Abstract
The purpose of this study was to examine the phonatory characteristics of pig, sheep, and cow excised larynges and to find out which of these animal species is the best model for human phonation. Excised pig, sheep, and cow larynges were prepared and mounted over a tapered tube on the excised bench that supplied pressurized, heated, and humidified air in a manner similar to that for excised canine models. Each excised larynx was subjected to a series of pressure-flow experiments with adduction as major control parameter. The subglottal pressure, electroglottograph (EGG), mean flow rate, audio signal, and sound pressure level were recorded during each experiment. EGG signal was used to extract the fundamental frequency. It was found that pressure-frequency relations were nonlinear for these species with large rate of frequency changes for the pig. The average oscillation frequencies for these species were 220±57 Hz for the pig, 102±33 Hz for the sheep, and 73±10 Hz for the cow. The average phonation threshold pressure for the pig was 7.4±2.0 cm H2O, 6.9±2.9 cm H2O for the sheep, and 4.4±2.3 cm H2O for the cow.
INTRODUCTION
Phonatory models are important tools that help in the systematic study of phonation. Biomechanical aspects of phonatory models are best described by animal larynges, whether in vivo or when mounted and oscillated on the laboratory bench. The major advantages of these models are their capability for self-oscillation and easy access for manipulation of the control parameters. The closer the geometric and morphological similarities between these animal models and the human larynx, the better the results can be applied and interpreted for human phonation. A widely used animal model has been the canine larynx because of its geometric similarities to human larynx. It has been extensively used to study many aspects of phonation either in vivo or with excised models. For example, Koyama et al. (1969, 1971) used in vivo canine models to investigate the mechanics of voice production including regulation of pitch and intensity. Berke et al., (1989a, 1989b) also used in vivo canine models to study the effect of recurrent laryngeal nerve and superior laryngeal nerve stimulation on the phonatory characteristics. Others have used excised canine larynges to study the phonatory physiology. Slavit et al. (1990) studied the effects of vocal fold tension and elongation on glottographic waveforms by using the excised canine larynx model. Alipour et al. (1997) used excised canine larynges to study the effects of adduction on laryngeal aerodynamics. Also, recently, the excised canine models have helped to quantify the aerodynamic and acoustic effects of the false folds and epiglottis [Alipour et al. (2007)] and pressure-frequency relations during phonation [Alipour and Scherer (2007)].
Besides the canine larynx, pig, sheep, and cow larynges may serve as other alternatives for phonatory models. Comparative anatomy of canine, pig, sheep, and human larynges [Kurita et al. (1983); Jiang et al. (2001); Hahn et al. (2005); Hahn et al., (2006a, 2000b)] has shown that the three animal species have certain similarities in their basic structures to human larynx as well as differences that would account for the differences in phonatory productions.
Jiang et al. (2001) reported anatomical measurements from pig, deer, dog, and human excised larynges. The pig larynges had larger thyroid cartilages than the dogs, but the size of the cricothyroid muscle was greater in the dogs than the pigs. They defined the vocal fold height as the perpendicular distance from the axis of rotation of cricothyroid (CT) joint to the longitudinal axis of the vocal fold, which they also referred to as an index of the mechanical advantage of the thyroid cartilage for the purpose of phonation. This vocal fold height and the range of rotation of the CT joint were found to be comparable in all the three species, and the authors concluded that the mechanical advantage for vocal fold lengthening was similar in these species. They concluded that from the structural perspective, the pig larynx is a superior model.
Kim et al. (2004) compared the laryngeal dimensions of sheep, dog, and human excised larynges. The ovine laryngeal height was significantly greater than those in dogs and humans due to its vertically elongated thyroid cartilage. In sheep, the CT gap was considerably smaller, suggesting a smaller range of fundamental frequency control in sheep (as the cricoid-thyroid rotation angle would be smaller as well because of the larger cricoid dimensions). The sheep larynx also lacked well-defined ventricles and vocal fold boundaries. They asserted that while canine larynx measurements were within the range of those of human larynges, the ovine larynx showed distinctive differences.
From a histological point of view, the animal species had a two-layered lamina propria structure unlike the three-layered structure in the humans [Kurita et al. 1983]. The pig vocal fold was assumed to have a greater resemblance to the human vocal folds [Jiang et al. (2001)] because of the similarity in mucosal thickness (0.9 mm compared to 1.1 mm in the human). Also, intrinsic laryngeal muscles of the pig were found to be similar to human in terms of their origins and insertions [Knight et al. (2005)]. Sheep laryngeal muscles had fiber types and composition comparable to humans [Happak et al. (1989); Zrunek et al. (1988)] and were therefore deemed as one of the models for physiological studies of phonation.
The geometrical differences between porcine, bovine, and ovine larynx compared to the canine and human larynx were not only in the laryngeal and vocal fold dimensions but also in certain protective modifications in the supraglottic structures. Unlike the canine, these species are herbivorous and bear a large epiglottis and arytenoids with high lateral walls that enable them to breathe and swallow at the same time [Harrison (1995)]. Consequently, there exists a long supraglottic duct about 1–2 in. long, that is formed between their larger arytenoids and supraglottic thyroid wall. The wall of this duct in these three species participates in the vocal fold oscillations in certain conditions and has influence on the overall acoustics of phonation. This functional difference may cause some limitation to the applicability and comparison of their phonatory data to human phonation.
