Abstract
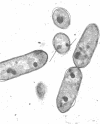
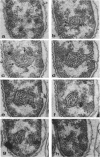
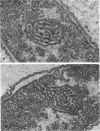
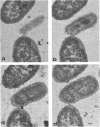
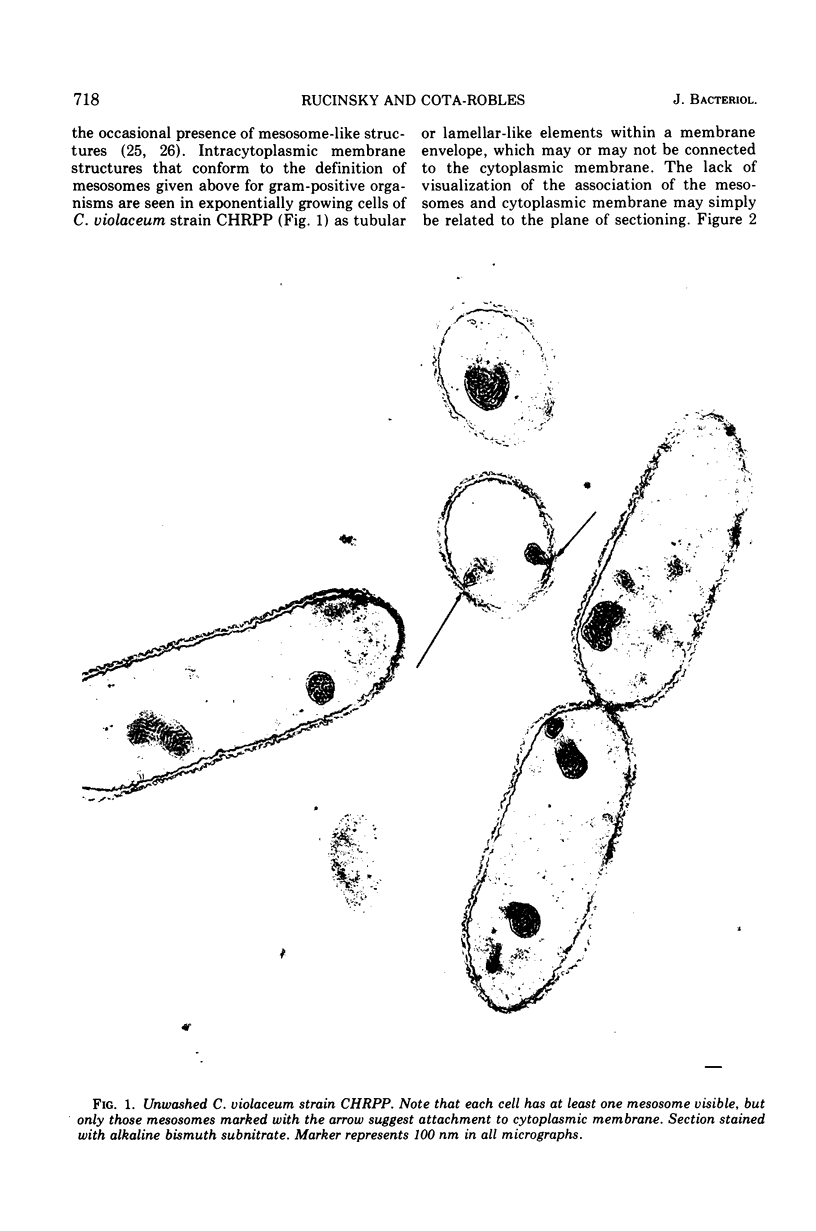
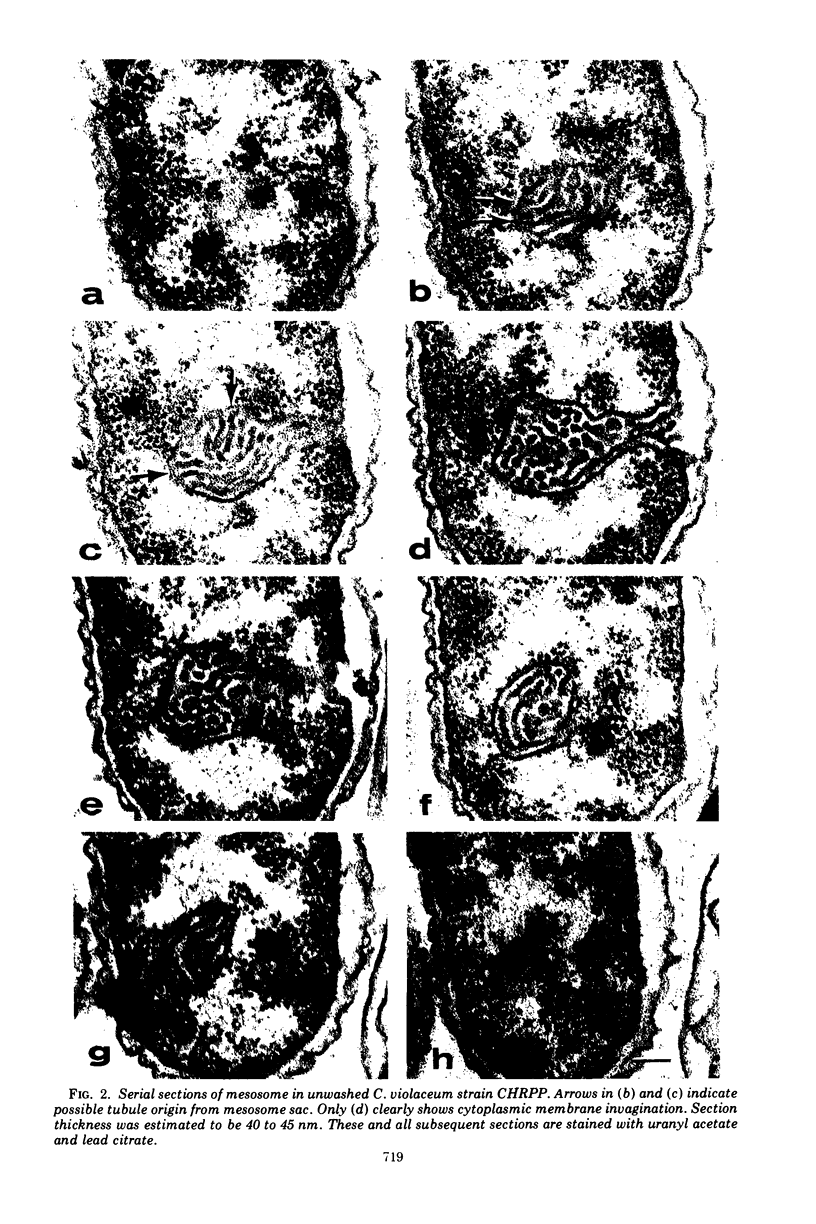
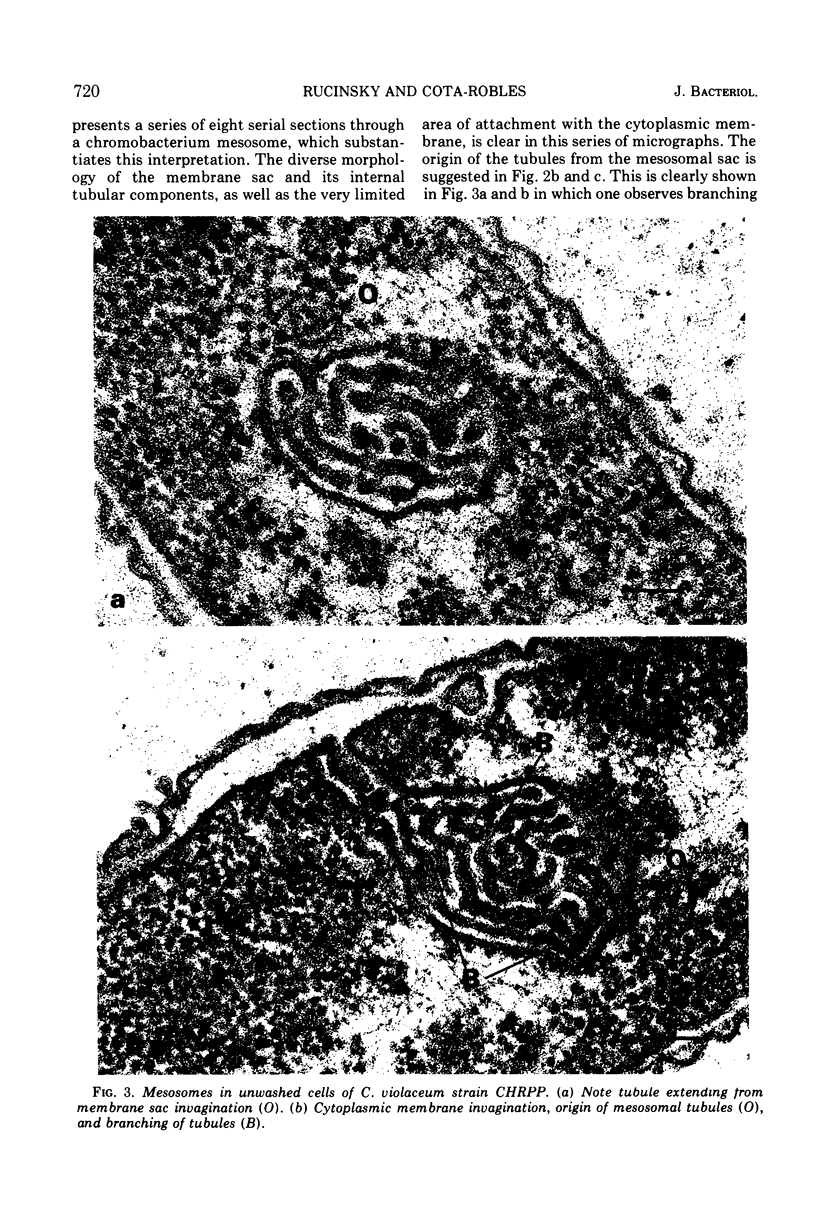
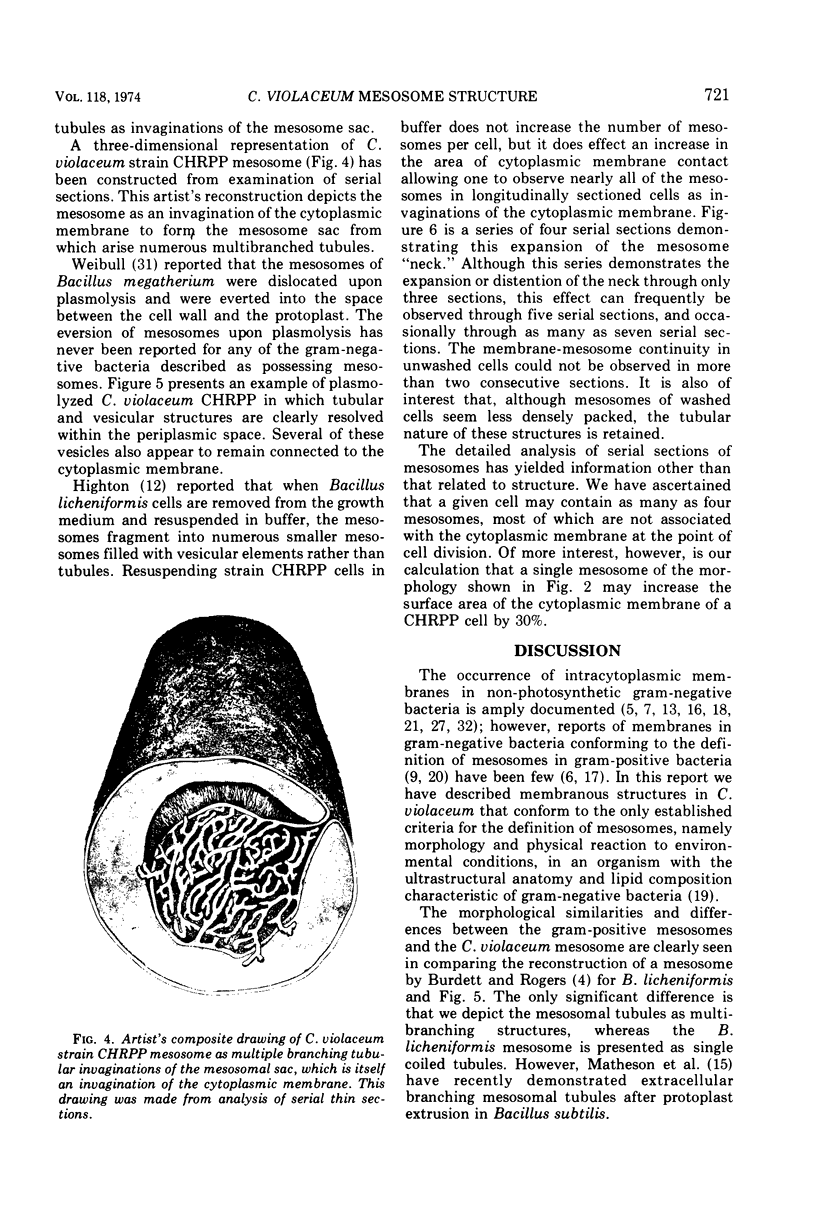
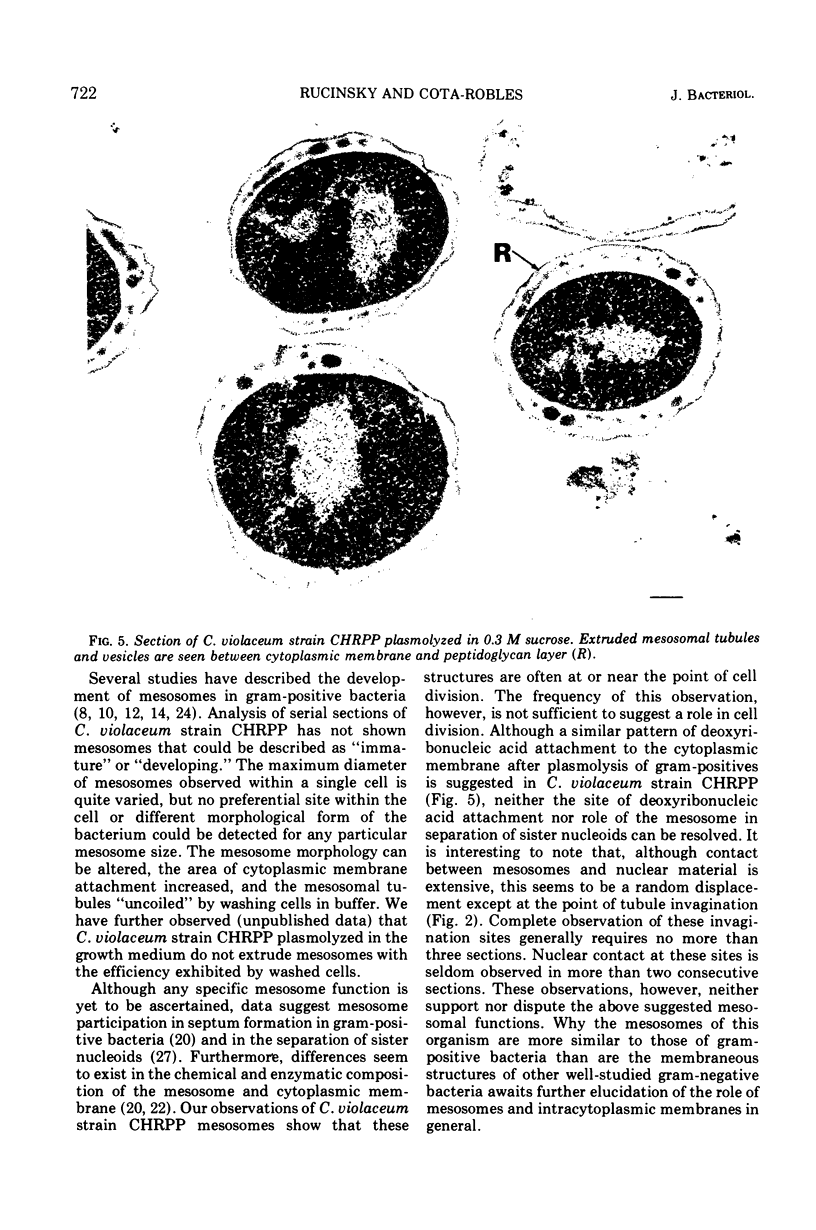
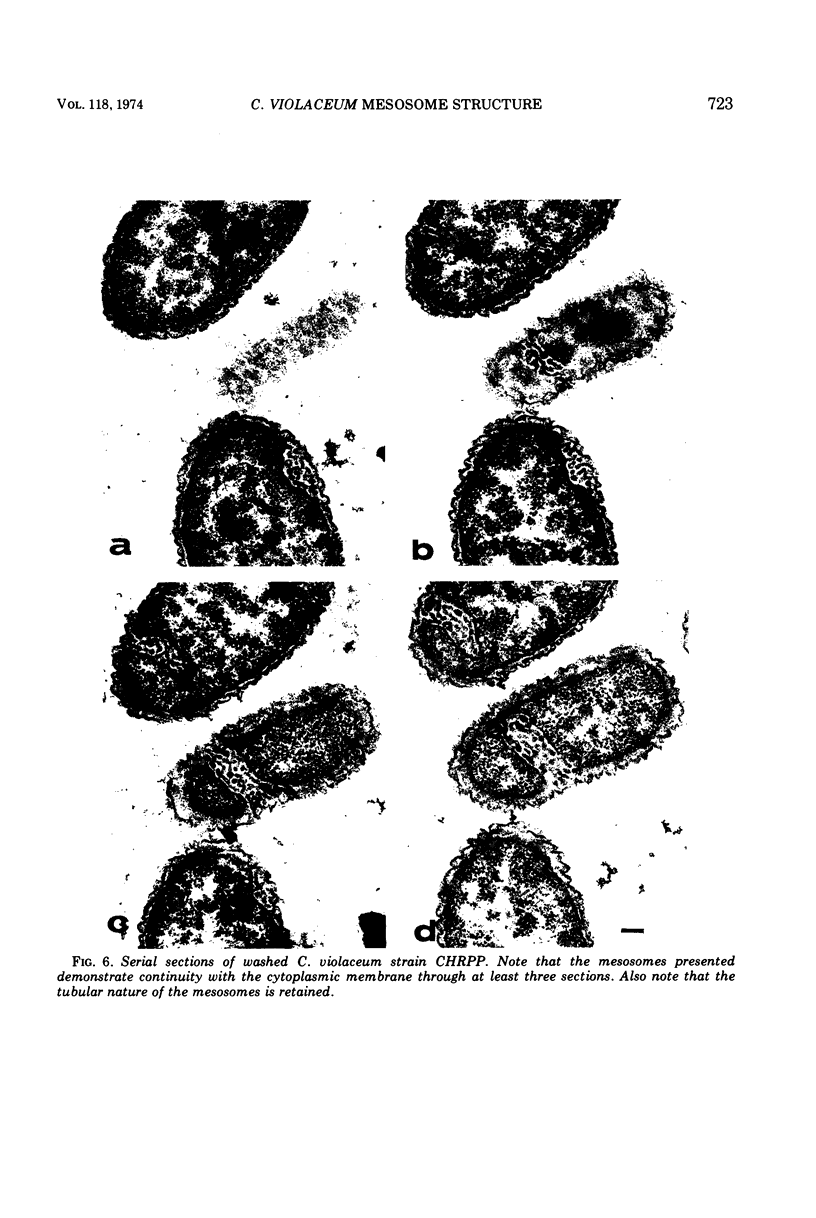
Exponentially growing cells of the gram-negative bacterium Chromobacterium violaceum demonstrate invaginations of the cytoplasmic membrane with a high frequency. These invaginations conform to the ultrastructural appearance of mesosomes of gram-positive bacteria. As many as four mesosomes are observed per cell, each of which may increase the total membrane surface of the cell by 30%. Washing of cells in dilute tris(hydroxymethyl)aminomethane buffer effects a distension of the mesosome “neck” and/or cytoplasmic membrane clarifying the association of the mesosome to the cytoplasmic membrane. Plasmolysis effects an eversion of the mesosome into the plasmolysis vacuole.