Abstract
This study used functional MRI (fMRI) to clarify the sites of brain activity associated with the antidepressant effects of sleep deprivation (SD). We hypothesized: (1) baseline perfusion in right and left amygdalae will be greater in responders than in nonresponders; (2) following partial sleep deprivation (PSD), perfusion in responders’ right and left amygdalae would decrease. Seventeen unmedicated outpatients with current major depression and eight controls received perfusion-weighted fMRI and structural MRI at baseline and following 1 night of late-night PSD. Baseline bilateral amygdalar perfusion was greater in responders than nonresponders. Clusters involving both amygdalae decreased from baseline to PSD specifically in responders. Right amygdalar perfusion diverged with PSD, increasing in nonresponders and decreasing in responders. These novel amygdalar findings are consistent with the overarousal hypothesis of SD as well as other functional imaging studies showing increased baseline amygdalar activity in depression and decreased amygdalar activity with remission or antidepressant medications.
Keywords: Magnetic resonance imaging, functional, Depressive disorder, major
1. Introduction
One night of total sleep deprivation (TSD) or partial sleep deprivation (PSD) produces temporary remission in 40–60% of patients with major depression (Wu and Bunney, 1990). Yet mechanisms of the antidepressant effects of sleep deprivation (SD), and explanations of why some individuals respond while others do not, remain unclear. Previous positron emission tomography (PET) and single photon emission computed tomography (SPECT) studies of SD report at least one area in which responders and nonresponders differ significantly at baseline, along with differential response patterns to SD in responders and nonresponders. In most cases, areas of differing baseline activity or unique change in responders were reported in ventral anterior cingulate, basal orbital, or medial frontal areas (Ebert et al., 1991, 1994; Wu et al., 1992, 1999; Volk et al., 1992). Similar PET findings in these areas have been linked with clinical response to antidepressant medications (Buchsbaum et al., 1997; Mayberg, 1997).
More recent work has employed functional magnetic resonance imaging (fMRI) to examine these effects with superior spatial resolution (Clark et al., 2001; Clark et al., submitted for publication). We recently (Clark et al., submitted for publication) used fMRI to clarify the exact medial frontal sites of brain activity associated with the antidepressant effects of SD in both region of interest (ROI) and voxelwise analyses. Using an arterial spin labeling (ASL) based fMRI method, we found greater baseline left ventral anterior cingulate (LVAC) perfusion in responders than nonresponders and a reduction of LVAC perfusion with PSD specific to the responder group. To test the anatomic specificity of these findings, we a priori divided the anterior cingulate and medial frontal cortex into dorsal, rostral, and ventral ROIs for each hemisphere, using ROIs derived from the Talairach daemon.
The “overarousal” hypothesis postulates that depression is associated with a pathological increase in physiologic arousal and that SD works by “de-arousing” depressed patients, or at least those who respond clinically. Aside from behavioral observations (Szuba et al., 1991), subjective reports of increased energy level (Van Den Burg et al., 1992), and neuroendocrine studies documenting increased hypothalamic–pituitary–adrenal (HPA) axis activity (Roy-Byrne et al., 1984), the main evidence for this theory comes from polysomnographic and functional neuroimaging data. Sleep abnormalities in depression, consistent with depressed patients’ reports of disturbed and nonrestorative sleep, include prolonged sleep latency, reduced total sleep time and sleep efficiency, and decreased slow wave (“deep”) sleep as well as increased REM sleep (Benca et al., 1992; Benca, 1996). Depressed patients have also been reported to exhibit increased nocturnal core temperature, especially those who respond to SD (Elsenga and van den Hoofdakker, 1988).
Functional brain-imaging data have shown elevated baseline limbic activity in depressed patients in wakefulness and sleep, with decreases in limbic activity in association with SD or other antidepressant treatments. Generally, reports of altered baseline limbic function in SD responders have focused primarily on medial frontal areas, including our own findings in the left ventral anterior cingulate area. Elevated baseline levels of limbic activity in depressed patients, whether in SD studies (Ebert et al., 1991, 1994; Wu et al., 1992) or other paradigms (Bench et al., 1992; Mayberg et al., 1997), are consistent with the concept of increased physiologic arousal. The overarousal hypothesis is also consistent with decreases in limbic activity in association with TSD (Ebert et al., 1991, 1994; Wu et al., 1992), PSD (Clark et al., submitted for publication) or other anti-depressant treatments (Scott et al., 1994; Buchsbaum et al., 1997).
The present study investigates the role of the amygdala in response to PSD in depressed individuals. Little has been reported about amygdalar function in connection with the antidepressant response to SD (Ebert et al., 1991; Wu and Gillin, 1992), and in our previous study we focused on the prefrontal cortex. However, increasing evidence suggests an important role for the amygdala in major depression (Whalen et al., 2002). Elevated resting baseline glucose metabolism has been reported in patients with major depression (Abercrombie et al., 1998; Drevets et al., 2002b). Treatment with antidepressant medications has been reported to modify affective response (Sheline et al., 2001; Davidson et al., 2003; Fu et al., 2004) and resting amygdalar activity (Drevets et al., 2002a) in major depression. Based on these observations, as well as the overarousal hypothesis, we predicted increased baseline perfusion in both the left and right amygdalae among PSD responders with major depression. We also hypothesized that perfusion in responders’ right and left amygdalae would decrease with SD.