There were some similarities and differences in the molecular composition and stiffness characteristics of the vocal folds as well. In the human vocal folds, the density of the collagen fibers progressively increased in the direction of the vocalis muscle laterally. The density of elastic fibers was greatest in the intermediate layer of the lamina propria and gradually decreased in the superficial and deep layers. Kurita et al. (1983) did a cross-species comparison of thickness of the mucosa and the density of collagen and elastic fibers in the lamina propria of the vocal folds. The vocal folds from larynges of dogs, pigs, sheep, and humans were sectioned, stained, and examined under a microscope. In contrast to the canine and sheep vocal folds, the pig larynx demonstrated the humanlike superficial layer with sparse fibrous components, with increasing collagenous fibers and decreasing elastic fibers close to the vocalis muscle. Hahn et al. (2005, 2006a, and 2006b) did a more quantitative immunohistochemical analyses to examine the collagen, elastin, and proteoglycan (PG) and associated glycosaminoglycan (GAG) content of the midmembrane vocal fold lamina propria (LP) in humans, dogs, and pigs. The comparison of LP distribution of specific PG∕GAG indicated that the canine LP subdivisions did not fit into the laminar arrangement characteristic of the human LP which may be indicative of the differences in the functional requirements.
Phonatory models require vocal fold dimensions and their viscoelastic properties for comparison to human subjects. Majority of these data were collected in the past on the canine tissues. Our preliminary studies of the vocal fold elastic properties of the pig, sheep, and cow [Alipour and Jaiswal (2006)] indicated significant differences between elastic properties among these species. The mean longitudinal Young’s modulus for the superior vocal folds of the porcine larynx was 20.2 kPa that was significantly larger than its inferior vocal fold Young’s modulus of 16.9 kPa (four samples, 18 cases, p=0.022). The bovine samples had a higher modulus value of 29.9 kPa (three samples, nine cases) but were also longer and thicker. The sheep vocal folds were more pliable with a value of 17.6 kPa (four samples, 27 cases). Although the number of samples were small, these preliminary data suggest that the fundamental frequency of canine vocal fold with low strain Young’s modulus of about 42 kPa has higher frequency sensitivity to elongation than pig vocal folds. Further investigation into the elastic properties of these vocal folds may be needed for a better comparison.
Interspecies comparison of phonatory characteristics is of interest as the similarities and differences across species allow for greater understanding of phonatory physiology. In addition, the elucidation of phonatory characteristics helps in the selection of appropriate model for experimentation. It also furthers the knowledge regarding phonatory modifications and its evolutionary bases. Despite the wide usage of pig, sheep, and cow larynges in laryngeal muscle physiology and comparative histology studies [Kurita et al. (1983); Happak et al. (1989); Zrunek et al. (1988)], these species have not been extensively used in phonation study. Scherer et al. (1985) used the bovine excised larynx model to measure contact pressure during phonation because of its large vocal folds and its stable oscillations. In a previous study, Alipour and Jaiswal (in press) quantified the glottal flow resistance in the pig, sheep, and cow excised larynges. They found that unlike the canine larynges, these species had nonlinear pressure-flow relations and oscillated in different ranges of frequencies. Cow larynx with its larger dimensions had the lowest oscillation range and the pig larynx had the highest range. Thus, this paper is a follow-up to the earlier work. The purpose of this study was to investigate phonatory characteristics of these species, to examine the relation between subglottal pressure and the fundamental frequency of vocal fold vibration, and to find out which of these animal species is the best model for human phonation. This was accomplished by measuring and reporting pressure-flow sweep data and comparing vocal fold vibration characteristics across the species.
METHODOLOGY
Larynges of pig (N=8), sheep (N=8), and cow (N=6) were procured from a local butcher shop. They were cleaned, and extraneous tissue and muscles were dissected and removed. Since the larynges were initially slow frozen at the butcher shop, the prepared tissue was slow frozen following the cleaning. They were packaged in plastic bags and stored in the freezer at −20 °C for a few days to a few weeks. Prior to the experiment, the specimen was thawed overnight in saline solution and mounted on a base (by the trachea) for better handling. The epiglottis was dissected out, and the resulting free ends of the lateral wall were sutured to the thyroid cartilage along its superior margin to prevent any interference with the experimental conditions. Sutures were placed on the larynges to stabilize the structures and simulate different degrees of adduction. Electrode plates from an electroglottograph (EGG) device (Synchrovoice) were placed on either side of the thyroid laminae to obtain the EGG signal during phonation. The EGG signal was later used to extract fundamental frequency.
Air from the building pipeline first entered a desiccating air filter (Devilbiss DEVDAD500) to remove dirt and water content, then passed through an in-line flow meter (Gilmont rotameter model J197) for airflow rate monitoring and a pneumatic flow meter (Rudolph 4700). The filtered air was then heated and humidified to about 37 °C and 100% humidity (ConchaTherm III, Hudson RCI) and entered the larynx via appropriately tapered tubing. A pressure tap located about 10 cm below the larynx was used to monitor the subglottal pressure through a well-type manometer (Dwyer model 1230-8).
Adduction was manipulated by simulating the action of the lateral cricoarytenoid muscle with sutures that medialized the vocal processes of the arytenoid cartilages. The sutures were placed on the muscular processes on either side, coursed parallel to the wall lateral to the vocal folds, and anteriorly emerging through the thyroid lamina. When the sutures were anteriorly pulled by addition of graded weights, the muscular process was rotated and consequently, the vocal processes medially moved, thereby increasing the degree of vocal fold adduction. Depending on the size of each larynx, adduction weights ranged from 20 to 200 g for the sheep, 50–300 g for the pig, and 200–1200 g for the cow larynges to provide graded low to high adductions levels. Each experiment started with one upward and one downward pressure-flow sweep of 20 s duration that determined the operational range of pressure, flow, and frequency for each larynx. The main control parameters were the levels of adduction and subglottal pressure. Due to the major differences in the dimensions and the angles of the vocal folds in these species, elongation was not considered in this study.
The time-varying subglottal pressure was recorded using a pressure transducer (Microswitch 136PC01G1) mounted in the tracheal tube across from the manometer pressure tap. The time-varying flow rate was recorded with a pneumatic flow meter and low-range pressure transducer (Validyne DP103) upstream of the humidifier. The sound intensity was measured with a sound level meter (Extec model 407738), placed 10–15 cm from the larynx. Analog signals from the EGG, microphone, pressure, and flow transducers were simultaneously recorded on a Sony SIR1000 digital tape recorder at a sampling rate of 40 kHz per channel and directly onto a computer using a DATAQ A∕D converter and WINDAQ software. The EGG signal and subglottal pressure were low-pass filtered at 500 Hz and monitored on a digital oscilloscope (Tektronix, TDS2014). The oscilloscope was set to show few cycles and the signal frequency. The superior view of the vocal fold oscillation was monitored with a stroboscopic light source on a television screen while it was video taped (Phaser Strobe, Monarch Instruments).