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ainsworth S. K., Ito S., Karnovsky M. J. Alkaline bismuth reagent for high resolution ultrastructural demonstration of periodate-reactive sites. J Histochem Cytochem. 1972 Dec;20(12):995–1005. doi: 10.1177/20.12.995. [DOI] [PubMed] [Google Scholar]
- Ainsworth S. K., Karnovsky M. J. An ultrastructural staining method for enhancing the size and electron opacity of ferritin in thin sections. J Histochem Cytochem. 1972 Mar;20(3):225–229. doi: 10.1177/20.3.225. [DOI] [PubMed] [Google Scholar]
- Burdett I. D. Bacterial mesosomes. Sci Prog. 1972 Winter;60(240):527–546. [PubMed] [Google Scholar]
- Burdett I. D., Rogers H. J. The structure and development of mesosomes studied in Bacillus licheniformis strain 6346. J Ultrastruct Res. 1972 Jan;38(1):113–133. doi: 10.1016/s0022-5320(72)90087-1. [DOI] [PubMed] [Google Scholar]
- Carrick L., Jr, Berk R. S. Membranous inclusions of Pseudomonas aeruginosa. J Bacteriol. 1971 Apr;106(1):250–256. doi: 10.1128/jb.106.1.250-256.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Bazire G., Kunisawa R., Poindexter J. S. The internal membranes of Caulobacter crescentus. J Gen Microbiol. 1966 Feb;42(2):301–308. doi: 10.1099/00221287-42-2-301. [DOI] [PubMed] [Google Scholar]
- Cota-Robles E. H. Internal membranes in cells of Escherichia coli. J Ultrastruct Res. 1966 Dec;16(5):626–639. doi: 10.1016/s0022-5320(66)80010-2. [DOI] [PubMed] [Google Scholar]
- Ellar D. J., Lundgren D. G., Slepecky R. A. Fine structure of Bacillus megaterium during synchronous growth. J Bacteriol. 1967 Oct;94(4):1189–1205. doi: 10.1128/jb.94.4.1189-1205.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITZ-JAMES P. C. Participation of the cytoplasmic membrane in the growth and spore fromation of bacilli. J Biophys Biochem Cytol. 1960 Oct;8:507–528. doi: 10.1083/jcb.8.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh B. K., Murray R. G. Fine structure of Listeria monocytogenes in relation to protoplast formation. J Bacteriol. 