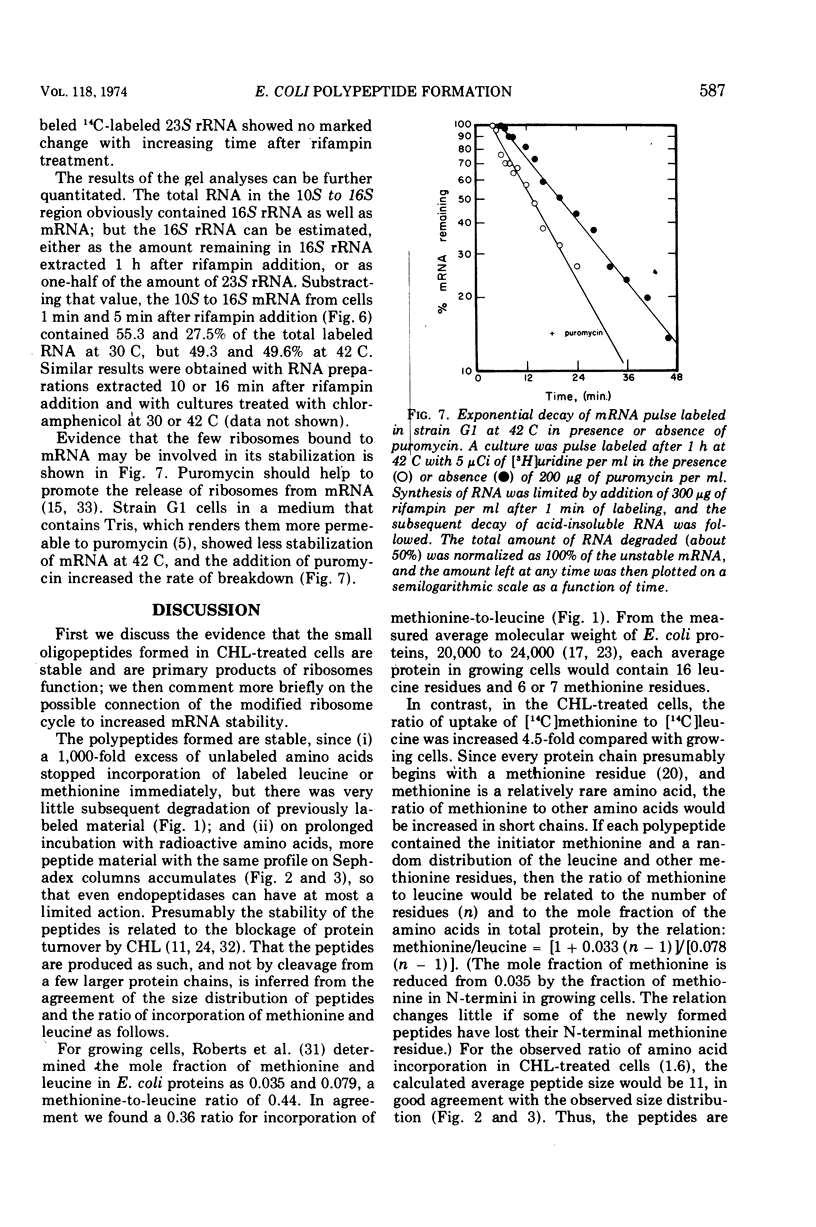
In Escherichia coli cultures maximally inhibited with chloramphenicol, formation of polypeptides still continued at a slow, constant rate for at least 90 min. The rate of leucine incorporation was reduced to 0.5%, but methionine was only reduced to 2%, suggesting that chains are normally initiated with methionine but are prematurely released at a short chain length. Consistent with this possibility was the distribution of the products on Sephadex columns: a range of peptides longer than 4 and shorter than 60 to 70 residues was seen. Less than 10% of the peptides broke down during a chase with cold amino acids, and during continuous labeling they accumulated progressively. On the average, one peptide was formed per ribosome every 5 min. Peptide synthesis in the presence of chloramphenicol was still dependent on ribosome translocation; it stopped in a mutant with an inactivated temperature-sensitive elongation factor G. But even in the absence of translocation, new messenger ribonucleic acid (mRNA) chains were found joined to one or a few ribosomes. The chains had a size distribution comparable to that of mRNA from polyribosomes of growing cells. They were stabilized for an average time of about 5 min, but were more rapidly degraded after puromycin was added to the cells. This suggests that stabilization may be related to the average time spent by a ribosome on an mRNA chain, with or without polypeptide formation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brunschede H., Bremer H. Synthesis and breakdown of proteins in Escherichia coli during amino-acid starvation. J Mol Biol. 1971 Apr 14;57(1):35–57. doi: 10.1016/0022-2836(71)90118-5. [DOI] [PubMed] [Google Scholar]
- Cameron H. J., Julian G. R. The effect of chloramphenicol on the polysome formation of starved stringent Escherichia coli. Biochim Biophys Acta. 1968 Dec 17;169(2):373–380. doi: 10.1016/0005-2787(68)90045-2. [DOI] [PubMed] [Google Scholar]
- Carter T., Newton A. New polarity suppressors in Escherichia coli: suppression and messenger RNA stability. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2962–2966. doi: 10.1073/pnas.68.12.2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corte G., Schlessinger D., Longo D., Venkov P. Transformation of 17 s to 16 s ribosomal RNA using ribonuclease II of Escherichia coli. J Mol Biol. 1971 Sep 14;60(2):325–338. doi: 10.1016/0022-2836(71)90297-x. [DOI] [PubMed] [Google Scholar]
- Craig E. Synthesis of Specific, Stabilized Messenger RNA When Translocation Is Blocked in ESCHERICHIA COLI. Genetics. 1972 Feb;70(2):331–336. doi: 10.1093/genetics/70.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischman J. B. A partial amino acid sequence in the heavy chain of a rabbit antibody to group C streptococcal carbohydrate. Biochemistry. 1971 Jul 6;10(14):2753–2761. doi: 10.1021/bi00790a016. [DOI] [PubMed] [Google Scholar]