2. Methods
2.1. Subjects
Seventeen unmedicated outpatients with current major depression and eight controls received perfusion-weighted fMRI and structural MRI at baseline and following 1 night of late-night PSD. To enter the study, depressed subjects (ages 18–55) had to meet full diagnostic criteria for current DSM-IV major depressive disorder (unipolar) and to have a baseline 17-item Hamilton Depression Rating Scale (HDRS-17) score of 16 or greater. Control subjects had no psychiatric disorders and were matched groupwise for age, sex, handedness, education, and tobacco use. Exclusion criteria included other Axis I disorders; conditions that might be exacerbated by sleep deprivation (e.g., epilepsy or bipolar disorder); potential safety contraindications to MRI (pacemakers, metal implants, pregnancy, lactation); irregular or deviant sleep cycle or primary sleep disorders; active substance abuse or dependence; history of alcohol or cocaine abuse or dependence; neurological problems or history of significant head injury; significant circulatory conditions (including hypertension) that could affect the cerebral circulation; and recent use of medications or substances that could affect sleep pattern, EEG, and/or patterns of brain blood flow.
All subjects received the Structured Clinical Interview for DSM-IV (SCID) (First et al., 1996) as well as a thorough medical and laboratory evaluation as previously described (Clark et al., 1999). All subjects were physically healthy and medication free for at least 2 weeks at the time of the study. (No patient took fluoxetine during the 2 months before to the study.) All subjects signed consent forms approved by the UCSD Human Research Protections Program.
2.2. General procedures
2.2.1. General study procedures
All subjects were required to keep and document (by completing sleep diaries) regular retiring and arising times for 5 nights preceding the study. Subjects spent an adaptation night, a baseline night, and a night of PSD in the sleep laboratory, with standard montage and scoring (Clark et al., 1999). On the PSD night, subjects had to remain awake beginning at 3 a.m. During SD subjects remained within constant visual supervision of study personnel and wore wrist activity and light meters (Sleep Watch) (Sadeh et al., 1994) to document wakefulness. Subjects also were required to fill out the Stanford Sleepiness Scale every 15 min (Hoddes et al., 1973).
The baseline and PSD polysomnography/fMRI blocks were performed in randomized counterbalanced order. Structural and perfusion MRI were performed on all subjects at approximately noon on baseline and sleep-deprived days. Mood ratings, including the HDRS-17, were administered on baseline and sleep-deprived days at 8 a.m. and before and after each scanning session.
Clinical response was measured by the decrease in the modified HDRS-17 (HDRS-17-Mod) (i.e., omitting the sleep and weight loss items) from baseline to the lowest score following PSD. Responders were defined by a decrease of 40% or greater in the HDRS-17-Mod (Wu et al., 1999).
2.2.2. fMRI procedures
All subjects were scanned on a General Electric Signa 1.5 T scanner, using a standard head coil. Subjects were instructed to relax but remain awake and motionless throughout the scans; they were fitted with soft earplugs and positioned carefully in the head coil with comfortable supports and cushions. To verify that subjects remained awake during scans, subjects were asked to keep their eyes open and to apply continuous gentle tonic pressure to a soft rubber bulb connected to a pressure transducer and computer monitor.
Structural scans were performed using the following T1-weighted SPGR sequence: TR: 20 ms, TE: minimum full, flip angle: 30°, FOV: 250 mm, NEX: 1, with 124 sagittal slices 1.5 mm thick and in-plane resolution of 0.9765×0.9765 mm.
Perfusion-weighted axial images were obtained using a spiral imaging (Li et al., 1999) refinement of the QUIPSS II pulsed arterial spin labeling (Wong et al., 1997, 1998b) sequence developed at UCSD. Arterial spin labeling techniques such as the fMRI pulse sequence described can be thought of as similar to diffusible tracer imaging techniques (e.g., H215O-PET), with “tagged” blood (e.g., blood that has been magnetically inverted proximal to the imaging region) as the “tracer”. Because of this endogenous contrast, no injections (e.g., gadolinium) are needed. The actual measured perfusion signal is the difference between measured tagged and untagged (control) signal intensity in the slice(s) of interest (Wong et al., 1997, 1998b; Clark et al., 2001). Because the spiral protocol is technically a series of fast single slices tagged individually, it avoids many potential problems with quantitation. Two slabs of nine 7-mm-thick axial slices (in-plane resolution of 1.875 mm by 1.875 mm) were obtained, covering the entire cerebrum, with the following parameters: TR: 3500 ms, TE: minimum; TI1: 700 ms; TI2: 1400 ms; flip angle: 90°, FOV: 240 mm, 100 repetitions.
2.2.3. Processing and analysis
Data were analyzed using Analysis of Functional Neuroimages (AFNI) 2.56 g (Cox, 1996). Images were bias-corrected with a nonparametric, nonuniform intensity normalization algorithm commonly referred to by its acronym as N3 (Sled et al., 1998). Preliminary skull stripping was performed with a Hybrid Watershed algorithm developed to be conservatively sensitive to the inclusion of brain (Dale et al., 1999; Segonne et al., 2004). This method uses a watershed algorithm to find local optima of image intensity gradients to segment images into connected components (Hahn and Peitgen, 2000). A deformable surface model is then used to find the boundary of the brain. Remaining non-brain tissue was removed with AFNI 3dIntracranial iteratively (with varying minimum signal intensity values) and manual editing where required. The 3dIntracranial routine fits a three-compartment Gaussian mixture mode to the gray scale intensity histogram to initially classify voxels into gray matter, white matter, and cerebrospinal fluid compartments. This step is followed by a series of steps that use global geometric constraints and neighborhood connectivity rules to arrive at a cohesive intracranial volume. The strategy of using two automated skull stripping programs that involve quite different segmentation algorithms often produces a more satisfactory intracranial volume than using these routines singularly (Fennema-Notestine et al., 2005).
