Abstract
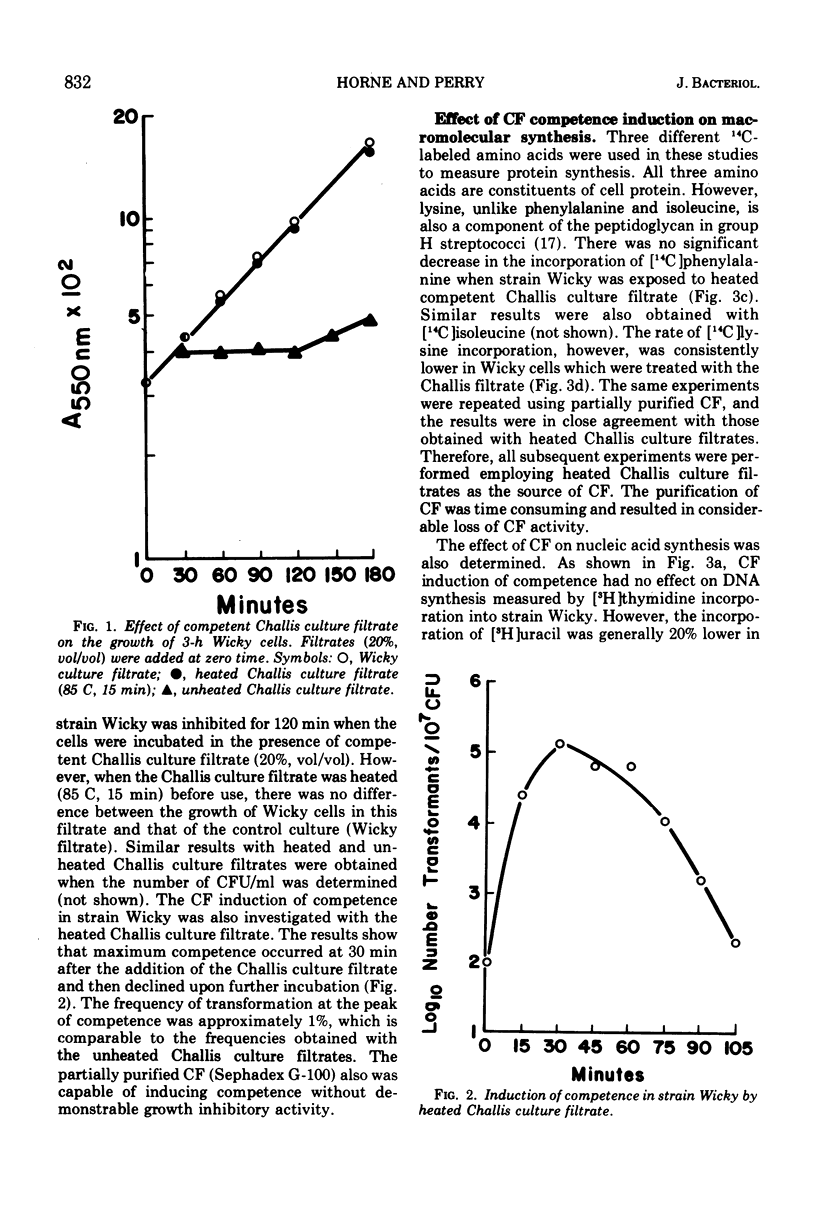
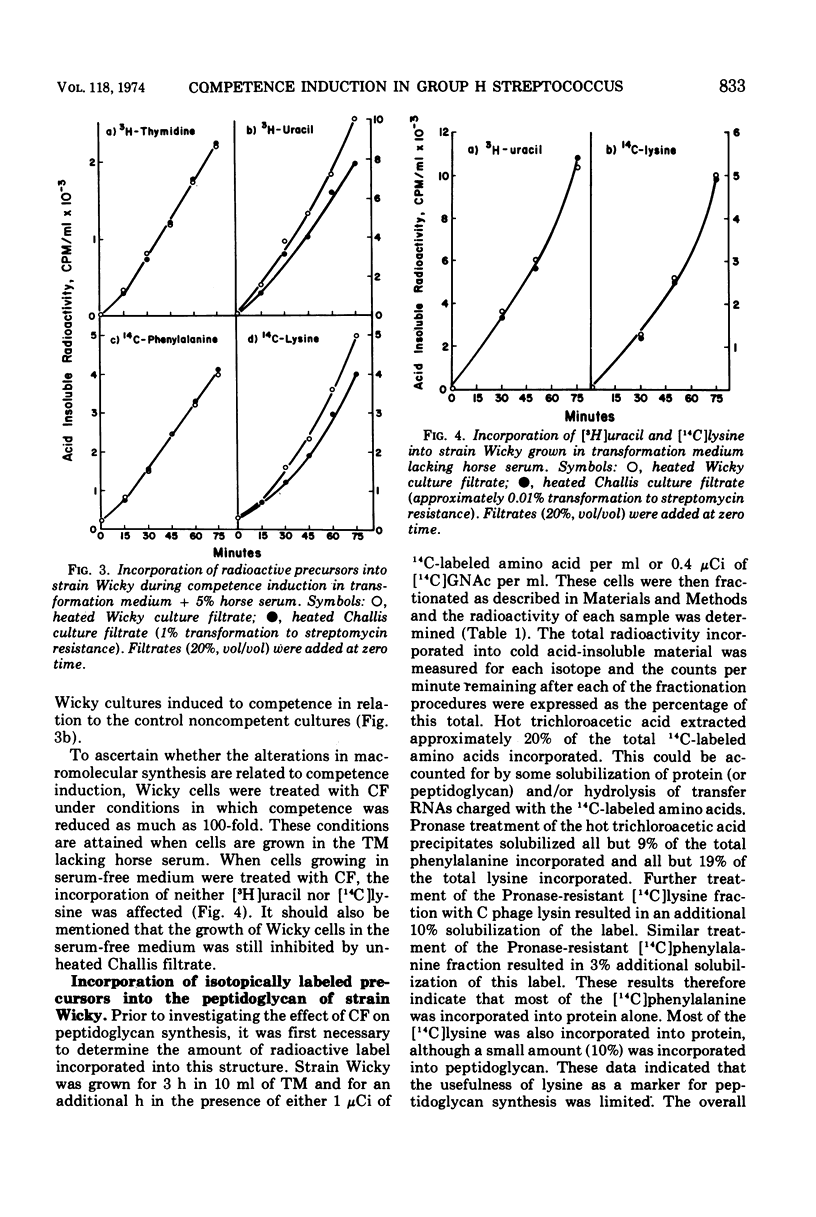
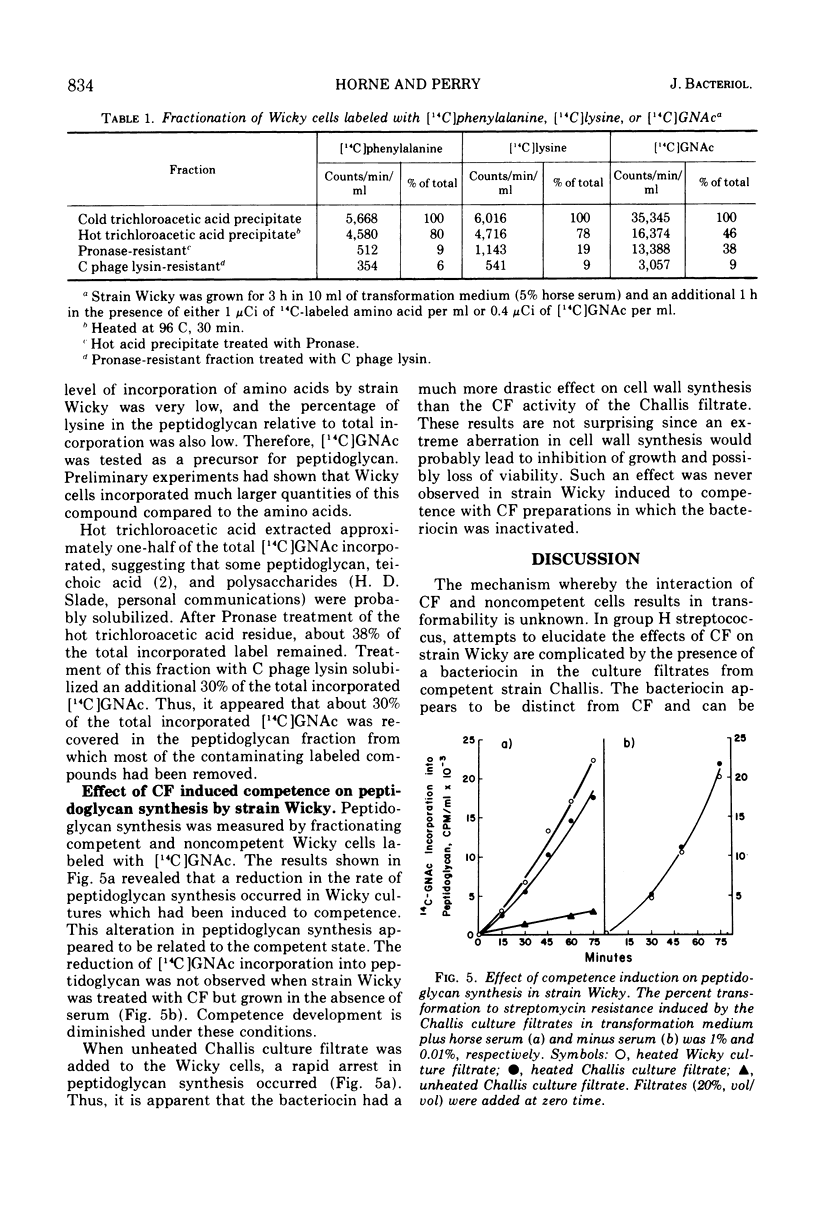
The effects of competence induction by competence factor (CF) on macromolecular synthesis in group H streptococcus strain Wicky were investigated. CF preparations (culture filtrates from competent group H streptococcus strain Challis) were either heated or partially purified to remove a bacteriocin. These preparations did not inhibit growth, although they induced high levels of competence in strain Wicky. The action of the CF preparations did not affect the overall rates of deoxyribonucleic acid and protein synthesis, but caused a reduction in the rates of ribonucleic acid (RNA) and peptidoglycan synthesis. When competence induction by CF was prevented, no alterations in RNA or peptidoglycan synthesis were observed, indicating that these changes are in fact related to the development of competence.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARKULIS S. S., SMITH C., BOLTRALIK J. J., HEYMANN H. STRUCTURE OF STREPTOCOCCAL CELL WALLS. IV. PURIFICATION AND PROPERTIES OF STREPTOCOCCAL PHAGE MURALYSIN. J Biol Chem. 1964 Dec;239:4027–4033. [PubMed] [Google Scholar]
- Boothby D., Daneo-Moore L., Shockman G. D. A rapid, guantitative, and selective estimation of radioactively labeled peptidoglycan in gram-positive bacteria. Anal Biochem. 1971 Dec;44(2):645–653. doi: 10.1016/0003-2697(71)90255-7. [DOI] [PubMed] [Google Scholar]
- Dooley D. C., Hadden C. T., Nester E. W. Macromolecular synthesis in Bacillus subtilis during development of the competent state. J Bacteriol. 1971 Nov;108(2):668–679. doi: 10.1128/jb.108.2.668-679.