For each excised larynx, the experiment started with two pressure-flow sweeps (upward and downward) for low, medium, and high adduction levels. Then, a series of sustained oscillation runs were made within the working range of pressure and flow to record and observe oscillation of vocal folds in slow motion visualized with strobe light. For some of the larynges, instead of adding graduated weights for discrete adduction levels, the adduction suture was attached to a micrometer with an attached strain gauge to measure the adduction force (in grams). The manipulation of the micrometer toward or away from the larynx generated continuous adduction variations in the form of adduction sweeps and was measured as adduction force on the strain gauge.
The recorded signals were then converted to physical values with MATLAB™ software routines and used for the aerodynamic and acoustic analyses. Mean values of subglottal pressure, flow rate, pressure amplitude, and fundamental frequency were calculated during each sweep in the following manner. First the highest value of fundamental frequency was estimated from the spectrogram of the EGG signal. With this period a time segment that could include 20 cycles was estimated. Then, the duration of each sweep was divided into these segments, and the mean subglottal pressure and mean flow rate were calculated for each segment. To calculate the fundamental frequency, the EGG signal was low-pass filtered at 150% of the previously estimated frequency. The fundamental frequency was calculated with a zero crossing method from the filtered EGG signal. In zero crossing method, first the signal dc offset was removed and then periods of all the cycles in the selected segment were calculated from consecutive zero crossings and averaged. Due to possible variation in the fundamental frequency during a sweep, the number of cycles in each segment could vary a few cycles above or below 20.
Once data were calibrated to their physical quantities in MATLAB environment, the mean values of major aerodynamic and acoustic information such as subglottal pressure, flow rate, glottal flow resistance, fundamental frequency, and pressure amplitudes were calculated and exported to EXCEL worksheets. Then statistical analysis was applied to these calculated mean values through the t-test for independent samples by variables in Basic Statistics of the STATISTICA package. Using a criteria of p=0.05, parameters were compared for significant differences. Also, whenever a correlation was established between two variables, its correlation coefficient (R2) was calculated.
RESULTS
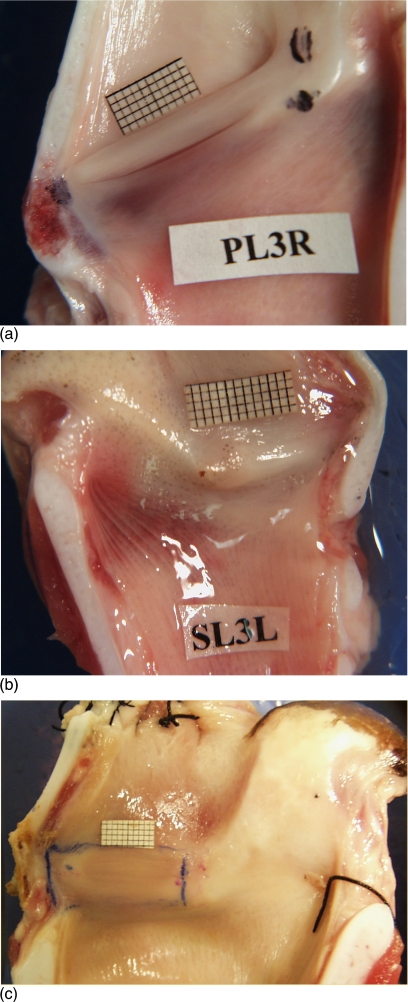
Figure 1a shows the internal cross-sectional view of the right half of a pig larynx. The pig larynx has a narrow slitlike, long ventricle that separates its vocal folds with well-differentiated boundaries. The two vocal folds are slanted at an angle of about 40° with the posterior end raised more than the anterior. The inferior vocal folds have a thinner mucosal cover with reddish muscular tissue visible underneath, especially in its inferior edge. The superior vocal folds are more distinct, usually 28±2 mm long with a thickness of about 3 mm. Video observation indicates that superior vocal fold is an active oscillator, which covers the inferior folds from top view. Unlike the canine and human larynges, this oscillator appears to differently behave from the false vocal fold because of its higher Young’s modulus than the inferior vocal folds. The elastic modulus for the false vocal folds measured for human larynx is reported to be much less than those of the true vocal folds [Chan et al. (2006)].
Figure 1.
(Color online) (a) Pig hemilarynx with a millimeter grid place above the superior fold. A large, modified arytenoid is attached to the superior and inferior folds on the upper right. A narrow ventricle separates the two folds. The vertically angled position of the vocal folds can be seen. (b) Sheep hemilarynx showing the padlike small vocal folds. (c) Cow hemilarynx showing the padlike large vocal folds.
Figures 1b, 1c show the internal cross-sectional view of the sheep and cow larynges, respectively. Sheep vocal folds are soft and pliable with an average length of 17.2±2 mm and thickness of 6 mm. Unlike the pig, sheep and cow lack a ventricle and their vocal folds are padlike with no sharp boundaries. The cow vocal folds are longer and stiffer with an average length of 37±0.7 mm and a thickness of about 18 mm. Observation of the superior view of these larynges indicates that oscillation in these two species generates large mucosal waves that travel both in vertical and horizontal directions.