Brains were segmented into gray, white, and cerebrospinal fluid compartments by fitting a three-compartment Gaussian mixture model to the intensity histogram with TriComp (Bondi et al., 2005). Resulting gray matter masks were transformed into Talairach space (Talairach and Tournoux, 1988) to create standardized yet individualized masks for perfusion data.
Perfusion images were coregistered to the 50th repetition (to minimize movement artifact). Next, for each repetition, images of the running difference between tagged and nontagged images were calculated and made into 4D (three spatial, one temporal) data sets using a script written by Eric Wong, M.D., Ph.D. For this pulse sequence, the difference between tagged and control images is directly proportional to brain blood flow in ml blood/ml tissue/minute units (Wong et al., 1997, 1998a; Clark et al., 2001) and is expressed in MR signal intensity units. At this point, spurious negative values were omitted for each repetition and each voxel. The first three repetitions and outlier repetitions were omitted for each voxel; the remaining repetitions were averaged. Thus, perfusion was calculated as mean perfusion-weighted signal averaged over each voxel.
We performed ROI-based analyses for right and left amygdalae blindly with respect to diagnosis and condition. Amygdala ROIs were produced on the AC-PC aligned tissue segmented gray matter mask produced by TriComp’s gray–white matter differentiation described above. Thus, anatomical designation required only the separation of amygdalar gray matter from other gray matter regions that were both contiguous and adjacent to the amygdala such as the superior boundary of the hippocampus. For example, the collateral sulcus was used as part of the inferolateral boundary of the amygdala. In addition to the gray matter mask, the registered T1-weighted image was used for anatomic reference (Fig. 1). The most posterior boundary of the amygdala was defined as the first slice in which the mammillary bodies were clearly visible. The most anterior slice on which the amygdala was designated was the slice in which the long columns of the fornix were most fully volumed. Reliability and boundary criteria were developed in conjunction with two experienced morphometrists (SLA, CFN). Reliability was established by one operator (CPC) performing the ROI analysis blindly on two brains 1 week apart and then analyzing the number of 1 mm3 voxel counts for each ROI. The average intrarater reliability was 89%; for both amygdalae on both brains, there was an average of 89% agreement for amygdalar volumes between both analyses (Jernigan et al., 2001).
Fig. 1.
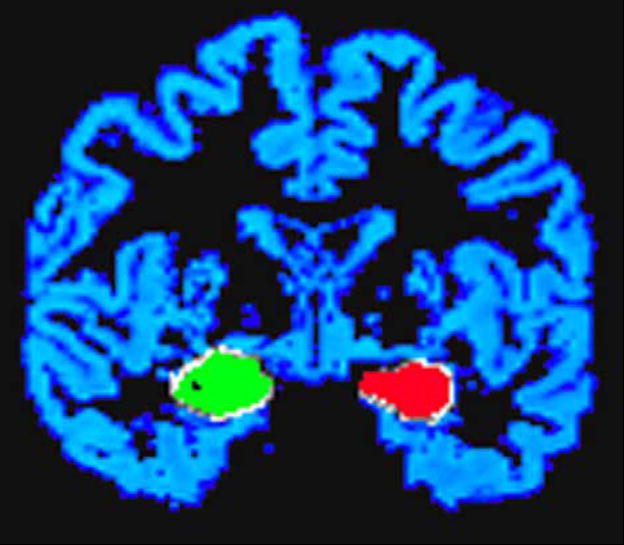
Hand-traced amygdala masks superimposed on the subject’s AC-PC aligned segmented gray matter mask.
For ROI analyses, we compared baseline right and left amygdalar perfusion between responders, nonresponders, and controls using a one-way analysis of variance (ANOVA). Because we were mainly interested in differences between responders and nonresponders, we used the multivariate model to perform a repeated measures analysis of variance (MANOVA) with condition (baseline vs. PSD) and hemisphere as within-group factors and response (responder vs. nonresponder) as the between-group factor. To test our a priori hypotheses that responders would show a greater change in amygdala perfusion than nonresponders, we performed one-tailed independent-group t-tests on change scores (baseline perfusion minus sleep-deprived perfusion) separately for right and left amygdalae.
For voxelwise analyses, we used within-sample or between-sample t-tests to test for the effect of PSD (Friston et al., 1994). We used a cluster threshold method to protect against Type I error (Forman et al., 1995). Our cluster threshold required a connectivity radius of 2.66 mm (e.g., the in-plane pixel length multiplied by the square root of 2), which identified face-to-face or edge-to-edge voxels as connected, and a minimum volume of 443 mm3 (18 voxels, based on Monte Carlo simulations). Perfusion data for individual scans were spatially smoothed with a Gaussian kernel of 3.75 mm (e.g., twice the in-plane pixel length).
3. Results
Seventeen patients (male/female 5/12; 42.8±9.5 years) and eight controls (male/female 4/4; 35.0±9.5 years) participated in the study. Patients and controls did not differ significantly on demographic measures. Responders (n =5) and nonresponders (n =12) did not differ on age, gender, or ethnicity. However, responders had significantly more years of education than nonresponders (t =10.6, df =1, P =0.000, between-groups t-test, 2-tailed). Interestingly, all five of the responders reported 16 years of education (see Table 1).