- Flessel C. P. Chloramphenicol protects polyribosomes. Biochem Biophys Res Commun. 1968 Aug 13;32(3):438–446. doi: 10.1016/0006-291x(68)90681-5. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L. A role of aminoacyl-tRNA in the regulation of protein breakdown in Escherichia coli. Proc Natl Acad Sci U S A. 1971 Feb;68(2):362–366. doi: 10.1073/pnas.68.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurgo C., Apirion D., Schlessinger D. Polyribosome metabolism in Escherichia coli treated with chloramphenicol, neomycin, spectinomycin or tetracycline. J Mol Biol. 1969 Oct 28;45(2):205–220. doi: 10.1016/0022-2836(69)90100-4. [DOI] [PubMed] [Google Scholar]
- Hill C. W., Echols H. Properties of a mutant blocked in inducibility of messenger RNA for the galactose operon of Escherichia coli. J Mol Biol. 1966 Aug;19(1):38–51. doi: 10.1016/s0022-2836(66)80048-7. [DOI] [PubMed] [Google Scholar]
- Imamoto F. Diversity of regulation of genetic transcription. I. Effect of antibiotics which inhibit the process of translation on RNA metabolism in Escherichia coli. J Mol Biol. 1973 Feb 25;74(2):113–136. doi: 10.1016/0022-2836(73)90102-2. [DOI] [PubMed] [Google Scholar]
- KUCAN Z., LIPMANN F. DIFFERENCES IN CHLORAMPHENICOL SENSITIVITY OF CELL-FREE AMINO ACID POLYMERIZATION SYSTEMS. J Biol Chem. 1964 Feb;239:516–520. [PubMed] [Google Scholar]
- Kaempfer R. Ribosomal subunit exchange during protein synthesis. Proc Natl Acad Sci U S A. 1968 Sep;61(1):106–113. doi: 10.1073/pnas.61.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennel D. Titration of the gene sites on DNA by DNA-RNA hybridization. II. The Escherichia coli chromosome. J Mol Biol. 1968 May 28;34(1):85–103. doi: 10.1016/0022-2836(68)90236-2. [DOI] [PubMed] [Google Scholar]
- Kiehn E. D., Holland J. J. Size distrbution of polypeptide chains in cells. Nature. 1970 May 9;226(5245):544–545. doi: 10.1038/226544a0. [DOI] [PubMed] [Google Scholar]
- Lark K. G. Initiation and termination of bacterial deoxyribonucleic acid replication in low concentrations of chloramphenicol. J Bacteriol. 1973 Feb;113(2):1066–1069. doi: 10.1128/jb.113.2.1066-1069.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A. J., Sinsheimer R. L. The process of infection with bacteriophage phiX174. XXV. Studies with bacteriophage phiX174 mutants blocked in progeny replicative form DNA synthesis. J Mol Biol. 1969 Feb 14;39(3):619–639. doi: 10.1016/0022-2836(69)90149-1. [DOI] [PubMed] [Google Scholar]
- Lu P., Rich A. The nature of the polypeptide chain termination signal. J Mol Biol. 1971 Jun 14;58(2):513–531. doi: 10.1016/0022-2836(71)90368-8. [DOI] [PubMed] [Google Scholar]