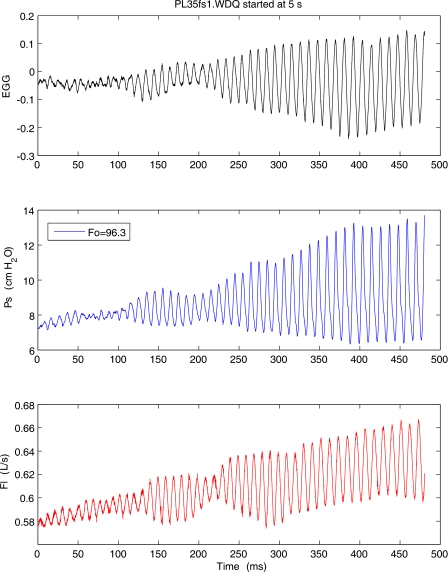
Figure 2 shows the beginning of typically recorded sweep for pig larynx, including from top to bottom are the following signals: EGG, subglottal pressure (Ps), and flow rate signal (F). These data correspond to oscillations at 96.3 Hz. The EGG data indicate some irregularity of oscillation that was typical of pig excised larynx. The fundamental frequency and pressure amplitude for each segment are calculated, such that each sweep generates a graph of glottal parameters such as the mean pressure, flow rate, fundamental frequency, and pressure amplitude as a function of time. The beginning upward sweep and the end of downward sweep data were used to calculate the onset and offset phonation threshold pressures (PTPs). These calculated PTP values complement their manually recorded values.
Figure 2.
(Color online) Initial portion of the pressure-flow sweep, including (from top to bottom) electroglottograph (EGG), subglottal pressure (Ps), and flow rate (Flow) signals.
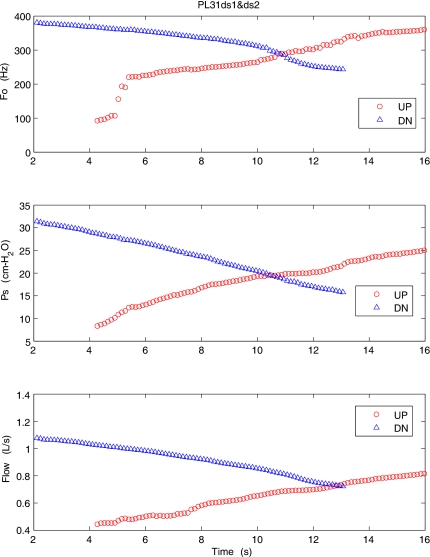
Figure 3 shows the variation of mean glottal parameters for a pig excised larynx (PL31) during an upward sweep (UP) and downward sweep (DN) for high adduction. The top graph shows the fundamental frequency (F0), the second graph shows the subglottal pressure (Ps), and the third graph is the mean flow rate (Flow) variation with time. For this pressure-flow sweep, the onset PTP was about 9 cm H2O and the maximum sound pressure level (SPL) at a distance of 15 cm from the larynx was 82.5 dB. During the upward sweep the mean fundamental frequency shows a sudden, rapid jump of about one octave and continues to rise to over 360 Hz. A mode change was observed at this point with an increase in audio pitch and sudden change in the oscillation amplitude observed in the video image from above. The downward sweep shows the decreasing trend across all the three parameters, though it did not reduce to the lower frequency range observed during the upward sweep. Pig larynges were usually found to oscillate with large amplitudes and loud SPLs.
Figure 3.
(Color online) Mean values of the fundamental frequency, subglottal pressure, and flow rate for an excised pig larynx during the upward (○) and downward (△) pressure-flow sweeps at high adduction level.
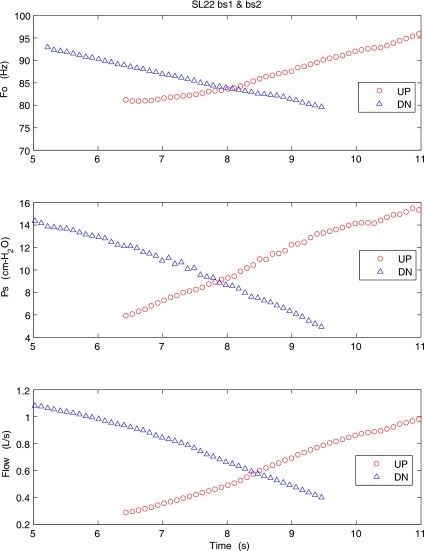
In a similar experiment, a sheep excised larynx (SL22) was subjected to the pressure-flow sweeps at high adduction (Fig. 4). All three mean values of glottal parameters (F0, Ps, and Flow) show monotonic changes with time either in upward sweep or downward sweep. This sheep larynx started to oscillate at about 6 cm H2O and ended at about 5 cm H2O. The maximum SPL at a distance of 15 cm from the larynx was 76.4 dB. In comparison to the previous case of pig larynx, this sheep larynx oscillated at lower ranges of subglottal pressure and fundamental frequency, but flow rates were comparable.
Figure 4.
(Color online) Mean values of the fundamental frequency, subglottal pressure, and flow rate for an excised sheep larynx during the upward (○) and downward (△) pressure-flow sweeps at high adduction level.
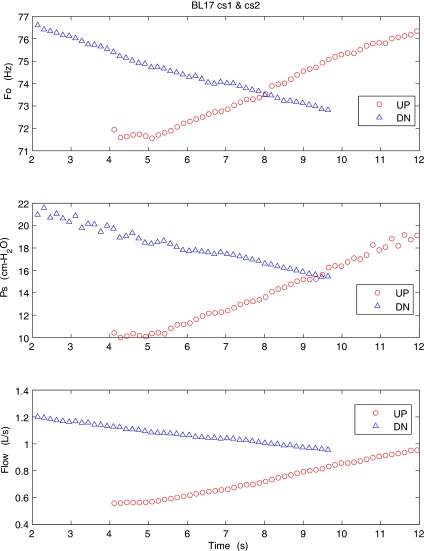
Figure 5 shows a similar pressure-flow sweeps in a cow excised larynx (BL17) at high adduction level. The maximum SPL at a distance of 15 cm from the larynx was 82.7 dB. The major difference observed in the cow oscillation is the smaller change in the fundamental frequency (about 9%, 4–5 Hz) from the beginning to the end of sweep. The cow excised larynx, thus, consistently showed sustained oscillation with steady frequency that did not drastically change with pressure variations. The typical frequency of the cow larynx oscillation was about 74 Hz. However, there were conditions in which the larynx initially oscillated at higher rate, and once the subglottal pressure increased, the frequency dropped to a lower value. The video observations of the oscillation with stroboscopic light indicated that at higher frequencies, only a segment of total length of the vocal fold oscillated with low amplitude, but once the subglottal pressure was increased, a greater portion or even the entire length of the vocal folds was set into large amplitude oscillation, resulting in the fundamental frequency drop.