Table 1.
Demographic measures (percent or mean±S.D.)
| Patients (n =17) | Responders (n =5) | Nonresponders (n =12) | Controls (n =8) | |
|---|---|---|---|---|
| Female | 70.6% | 60.0% | 75.0% | 50% |
| Caucasian | 70.6% | 80.0% | 66.7% | 75% |
| Age | 42.4±9.5 | 43.4±6.1 | 42.0±10.8 | 35.0±9.5 |
| Years of education | 14.3±1.3 | 16.0±0.0* | 13.6±0.8* | 15.1±1.3 |
Responders are defined by having a decrease in the modified 17-item Hamilton Depression Rating Scale of at least 40% with sleep deprivation.
t =10.6, df =11, P =0.000 (between-groups t-test, 2-tailed).
Baseline HDRS-17 and HDRS-17-Mod did not differ between responders (e.g., patients with a decrease of at least 40% in the HDRS-17-Mod with PSD) and nonresponders. However, HDRS-17-Mod diverged with PSD (see Table 2). A group by time analysis of variance between these two groups and conditions revealed a significant interaction effect (F =11.0, df =1,15, P =0.005, η2=0.42, Table 2). Five of the seventeen patients were responders, e.g., patients whose HDRS-17-Mod decreased by 40% or more from baseline to PSD.
Table 2.
Modified Hamilton scoresa (mean±S.D.)
| Baseline | Sleep-deprived | |
|---|---|---|
| Responders (5) | 16.6±1.5 | 8.0±2.5 |
| Nonresponders (12) | 15.3±4.8 | 12.9±3.7 |
Responders are defined by having a decrease in the modified 17-item Hamilton Depression Rating Scale of at least 40% with sleep deprivation.
17-item Hamilton Depression Rating Scale omitting sleep and weight loss items.
3.1. ROI analyses
3.1.1. Baseline perfusion
In the one-way ANOVA, the group effect was significant for right amygdala (F =7.5, df =2,24, P =0.003); follow-up t-tests indicated that mean right amygdala perfusion was greater in responders than in either nonresponders (P =0.03) or controls (P =0.028) (Bonferroni correction). There was no significant group effect for left amygdala. However, in direct comparisons of responders with nonresponders, baseline perfusion was significantly greater in responders in the left (t =2.2, df =15, P < 0.048, η2=0.24) and also in the right (t =4.3, df =15, P < 0.001, η2=0.54) amygdala (Table 3).
Table 3.
Amygdala perfusion (mean±S.D.)
| Baseline
|
Sleep-deprived
|
BL − SD
|
||||
|---|---|---|---|---|---|---|
| Right | Left | Right | Left | Right | Left | |
| NCs (8) | 88.1±39.0 | 147.2±84.1 | 109.9±60.5 | 129.1±60.9 | − 21.9±45.6 | 18.1±66.1 |
| Pts (17) | 94.1±43.2 | 155.8±65.8 | 110.0±41.3 | 173.8±66.1 | − 11.7±34.1 | − 14.5±80.1 |
| Rs (5) | 142.1±43.1 | 203.9±56.4 | 130.4±45.7 | 203.4±65.7 | 11.8±16.7 | 0.5±86.0 |
| NRs (13) | 74.1±23.7 | 135.7±60.5 | 99.1±38.5 | 154.8±61.8 | − 25.0±35.2 | − 19.2±84.0 |
NC: normal control, Pts: patients, Rs: responders, NRs: nonresponders, BL: baseline, SD: sleep-deprived, BL − SD: baseline minus sleep-deprived. Values are in gray scale MR units.
3.1.2. Sleep deprivation
In the condition by hemisphere by group (responder vs. nonresponder) repeated-measures MAN-OVA, there were no significant interaction effects or significant effects for the sleep deprivation condition. However, the main effect for hemisphere was greater on the left than the right regardless of group or condition (F =22.37, df =1,15, P < 0.001). Moreover, the group effect was significant (F =11.71, df =1,15, P =0.003), with responders experiencing greater perfusion on both baseline and sleep-deprived days than nonresponders.
When directly compared, right amygdala change scores (calculated as baseline minus sleep-deprived perfusion) were significantly different between non-responders and responders (t =2.3, df =15, P < 0.033, η2=0.19, between-groups t-test, 2-tailed). In examinations of right amygdala perfusion means, responders appeared to decrease, whereas nonresponders increased. The nonresponders’ right amygdala perfusion significantly increased with PSD (t =− 2.6, df =11, P < 0.023, η2=0.38) and carried a similar trend (t =− 1.8, df =16, P < 0.098) in the major depression group as a whole (within-group t-test, 2-tailed). The left amygdala did not show any significant change between baseline and PSD conditions for any subject group.
3.2. Voxelwise analyses
3.2.1. Baseline
Cluster threshold adjusted t-test maps revealed small areas including the right amygdala in which responders’ perfusion was greater than that of nonresponders. No significant difference between responders and nonresponders was apparent in the left amygdala.
3.2.2. Sleep deprivation
Cluster threshold adjusted t-test maps revealed clusters involving both amygdalae (more pronounced on the right), which also showed a significant decrease from baseline to PSD specifically in responders (Fig. 2). Nonresponders and controls showed virtually no clusters in which perfusion changed significantly from baseline to PSD.
Fig. 2.