- MANDELSTAM J. Turnover of protein in growing and non-growing populations of Escherichia coli. Biochem J. 1958 May;69(1):110–119. doi: 10.1042/bj0690110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiarotti G., Apirion D., Schlessinger D. Selection of sucrose-dependent Escherichia coli to obtain envelope mutants and fragile cultures. Science. 1966 Aug 19;153(3738):892–894. doi: 10.1126/science.153.3738.892. [DOI] [PubMed] [Google Scholar]
- Mangiarotti G., Schlessinger D., Kuwano M. Initiation of ribosome-dependent breakdown of T4-specific messenger RNA. J Mol Biol. 1971 Sep 28;60(3):441–452. doi: 10.1016/0022-2836(71)90180-x. [DOI] [PubMed] [Google Scholar]
- Mole L. E., Jackson S. A., Porter R. R., Wilkinson J. M. Allotypically related sequences in the Fd fragment of rabbit immunoglobulin heavy chains. Biochem J. 1971 Sep;124(2):301–318. doi: 10.1042/bj1240301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse D. E. Polarity induced by chloramphenicol and relief by suA. J Mol Biol. 1971 Jan 14;55(1):113–118. doi: 10.1016/0022-2836(71)90285-3. [DOI] [PubMed] [Google Scholar]
- Pato M. L., Bennett P. M., von Meyenburg K. Messenger ribonucleic acid synthesis and degradation in Escherichia coli during inhibition of translation. J Bacteriol. 1973 Nov;116(2):710–718. doi: 10.1128/jb.116.2.710-718.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka S. Inhibitors of ribosome functions. Annu Rev Microbiol. 1971;25:487–562. doi: 10.1146/annurev.mi.25.100171.002415. [DOI] [PubMed] [Google Scholar]
- Schlessinger D., Ben-Hamida F. Turnover of protein in Escherichia coli starving for nitrogen. Biochim Biophys Acta. 1966 Apr 18;119(1):171–182. doi: 10.1016/0005-2787(66)90048-7. [DOI] [PubMed] [Google Scholar]
- Schlessinger D., Mangiarotti G., Apirion D. The formation and stabilization of 30S and 50S ribosome couples in Escherichia coli. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1782–1789. doi: 10.1073/pnas.58.4.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takanami M., Yan Y., Jukes T. H. Studies on the site of ribosomal binding of f2 bacteriophage RNA. J Mol Biol. 1965 Jul;12(3):761–773. doi: 10.1016/s0022-2836(65)80325-4. [DOI] [PubMed] [Google Scholar]
- Tocchini-Valentini G. P., Mattoccia E. A mutant of E. coli with an altered supernatant factor. Proc Natl Acad Sci U S A. 1968 Sep;61(1):146–151. doi: 10.1073/pnas.61.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M. J., DeMoss J. A. The inhibition by chloramphenicol of nascent protein formation in E. coli. Proc Natl Acad Sci U S A. 1966 May;55(5):1224–1230. doi: 10.1073/pnas.55.5.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]