Figure 5.
(Color online) Mean values of the fundamental frequency, subglottal pressure, and flow rate for an excised cow larynx during the upward (○) and downward (△) pressure-flow sweeps at high adduction level.
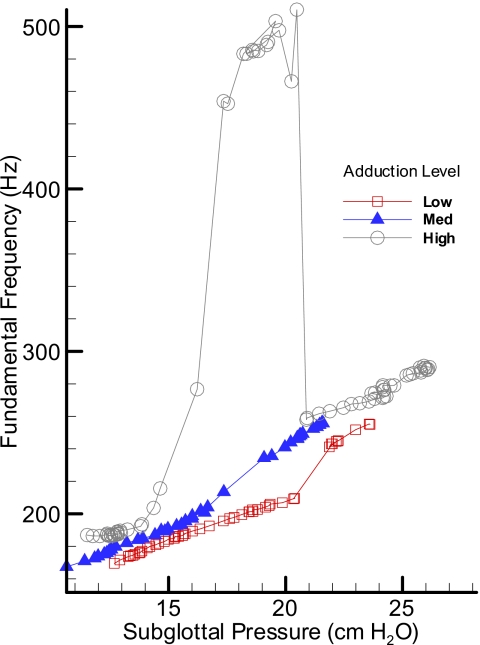
Figures 678 show the pressure-frequency relations for the excised pig, sheep, and cow larynges during three upward sweeps, respectively. These graphs are based on the mean values of subglottal pressure and fundamental frequency calculated from pressure-flow sweep experiments. The pig larynx (PL34, Fig. 6) showed a wider range of oscillation with frequency ranging from 160 to 300 Hz excluding the mode change and up to 500 Hz with mode change. The high adduction condition showed a mode change with a sharp increase in the fundamental frequency between 15 and 17 cm H2O subglottal pressure. The duration of this high frequency oscillation is short, but suggests nonlinear behavior in the pig vocal folds. Nonlinearity in the pressure-frequency relations is observable even in the average curves without the mode changes.
Figure 6.
(Color online) Pressure-frequency relationship for an oscillating pig larynx at three adduction levels: low, medium, and high.
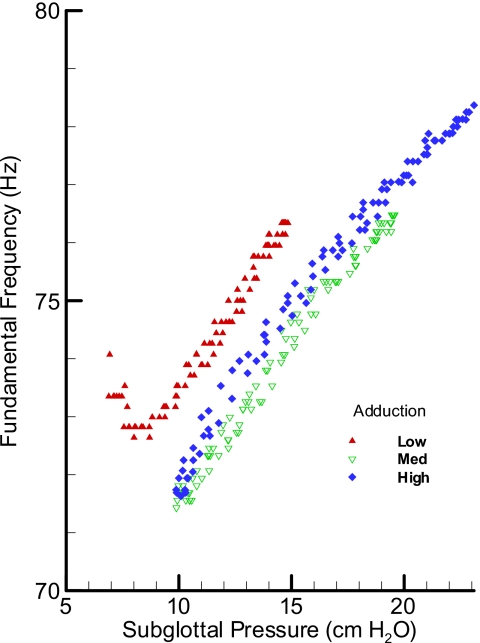
Figure 7.
(Color online) Pressure-frequency relationship for an oscillating sheep larynx at three adduction levels: low, medium, and high.
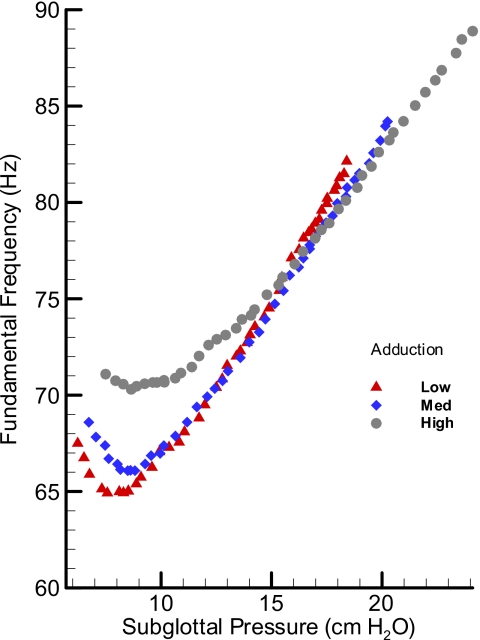
Figure 8.
(Color online) Pressure-frequency relationship for an oscillating cow larynx at three adduction levels: low, medium, and high.
The sheep larynx pressure-frequency relationship (SL15) showed slightly different behavior for low pressures in comparison to high pressures (Fig. 7). At higher subglottal pressure ranges, the pressure-frequency appeared to be fairly linear with very little sensitivity to the degree of adduction. However, at the lower subglottal pressure ranges, a more nonlinear relation was observed with the frequency showing minima that was also dependent on the adduction level with very low and high adduction levels showing the least drop in frequency. The negative values of slope (dF∕dP) at beginning of the oscillation may be related to the decrease in the oscillation frequency with greater extent of participation of vocal fold masses for oscillation, as seen in lower levels of adduction. The average oscillation frequency in the sheep larynges was lower with smaller range than the pig larynges (65–90 Hz).