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ephrussi-Taylor H., Freed B. A. Incorporation of thymidine and amino acids into deoxyribonucleic acid and acid-insoluble cell structures in pneumococcal cultures synchronized for competence to transform. J Bacteriol. 1964 May;87(5):1211–1215. doi: 10.1128/jb.87.5.1211-1215.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy C., Nester E. W. Macromolecular synthesis in newly transformed cells of Bacillus subtilis. J Bacteriol. 1967 Jul;94(1):131–140. doi: 10.1128/jb.94.1.131-140.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NESTER E. W. PENICILLIN RESISTANCE OF COMPETENT CELLS IN DEOXYRIBONUCLEIC ACID TRANSFORMATION OF BACILLUS SUBTILIS. J Bacteriol. 1964 Apr;87:867–875. doi: 10.1128/jb.87.4.867-875.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J. Structure and biosynthesis of the bacterial cell wall. Annu Rev Biochem. 1969;38:501–538. doi: 10.1146/annurev.bi.38.070169.002441. [DOI] [PubMed] [Google Scholar]
- Osowiecki H., Nalecz J., Dobrzański W. T. The mechanism of competence in the transformation of Streptococci of serological group H. Purification and some properties of the competence factor. Mol Gen Genet. 1969;105(1):16–20. doi: 10.1007/BF00750310. [DOI] [PubMed] [Google Scholar]
- PARK J. T., HANCOCK R. A fractionation procedure for studies of the synthesis of cell-wall mucopeptide and of other polymers in cells of Staphylococcus aureus. J Gen Microbiol. 1960 Feb;22:249–258. doi: 10.1099/00221287-22-1-249. [DOI] [PubMed] [Google Scholar]
- Pakula R., Spencer L. R. Some features of competent streptococci. Can J Microbiol. 1972 Apr;18(4):487–491. doi: 10.1139/m72-075. [DOI] [PubMed] [Google Scholar]