1967 Jan;93(1):411–426. doi: 10.1128/jb.93.1.411-426.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Model for cell wall growth of Streptococcus faecalis. J Bacteriol. 1970 Feb;101(2):643–648. doi: 10.1128/jb.101.2.643-648.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highton P. J. An electron microscopic study of cell growth and mesosomal structure of Bacillus licheniformis. J Ultrastruct Res. 1969 Jan;26(1):130–147. doi: 10.1016/s0022-5320(69)90040-9. [DOI] [PubMed] [Google Scholar]
- Hoffmann H. P., Geftic S. G., Heymann H., Adair F. W. Mesosomes in Pseudomonas aeruginosa. J Bacteriol. 1973 Apr;114(1):434–438. doi: 10.1128/jb.114.1.434-438.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakefuda T., Holden J. T., Utech N. M. Ultrastructure of the membrane system in Lactobacillus plantarum. J Bacteriol. 1967 Jan;93(1):472–482. doi: 10.1128/jb.93.1.472-482.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheson A., Yang M. K., Smith R. P. Demonstration of ribosomes in mesosomes associated with Bacillus subtilis protoplasts. J Bacteriol. 1973 Jul;115(1):349–357. doi: 10.1128/jb.115.1.349-357.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangborn J., Marr A. G., Robrish S. A. LOCALIZATION OF RESPIRATORY ENZYMES IN INTRACYTOPLASMIC MEMBRANES OF AZOTOBACTER AGILIS. J Bacteriol. 1962 Oct;84(4):669–678. doi: 10.1128/jb.84.4.669-678.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate J. L., Ordal E. J. The fine structure of Chondrococcus columnaris. I. Structure and formation of mesosomes. J Cell Biol. 1967 Oct;35(1):1–13. doi: 10.1083/jcb.35.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontefract R. D., Bergeron G., Thatcher F. S. Mesosomes in Escherichia coli. J Bacteriol. 1969 Jan;97(1):367–375. doi: 10.1128/jb.97.1.367-375.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYTER A., JACOB F. ETUDE AU MICROSCOPE 'ELECTRONIQUE DE LA LIAISON ENTRE NOYAU ET M'ESOSOME CHEZ BACILLUS SUBTILIS. Ann Inst Pasteur (Paris) 1964 Sep;107:384–400. [PubMed] [Google Scholar]