In Fig. 8, the pressure-frequency relationship for the cow larynx BL17 is presented. The graph suggests that not only the oscillation frequency range was limited (70–80 Hz); its rate of change with pressure (dF∕dP) did not show a similar relationship to the adduction level as in the pig larynx. The widest range of oscillation was observed for the highest adduction level with the lowest range for the low adduction level. The fact that frequency did not show any nonlinearity associated with oscillation condition makes the cow larynx a suitable oscillator for the aerodynamic and acoustic studies.
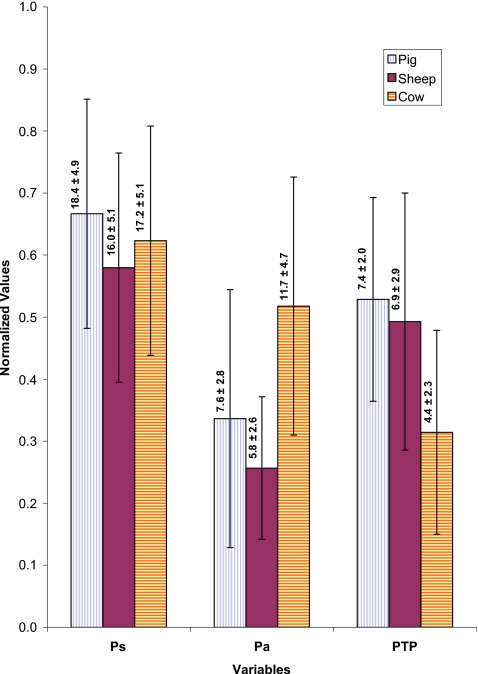
The mean glottal parameters of these larynges during the pressure-flow sweeps across different samples and at various adduction levels were calculated and statistical analysis was applied to these calculated means through the t-test for independent samples by variables in Basic Statistics of the STATISTICA package. Figure 9 compares the mean values of subglottal pressure (Ps), pressure amplitude (Pa), and PTP for the pig (eight samples, 38 sweep cases), sheep (eight samples, 34 sweep cases), and cow (six samples, 27 sweep cases). The operating mean subglottal pressures were in the same range, but the cow larynx had significantly larger pressure amplitude than pig and sheep (p<0.01). This can be related to the almost rectangular glottis in the cow larynx with large surface area and padlike vocal folds that displaced a larger volume of air during the oscillation. The cow larynx had the PTP range of 2–10 cm H2O with the lowest average value of 4.4±2.3 cm H2O, sheep larynx had PTP range of 3–14 cm H2O with average value of 6.9±2.9 cm H2O, and pig larynx had PTP range of 4–12.3 cm H2O with an average of 7.4±2.0 cm H2O. The same reason for the pressure amplitudes may also explain why the cow larynx has a significantly smaller PTP than pig and sheep (p<0.001).
Figure 9.
(Color online) Normalized composite profile of different pressure variables during pressure-flow sweeps of pig, sheep, and cow larynges. Ps is subglottal pressure, Pa is the pressure amplitude, and PTP is phonation threshold pressure. All pressure values are in cm H2O. The mean value for each condition is provided over the corresponding bar.
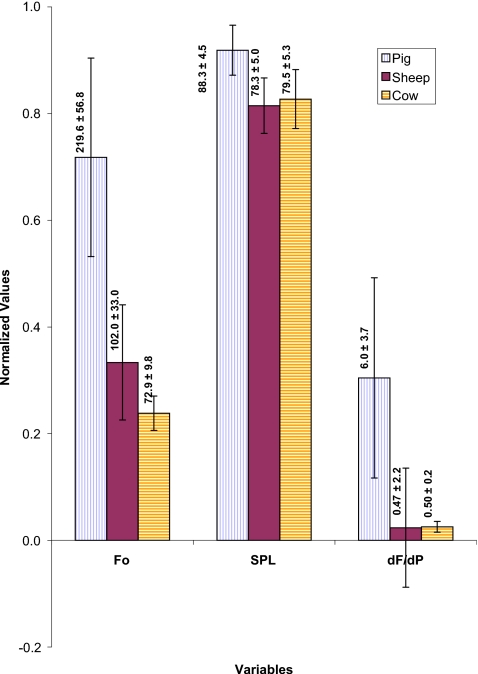
Figure 10 compares the mean values fundamental frequency (F0), SPL, and the rate of frequency changes with pressure (dF∕dP) for these larynges. The mean oscillation frequency for the pig was 220±57 Hz and for the sheep was 102±33 Hz, and 73±10 Hz for the cow. The pig larynx has significantly higher F0 than sheep larynx (p<0.0001) and sheep larynx has significantly higher F0 than cow larynx (p<0.0001). The pig larynx usually generated the loudest sound (p<0.0001) that could reach as high as 96.1 dB with an average of 88.3±4.5 dB. The sheep and cow larynges had produced sounds within similar intensity ranges. The SPL for the sheep was 78.3±5.0 dB and for the cow was 79.5±5.3 dB. Finally, the pig larynx had the highest dynamic range of frequency (dF∕dP=1.9–19.7) with an average of 6.0 Hz∕cm H2O which is significantly larger than others (p<0.0001). The sheep larynx had a peculiar pattern with a negative region and a range of −6.5–5.5 with an average of 0.47 Hz∕cm H2O. The cow larynx had the lowest range of 0.1–0.9 with an average of 0.5 Hz∕cm H2O.
Figure 10.
(Color online) Normalized composite profile of different phonatory variables during pressure-flow sweeps of pig, sheep, and cow larynges. F0 is the fundamental frequency (Hz), SPL is sound pressure level (dB), and dF∕dP is the rate of change of frequency with pressure (Hz/cm H2O). The mean value for each condition is provided over the corresponding bar.