- Perry D. Effect of D-cycloserine on transformation in group H streptococcus, strain Challis. J Bacteriol. 1972 Mar;109(3):1014–1019. doi: 10.1128/jb.109.3.1014-1019.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry D., Slade H. D. Effect of filtrates from transformable and nontransformable streptococci on the transformation of streptococci. J Bacteriol. 1966 Jun;91(6):2216–2222. doi: 10.1128/jb.91.6.2216-2222.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry D. Transformability of streptomycin-resistant group H streptococci. J Bacteriol. 1968 Jan;95(1):132–138. doi: 10.1128/jb.95.1.132-138.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranhand J. M., Leonard C. G., Cole R. M. Autolytic activity associated with competent group H streptococci. J Bacteriol. 1971 Apr;106(1):257–268. doi: 10.1128/jb.106.1.257-268.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel R., Slade H. D. Bacteriocin production by transformable group H streptococci. J Bacteriol. 1972 Nov;112(2):824–829. doi: 10.1128/jb.112.2.824-829.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel R., Slade H. D. Properties of a Streptococcus sanguis (group H) bacteriocin and its separation from the competence factor of transformation. J Bacteriol. 1973 Aug;115(2):655–661. doi: 10.1128/jb.115.2.655-661.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade H. D., Slamp W. C. Peptidoglycan composition and taxonomy of group D, E, and H streptococci, and Streptococcus mutans. J Bacteriol. 1972 Feb;109(2):691–695. doi: 10.1128/jb.109.2.691-695.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A. Cellular metabolism in genetic transformation of pneumococci: requirement for protein synthesis during induction of competence. J Bacteriol. 1970 Mar;101(3):860–871. doi: 10.1128/jb.101.3.860-871.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]