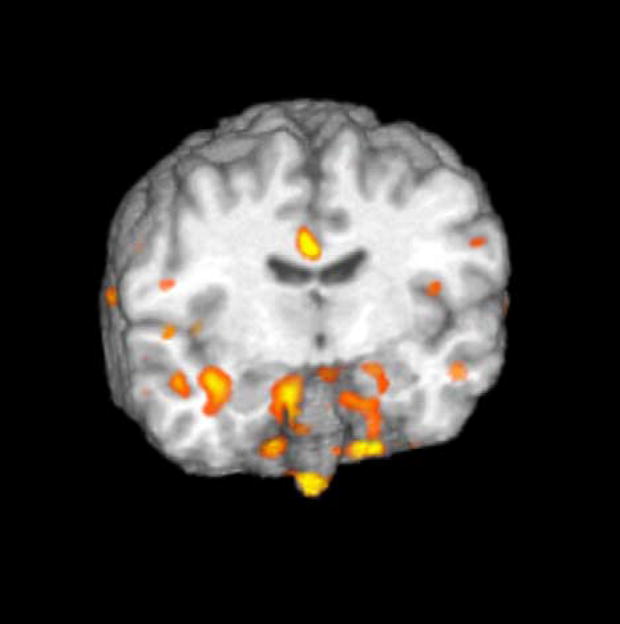
Areas including amygdalae in which responders’ perfusion decreased from baseline to sleep-deprived scans.
4. Discussion
Our most important findings from this study include greater baseline amygdalar perfusion in responders than nonresponders and differential amygdalar perfusion changes with SD between responders and nonresponders. Increased baseline perfusion in responders was apparent bilaterally in ROI analysis on between-group t-tests; however, only the stronger right amygdala difference was evident on voxelwise analysis. The MAN-OVA indicated that the larger perfusion values for the responders were maintained at follow-up. In response to PSD, responders showed clusters of significantly decreased perfusion in both amygdalae, although neither amygdala significantly changed based on ROI analysis. On the other hand, nonresponders significantly increased right amygdalar perfusion with PSD when ROI data were examined. Because of the relatively small subject numbers, particularly for responders, we examined Tukey’s box plots (Tukey, 1977) of all amygdalar ROI variables to verify that between-and within-group differences were not spurious results caused by statistical outliers.
A review of previous imaging studies of sleep in depression revealed higher left amygdala relative glucose metabolism in patients with major depression than healthy controls in waking and non-REM sleep (Nofzinger, 2004). In addition, the increase in glucose metabolism from waking to REM sleep was more pronounced in depressed patients than controls in a widespread area including left amygdala (Nofzinger et al., 1997). Taken together with reports of increased REM sleep in depression (Benca et al., 1992), these may contribute to greater waking amygdalar perfusion in some depressed patients.
Elevated baseline amygdalar perfusion in responders, like increased baseline activity elsewhere in the limbic system, is consistent with the overarousal hypothesis of SD (Gillin et al., 1995). In addition, our amygdalar findings may be related to the effects of depression itself on the amygdala. Drevets et al. (2002b) found increased left amygdalar glucose metabolic rate (compared with normal controls) in bipolar depressed patients and in unipolar major depression patients with positive family histories. They also reported positive correlations between left amygdalar glucose metabolic rate and stressed plasma cortisol levels in unmedicated patients with unipolar depression. In another study of unmedicated patients with major depression, right amygdalar metabolism correlated positively with the intensity of negative affect (Abercrombie et al., 1998).
Clusters in which responders’ perfusion significantly decreased with PSD in both amygdalae are consistent with the concept that SD “de-arouses” responders, as evidenced by decreased activity in other parts of the limbic system as well. Based on effect sizes, a larger group of responders may be needed to see a significant perfusion decrease in ROI analyses. It is also interesting that nonresponders, at least in ROI statistics, responded in the opposite way, with a significant between-group difference in right amygdala change score driven by the nonresponder group’s significant increase in amygdalar perfusion. This is consistent with previous functional imaging data (Wu et al., 1999; Gillin et al., 2001) suggesting that responders and nonresponders represent two distinct groups of depressed patients.
Other studies report decreasing left amygdalar activity with remission in patients with major depression. The increased left amygdalar response to masked, especially fearful, faces (as compared with controls) resolves with antidepressant medications (Sheline et al., 2001). In another fMRI study, the left amygdalar response to viewing sad faces diminished with antidepressant medication (Fu et al., 2004). Finally, in bipolar patients experiencing a major depressive episode, resting left amygdala CBF decreased with remission in patients taking mood stabilizers, but it remained elevated in those not on mood stabilizers (Drevets et al., 2002a). Despite the focus of these findings on the left amygdala, the variability in the laterality of amygdalar findings in normal controls (Phillips et al., 2001; Baas et al., 2004), as well as in major depression, its treatment, and remission (Abercrombie et al., 1998; Drevets et al., 2002b), precludes definitive conclusions about lateralized amygdalar perfusion in depression.