DISCUSSION
The purpose of this study was to examine the phonatory characteristics of pig, sheep, and cow excised larynges and to examine the relation between subglottal pressure and the fundamental frequency of vocal fold vibration in these species. Each one of these species demonstrated different oscillation ranges and pressure-frequency behavior. The presence of two oscillating vocal folds in addition to the supraglottic structure wall was probably responsible for the greater dynamic range of F0 in pig larynx. This was possibly responsible for greater instability in oscillation as well. Mode changes in the form of sudden variations in oscillation frequency during the pressure sweep were observed in pig and in some cases in sheep larynges as well. The physiological explanation of the phenomenon can be attributed to the participation of additional masses, whether in the form of superior∕inferior vocal folds and glottal wall in the pigs or supraglottic structures in the sheep in the oscillation.
One thing that was common in all three species was the existence of a supraglottic duct with an approximately 2–4 cm length. The combination of this duct and the spout formed by the large epiglottis and arytenoids in these species creates an inertive load that helps stabilize their phonation. The low frequency oscillations of the supraglottic wall could have supplemented the glottal oscillations. These three larynges considerably varied in terms of dimensions of the cartilages and the vocal folds. The lack of well-defined vocal fold boundaries in the sheep and cow larynges made it difficult to accurately measure their vocal fold dimensions.
In a previous study reported on these larynges [Alipour and Jaiswal (in press)], it was reported that these larynges operated in almost the same ranges of maximum pressure and maximum flow rate. The most obvious difference observed was their maximum frequency ranges, with the highest value for the pig and the lowest for the cow larynx. Similarly, in this study, the mean fundamental frequency is reported for each larynx during the sweep. The pig larynx with well-defined superior and inferior vocal folds and a ventricle in between had higher frequency because its narrow and stiff superior vocal folds acted as the main oscillator. This phonation type probably matched with the high frequency natural “squeal” of the pig vocalization. Participation of the supraglottic wall and other structures probably results in the lower pitched “grunt.”
These pressure-frequency behaviors suggest that pig larynx with its widest operating range of frequency, large amplitude, and higher dF∕dP is a good model for the study of pitch control. Pig larynx can oscillate from 100 to 300 Hz with large amplitude and loud intensity, but most often with large frequency jitter. Histological studies have suggested a closer similarity of its LP to the human vocal folds than canine. However, the steeply angled position of the pig vocal folds creates a big challenge for the image analysis and vocal amplitude measurements. Moreover, the previous histological studies seemed to have focused on the inferior folds, assuming the superior folds to be nonparticipatory as in the human and canine false folds. The phonatory data provide evidence that the superior folds are actually one of the primary vibratory sources. More information regarding the histological and biomechanical nature of the superior folds is required to provide physiological basis to the acoustic output. Moreover, possible participation of the supraglottic wall can further create phonatory instability.
The cow larynx with low PTP and almost steady pitch is suitable for studies involving aerodynamic measurements. Also, the SPL values of cow larynges seem to correlate well with their pressure amplitude (R2=0.81) and the subglottal pressure (R2=0.64). The pressure-amplitude dependence was reported for the canine larynges by Titze (1989). Thus, cow larynges can be a good model for studies involving measurements of oscillation amplitude and intraglottal [Alipour et al. (2001)] pressure due to its large dimensions and steady pitch profile. The larger dimension has allowed for the insertion of contact pressure transducers in the larynx in the past [Scherer et al. (1985)].
The sheep larynx with a soft and pliable vocal fold tissue produced large vibrational amplitudes with big mucosal waves that could serve as a useful phonatory model as well. The similarity of its vocal fold length, laryngeal dimensions, and tissue histochemistry to human vocal folds make it a good physiological model. It would also serve as suitable phonatory model for studies requiring smaller frequency ranges.
The findings from this study suggest that the pig, sheep, and cow larynges with their distinctive aerodynamic and acoustic behaviors could be used as phonatory and aerodynamic models, especially when different frequency ranges and sizes of the larynx are crucial factors. When replacing pig larynx with canine, the angled position of the vocal folds, longer dimensions of vocal folds, smaller CT size, and lower mechanical advantage in pig larynx would need to be considered. The variations in pitch with elongation may not be as clear as in the canine larynx. The results of this study also provide some clues about the phonatory effects of evolutionary modifications of the larynx. This is especially reflected in the shapes and sizes of the supraglottal duct and its participation in phonation. Some of the variability in the data may be attributed to the use of some samples that were slow frozen, and their mechanical properties were deteriorated by the ice crystals. Future works should use fresh or fast frozen samples to avoid this problem.
SUMMARY AND CONCLUSIONS
Pressure-frequency relations were obtained for pig (eight samples), sheep (eight samples), and cow (six samples) larynges through a series of pressure-flow sweep experiments that were conducted on the excised bench. Vocal fold adduction was the major control parameter. The following was found.
-
(1)
Pressure-frequency relations were nonlinear for these species, with large ranges in df∕dp slope for the pig and sheep excised larynges. Sheep larynx showed negative slope under low pressure conditions that stabilized with increase in subglottal pressure.
-
(2)
The average oscillation frequencies for these species were 220±57 Hz for the pig, 102±33 Hz for the sheep, and 73±10 Hz for the cow.
-
(3)
The average PTP for the pig was 7.4±2.0 cm H2O, 6.9±2.9 cm H2O for the sheep, and 4.4±2.3 cm H2O for the cow.
ACKNOWLEDGMENTS
National Institute on Deafness and other Communication Disorders, Grant No. DC03566 supported this work. The authors would like to thank Jaclyn Curiel for assistance in data collection and Dr. Anders Löfqvist and two anonymous reviewers for their helpful comments.
References
- Alipour, F., Scherer, R. C., and Finnegan, E. (1997). “Pressure-flow relationships during phonation as a function of adduction,” J. Voice 10.1016/S0892-1997(97)80077-X 11, 187–194. [DOI] [PubMed] [Google Scholar]
- Alipour, F., Montequin, D., and Tayama, N. (2001). “Aerodynamic profiles of a hemilarynx with vocal tract,” Ann. Otol. Rhinol. Laryngol. 110, 550–555. [DOI] [PubMed] [Google Scholar]
- Alipour, F., and Jaiswal, S. (2006). “Vocal Fold Elasticity of pig, sheep and cow larynges,” Proceedings of the Fifth International Conference on Voice Physiology and Biomechanics, Tokyo, Japan, July 12–14 pp. 28–29.