- RYTER A., KELLENBERGER E., BIRCHANDERSEN A., MAALOE O. Etude au microscope électronique de plasmas contenant de l'acide désoxyribonucliéique. I. Les nucléoides des bactéries en croissance active. Z Naturforsch B. 1958 Sep;13B(9):597–605. [PubMed] [Google Scholar]
- Randle C. L., Albro P. W., Dittmer J. C. The phosphoglyceride composition of Gram-negative bacteria and the changes in composition during growth. Biochim Biophys Acta. 1969;187(2):214–220. doi: 10.1016/0005-2760(69)90030-7. [DOI] [PubMed] [Google Scholar]
- Remsen C. C., Valois F. W., Watson S. W. Fine structure of the cytomembranes of Nitrosocystis oceanus. J Bacteriol. 1967 Aug;94(2):422–433. doi: 10.1128/jb.94.2.422-433.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusch V. M., Jr, Burger M. M. The bacterial mesosome. Biochim Biophys Acta. 1973 Apr 3;300(1):79–104. doi: 10.1016/0304-4157(73)90012-9. [DOI] [PubMed] [Google Scholar]
- Rogers H. J. Bacterial growth and the cell envelope. Bacteriol Rev. 1970 Jun;34(2):194–214. doi: 10.1128/br.34.2.194-214.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucinsky T. E., Gregory J. P., Cota-Robles E. H. Organization of bacteriophage tail-like particles in cells of Chromobacterium violaceum. J Bacteriol. 1972 May;110(2):754–757. doi: 10.1128/jb.110.2.754-757.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter A. Association of the nucleus and the membrane of bacteria: a morphological study. Bacteriol Rev. 1968 Mar;32(1):39–54. doi: 10.1128/br.32.1.39-54.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- WEIBULL C. PLASMOLYSIS IN BACILLUS MEGATERIUM. J Bacteriol. 1965 Apr;89:1151–1154. doi: 10.1128/jb.89.4.1151-1154.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigand R. A., Holt S. C., Shively J. M., Decker G. L., Greenawalt J. W. Ultrastructural properties of the extra membranes of Escherichia coli O111a as revealed by freeze-fracturing and negative-staining techniques. J Bacteriol. 1973 Jan;113(1):433–444. doi: 10.1128/jb.113.1.433-444.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]