Our amygdalar findings are interesting in light of previous SD studies. In their initial PET study, Wu et al. (1992) reported greater bilateral amygdalar baseline metabolism, with no change in amygdalar metabolism with TSD in any group. However, a subsequent article (Wu et al., 1999) utilizing additional subjects did not report significant amygdalar findings. Most PET and SPECT studies of SD in depression that reported any temporal lobe findings (Ebert et al., 1991; Volk et al., 1992; Kaendler et al., 1993), involved template-based ROIs or manually placed rectangular ROIs in predefined levels axially. In some cases (Volk et al., 1992; Kaendler et al., 1993), these ROIs did not permit any conclusions at all specific to amygdalar function. Ebert et al. (1991) found greater baseline infratemporal hexamethylpropyleneamine oxime (HMPAO) activity bilaterally in responders than nonresponders, with responders uniquely showing decreased bilateral infratemporal HMPAO activity after TSD. They reported an area of greater baseline activity in responders than controls in an area including right amygdala and portions of hippocampus and parahippocampus. In the responder group only, HMPAO activity decreased after TSD in the right amygdala/hippocampal/parahippocampal area.
It is possible that the relative paucity of significant amygdalar findings in SD studies of depression reflect spatial resolution limitations of PET and SPECT and/or different ROI implementation methods, none of which utilized hand tracing based on structural MRI data.
We also considered whether differences in imaging conditions might explain why our amygdalar findings have not previously been reported. However, previous studies of the antidepressant response to SD have imaged subjects during resting conditions (e.g., eyes closed or looking at a white screen in a quiet room) (Ebert et al., 1991; Volk et al., 1992) or performing a visual version of the Continuous Performance Task (Wu et al., 1992; Clark et al., 1998). In our study, subjects were imaged while applying gentle tonic pressure to a soft rubber bulb connected to a pressure transducer and computer monitor. (Continuous visualization of the computer monitor during scanning allowed us to verify that subjects maintained wakefulness at this time.) None of these conditions would be considered affective tasks in any way.
In summary, our findings are consistent with the overarousal hypothesis of depression and with the amygdala’s key role in major depression (Whalen et al., 2002). Interestingly, our amygdalar findings of increased baseline bilateral perfusion in responders (by ROI and voxelwise analyses) parallel our left ventral anterior cingulate findings (Clark et al., submitted for publication).
Further study, perhaps using an emotional “challenge” such as presentation of affective stimuli in baseline and sleep-deprived conditions, will be necessary to delineate the relationship between the amygdala’s role in depression and its activity in association with the antidepressant response to SD.
Acknowledgments
This work was supported by 5 K08 MH01642, M01RR00827, and the VISN 22 Mental Illness, Research, Education and Clinical Center. We thank Lesley Wetherell, Anna Demodena, and Dexter Walpole for their help in conducting this study.
References
- Abercrombie HC, Schaefer SM, Larson CL, Oakes TR, Lindgren KA, Holden JE, Perlman SB, Turski PA, Krahn DD, Benca RM, Davidson RJ. Metabolic rate in the right amygdala predicts negative affect in depressed patients. NeuroReport. 1998;9:3301–3307. doi: 10.1097/00001756-199810050-00028. [DOI] [PubMed] [Google Scholar]
- Baas D, Aleman A, Kahn RS. Lateralization of amygdala activation: a systematic review of functional neuroimaging studies. Brain Research Reviews. 2004;45:96–103. doi: 10.1016/j.brainresrev.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Benca RM. Sleep in psychiatric disorders. Neurologic Clinics. 1996;14:739–764. doi: 10.1016/s0733-8619(05)70283-8. [DOI] [PubMed] [Google Scholar]
- Benca RM, Obermeyer WH, Thisted RA, Gillin JC. Sleep and psychiatric disorders: a meta-analysis. Archives of General Psychiatry. 1992;49:651–668. doi: 10.1001/archpsyc.1992.01820080059010. [DOI] [PubMed] [Google Scholar]
- Bench CJ, Friston KJ, Brown RG, Scott LC, Frackowiak RSJ, Dolan RJ. The anatomy of melancholia—focal abnormalities of cerebral blood flow in major depression. Psychological Medicine. 1992;22:607–615. doi: 10.1017/s003329170003806x. [DOI] [PubMed] [Google Scholar]
- Bondi MW, Houston WS, Eyler LT, Brown GG. fMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer’s disease. Neurology. 2005;64:501–508. doi: 10.1212/01.WNL.0000150885.00929.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchsbaum MS, Wu JC, Siegel BW, Hackett E, Trenary M, Abel L, Reynolds C. Effect of sertraline on regional metabolic rate in patients with affective disorders. Biological Psychiatry. 1997;41:15–22. doi: 10.1016/s0006-3223(96)00097-2. [DOI] [PubMed] [Google Scholar]
- Clark C, Dupont R, Lehr P, Yeung D, Halpern S, Golshan S, Gillin JC. Is there a relationship between delta sleep at night and afternoon cerebral blood flow, assessed by HMPAO-SPECT, in depressed patients and normal controls? Preliminary data. Psychiatry Research: Neuroimaging. 1998;84:89–99. doi: 10.1016/s0925-4927(98)00049-3. [DOI] [PubMed] [Google Scholar]