- Alipour, F., Jaiswal, S., and Finnegan, E. (2007). “Aerodynamic and acoustic effects of false vocal folds and epiglottis in excised larynx models,” Ann. Otol. Rhinol. Laryngol. 116, 135–144. [DOI] [PubMed] [Google Scholar]
- Alipour, F., and Jaiswal, S. (2008). “Glottal airflow resistance in excised pig, sheep and cow larynges,” J. Voice (in press). [DOI] [PubMed]
- Alipour, F., and Scherer, R. C. (2007). “On pressure-frequency relations in the excised larynx,” J. Acoust. Soc. Am. 124, 2296–2305. [DOI] [PubMed] [Google Scholar]
- Berke, G. S., Moore, D. M., Gerratt, B. R., Hanson, D. G., and Natividad, M. (1989a). “Effect of superior laryngeal nerve stimulation on phonation in an in vivo canine model,” Am. J. Otolaryngol. 10, 181–187. [DOI] [PubMed] [Google Scholar]
- Berke, G. S., Moore, D. M., Gerratt, B. R., Hanson, D. G., Bell, T. S., and Natividad, M. (1989b). “The effect of recurrent laryngeal nerve stimulation on phonation in an in vivo canine model,” Laryngoscope 99, 977–982. [DOI] [PubMed] [Google Scholar]
- Chan, R. W., Fu, M., and Tirunagari, N. (2006). “Elasticity of the human false vocal fold,” Ann. Otol. Rhinol. Laryngol. 115, 370–381. [DOI] [PubMed] [Google Scholar]
- Hahn, M. S., Kobler, J. B., Zeitels, S. M., and Langer, R. (2005). “Midmembranous vocal fold lamina propria proteoglycans across selected species,” Ann. Otol. Rhinol. Laryngol. 114, 451–462. [DOI] [PubMed] [Google Scholar]
- Hahn, M. S., Kobler, J. B., Starcher, B. C., Zeitels, S. M., and Langer, R. (2006a). “Quantitative and comparative studies of the vocal fold extracellular matrix. I: Elastic fibers and hyaluronic acid,” Ann. Otol. Rhinol. Laryngol. 115, 156–164. [DOI] [PubMed] [Google Scholar]
- Hahn, M. S., Kobler, J. B., Zeitels, S. M., and Langer, R. (2006b). “Quantitative and comparative studies of the vocal fold extracellular matrix II: Collagen,” Ann. Otol. Rhinol. Laryngol. 115, 225–232. [DOI] [PubMed] [Google Scholar]
- Happak, W., Zrunek, M., Pechmann, U., and Streinzer, W. (1989). “Comparative histochemistry of human and sheep laryngeal muscles,” Acta Oto-Laryngol. 107, 283–288. [DOI] [PubMed] [Google Scholar]
- Harrison, D. F. N. (1995). The Anatomy and Physiology of the Mammalian Larynx (Cambridge University Press, Cambridge: ). [Google Scholar]
- Jiang, J. J., Raviv, J. R., and Hanson, D. G. (2001). “Comparison of the phonation related structures among pig, dog, white-tailed deer, and human larynges,” Ann. Otol. Rhinol. Laryngol. 110, 1120–1125. [DOI] [PubMed] [Google Scholar]
- Kim, M. J., Hunter, E. J., and Titze, I. R. (2004). “Comparison of human, canine, and ovine laryngeal dimensions,” Ann. Otol. Rhinol. Laryngol. 113, 60–68. [DOI] [PubMed] [Google Scholar]
- Knight, M. J., McDonald, S. E., and Birchall, M. A. (2005). “Intrinsic muscles and distribution of the recurrent laryngeal nerve in the pig larynx,” Eur. Arch. Otorhinolaryngol. 262, 281–5. [DOI] [PubMed] [Google Scholar]
- Koyama, T., Kawasaki, M., and Ogura, J. H. (1969). “Mechanics of voice production I. Regulation of vocal intensity,” Laryngoscope 79, 337–354. [DOI] [PubMed] [Google Scholar]
- Koyama, T., Harvey, J. E., and Ogura, J. H. (1971). “Mechanics of voice production II. Regulation of pitch,” Laryngoscope 10.1288/00005537-197101000-00005 81, 45–65. [DOI] [PubMed] [Google Scholar]
- Kurita, S., Nagata, K., and Hirano, H. (1983). “A comparative study of the layer structure of the vocal fold,” in Vocal Fold Physiology: Contemporary Research and Clinical Issues, edited by Bless, D. M., and Abbs, J. H. (College Hill, San Diego: ), pp. 3–21. [Google Scholar]
- Scherer, R. C., Cooper, D., Alipour-Haghighi, F., and Titze, I. R. (1985). “Contact pressure between the vocal processes of an excised bovine larynx,” in Vocal Fold Physiology: Biomechanics, Acoustics and Phonatory Control, edited by Titze I. R., and Scherer R. C.(The Denver Center for the Performing Arts, Denver: ), pp. 292–303. [Google Scholar]
- Slavit, D. H., Lipton, R. J., and McCaffrey, T. V. (1990). “Glottographic analysis of phonation in the excised canine larynx,” Ann. Otol. Rhinol. Laryngol. 99, 396–402. [DOI] [PubMed] [Google Scholar]
- Titze, I. R. (1989). “On the relation between subglottal pressure and fundamental frequency in phonation,” J. Acoust. Soc. Am. 10.1121/1.397562 85, 901–906. [DOI] [PubMed] [Google Scholar]
- Zrunek, M., Happak, W., Hermann, M., and Streinzer, W. (1988). “Comparative anatomy of human and sheep laryngeal skeleton,” Acta Oto-Laryngol. 105, 155–162. [DOI] [PubMed] [Google Scholar]