- Clark CP, Gillin JC, Golshan S, Demodena A, Smith TL, Danowski S, Irwin M, Schuckit M. Polysomnography and depressive symptoms in primary alcoholics with and without a lifetime diagnosis of secondary depression and in patients with primary major depression. Journal of Affective Disorders. 1999;52:177–185. doi: 10.1016/s0165-0327(98)00078-0. [DOI] [PubMed] [Google Scholar]
- Clark CP, Frank LR, Brown GG. Sleep deprivation, EEG, and functional MRI in depression: preliminary results. Neuropsychopharmacology. 2001;25:S79–S84. doi: 10.1016/S0893-133X(01)00324-4. [DOI] [PubMed] [Google Scholar]
- Clark CP, Brown GG, Frank L, Thomas L, Sutherland AN, Gillin JC. Improved anatomic delineation of the antidepressant response to partial sleep deprivation in medial frontal cortex using perfusion-weighted functional MRI. Psychiatric Research: Neuroimaging. doi: 10.1016/j.pscychresns.2005.12.008. submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis: I. Segmentation and surface reconstruction. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Irwin W, Anderle MJ, Kalin NH. The neural substrates of affective processing in depressed patients treated with venlafaxine. American Journal of Psychiatry. 2003;160:64–75. doi: 10.1176/appi.ajp.160.1.64. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Bogers W, Raichle ME. Functional anatomical correlates of antidepressant drug treatment assessed using PET measures of regional glucose metabolism. European Neuropsychopharmacology. 2002a;12:527–544. doi: 10.1016/s0924-977x(02)00102-5. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Bardgett ME, Reich T, Todd RD, Raichle ME. Glucose metabolism in the amygdala in depression: relationship to diagnostic subtype and plasma cortisol levels. Pharmacology, Biochemistry and Behavior. 2002b;71:431–447. doi: 10.1016/s0091-3057(01)00687-6. [DOI] [PubMed] [Google Scholar]
- Ebert D, Feistel H, Barocka A. Effects of sleep deprivation on the limbic system and the frontal lobes in affective disorders: a study with Tc-99m-HMPAO SPECT. Psychiatry Research: Neuroimaging. 1991;40:247–251. doi: 10.1016/0925-4927(91)90016-j. [DOI] [PubMed] [Google Scholar]
- Ebert D, Feistel H, Barocka A, Kaschka W. Increased limbic blood flow and total sleep deprivation in major depression with melancholia. Psychiatry Research: Neuroimaging. 1994;55:101–109. doi: 10.1016/0925-4927(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Elsenga S, van den Hoofdakker R. Body core temperature and depression during total sleep deprivation in depressives. Biological Psychiatry. 1988;24:531–540. doi: 10.1016/0006-3223(88)90164-3. [DOI] [PubMed] [Google Scholar]
- Fennema-Notestine C, Ozyurt I, Clark CP, Morris S, Bischoff-Grethe A, Bondi MW, Jernigan TL, Fischl B, Segonne F, Shattuck DW, Leahy RM, Rex DE, Toga AW, Zou KH, Morphometry BIRN, Brown GG. Quantitative evaluation of automated skull-stripping methods applied to contemporary and legacy images: effects of diagnosis, bias correction, and slice location. Human Brain Mapping. 2005 doi: 10.1002/hbm.20161. June 28 [Electronic publication ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Williams JBW. Structured Clinical Interview for DSM-IVAxis I Disorders-Patient Edition (SCID-I/P, Version 2.0) Biometrics Research Department, New York State Psychiatric Institute; New York, NY: 1996. [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Jezzard P, Turner R. Analysis of fMRI time-series. Human Brain Mapping. 1994;1:153–171. [Google Scholar]
- Fu CH, Williams SC, Cleare AJ, Brammer MJ, Walsh ND, Kim J, Andrew CM, Pich EM, Williams PM, Reed LJ, Mitterschiffthaler MT, Suckling J, Bullmore ET. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Archives of General Psychiatry. 2004;61:877–889. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- Gillin JC, Ho AP, Buchsbaum MS, Wu J, Abel L, Bunney WE., Jr Functional brain imaging, sleep, and sleep deprivation: contributions to the “overarousal” hypothesis of depression. Acta Neuropsychiatrica. 1995;7:33–34. doi: 10.1017/S0924270800037492. [DOI] [PubMed] [Google Scholar]
- Gillin JC, Buchsbaum M, Wu J, Clark C, Bunney W., Jr Sleep deprivation as a model experimental antidepressant treatment: findings from functional brain imaging. Depression and Anxiety. 2001;14:37–49. doi: 10.1002/da.1045. [DOI] [PubMed] [Google Scholar]
- Hahn H, Peitgen HO. The skull stripping problem in MRI solved by a single 3D watershed transform. Paper presented at the Proceedings of the MICCAI, LNCS. 2000;1935:134–143. [Google Scholar]
- Hoddes E, Zarcone V, Smyth H. Quantification of sleepiness: a new approach. Psychophysiology. 1973;10:431–436. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J, Hesselink JR. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiology of Aging. 2001;22:581–594. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- Kaendler SH, Volk S, Maul FD, Weber R, Georgi K, Hertel A, Pflug B, Hor G. In: Evaluation of Total Sleep Deprivation by Single Photon Emission Computerized Tomography. Maurer K, editor. Springer-Verlag; Berlin: 1993. pp. 115–120. [Google Scholar]
- Li T-Q, Takahashi A, Moseley ME, Glover GH. A single-shot dual-echo spiral FAIR sequence for simultaneous measurements of CBF and oxygenation in fMRI. Paper presented at the International Society for Magnetic Resonance in Medicine, 1731.1999. [Google Scholar]
- Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. Journal of Neuropsychiatry. 1997;9:1–11. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, Silva A, McGinnis S, Glass TG, Martin CC, Fox PT. Cingulate function in depression: a potential predictor of treatment response. NeuroReport. 1997;8:1057–1061. doi: 10.1097/00001756-199703030-00048. [DOI] [PubMed] [Google Scholar]
- Nofzinger EA. What can neuroimaging findings tell us about sleep disorders? Sleep Medicine. 2004;5(Supplement 1):S16–S22. doi: 10.1016/s1389-9457(04)90003-2. [DOI] [PubMed] [Google Scholar]
- Nofzinger EA, Mintun MA, Wiseman MB, Kupfer DJ, Moore RY. Forebrain activation in REM sleep: an FDG PET study. Brain Research. 1997;770:192–201. doi: 10.1016/s0006-8993(97)00807-x. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Medford N, Young AW, Williams L, Williams SC, Bullmore ET, Gray JA, Brammer MJ. Time courses of left and right amygdalar responses to fearful facial expressions. Human Brain Mapping. 2001;12:193–202. doi: 10.1002/1097-0193(200104)12:4<193::AID-HBM1015>3.0.CO;2-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy-Byrne PG, Uhde TW, Post RM. In: Antidepressant Effects of One Night’s Sleep Deprivation: Clinical and Theoretical Implications. Post RM, Ballenger J, editors. William and Wilkins; Baltimore: 1984. pp. 817–835. [Google Scholar]
- Sadeh A, Sharkey K, Carskadon M. Activity-based sleep–wake identification: an empirical test of methodological issues. Sleep. 1994;17:201–207. doi: 10.1093/sleep/17.3.201. [DOI] [PubMed] [Google Scholar]
- Scott AIF, Dougall N, Ross M, O’Carroll RE, Riddle W, Ebmeier KP, Goodwin GM. Short-term effects of electroconvulsive treatment on the uptake of 99mTc-exametazime into brain in major depression shown with single photon emission tomography. Journal of Affective Disorders. 1994;30:27–34. doi: 10.1016/0165-0327(94)90148-1. [DOI] [PubMed] [Google Scholar]
- Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B. A hybrid approach to the skull stripping problem in MRI. NeuroImage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with anti-depressant treatment: an fMRI study. Biological Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Transactions on Medical Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Szuba MP, Baxter LR, Jr, Fairbanks LA, Guze BH, Schwartz JM. Effects of partial sleep deprivation on the diurnal variation of mood and motor activity in major depression. Biological Psychiatry. 1991;30:817–829. doi: 10.1016/0006-3223(91)90237-g. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. Thieme Medical Publishers; New York: 1988. [Google Scholar]
- Tukey JW. Exploratory Data Analysis. Addison-Wesley; Reading, MA: 1977. [Google Scholar]
- Van Den Burg W, Beersma DGM, Bouhuys AL, Van Den Hoofdakker RH. Self-rated arousal concurrent with the antidepressant response to total sleep deprivation of patients with a major depressive disorder: a disinhibition hypothesis. Journal of Sleep Research. 1992;1:211–222. doi: 10.1111/j.1365-2869.1992.tb00042.x. [DOI] [PubMed] [Google Scholar]
- Volk S, Kaendler SH, Weber R, Georgi K, Maul F, Hertel A, Pflug B, Hoer G. Evaluation of the effects of total sleep deprivation on cerebral blood flow using single photon emission computerized tomography. Acta Psychiatrica Scandinavica. 1992;86:478–483. doi: 10.1111/j.1600-0447.1992.tb03301.x. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Shin LM, Somerville LH, McLean AA, Kim H. Functional neuroimaging studies of the amygdala in depression. Seminars in Clinical Neuropsychiatry. 2002;7:234–242. doi: 10.1053/scnp.2002.35219. [DOI] [PubMed] [Google Scholar]
- Wong EC, Buxton RB, Frank LR. Implementation of quantitative perfusion imaging techniques for functional brain mapping using pulsed arterial spin labeling. NMR in Biomedicine. 1997;10:237–249. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<237::aid-nbm475>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Wong EC, Buxton RB, Frank LR. A theoretical and experimental comparison of continuous and pulsed arterial spin labeling techniques for quantitative perfusion imaging. Magnetic Resonance in Medicine. 1998a;40:348–355. doi: 10.1002/mrm.1910400303. [DOI] [PubMed] [Google Scholar]
- Wong EC, Buxton RB, Frank LR. Quantitative imaging of perfusion using a single subtraction (QUIPSS and QUIPSS II) Magnetic Resonance in Medicine. 1998b;39:702–708. doi: 10.1002/mrm.1910390506. [DOI] [PubMed] [Google Scholar]
- Wu J, Buchsbaum MS, Gillin JC, Tang C, Cadwell S, Keator D, Fallon JH, Wiegand M, Najafi A, Klein E, Hazen K, Bunney WE., Jr Prediction of antidepressant effects of sleep deprivation by metabolic rates in the ventral anterior cingulate and medial prefrontal cortex. American Journal of Psychiatry. 1999;156:1149–1158. doi: 10.1176/ajp.156.8.1149. [DOI] [PubMed] [Google Scholar]
- Wu JC, Bunney WE., Jr The biological basis of an antidepressant response to sleep deprivation and relapse: review and hypothesis. American Journal of Psychiatry. 1990;147:14–21. doi: 10.1176/ajp.147.1.14. [DOI] [PubMed] [Google Scholar]
- Wu JC, Gillin JC. Elevated cingulate cortex in subtype of depression which improves with sleep deprivation. Sleep Research. 1992;21:326. [Google Scholar]
- Wu JC, Gillin JC, Buchsbaum MS, Hershey T, Johnson JC, Bunney WE., Jr Effect of sleep deprivation on brain metabolism of depressed patients. American Journal of Psychiatry. 1992;149:538–543. doi: 10.1176/ajp.149.4.538. [DOI] [PubMed] [Google Scholar]


