Abstract
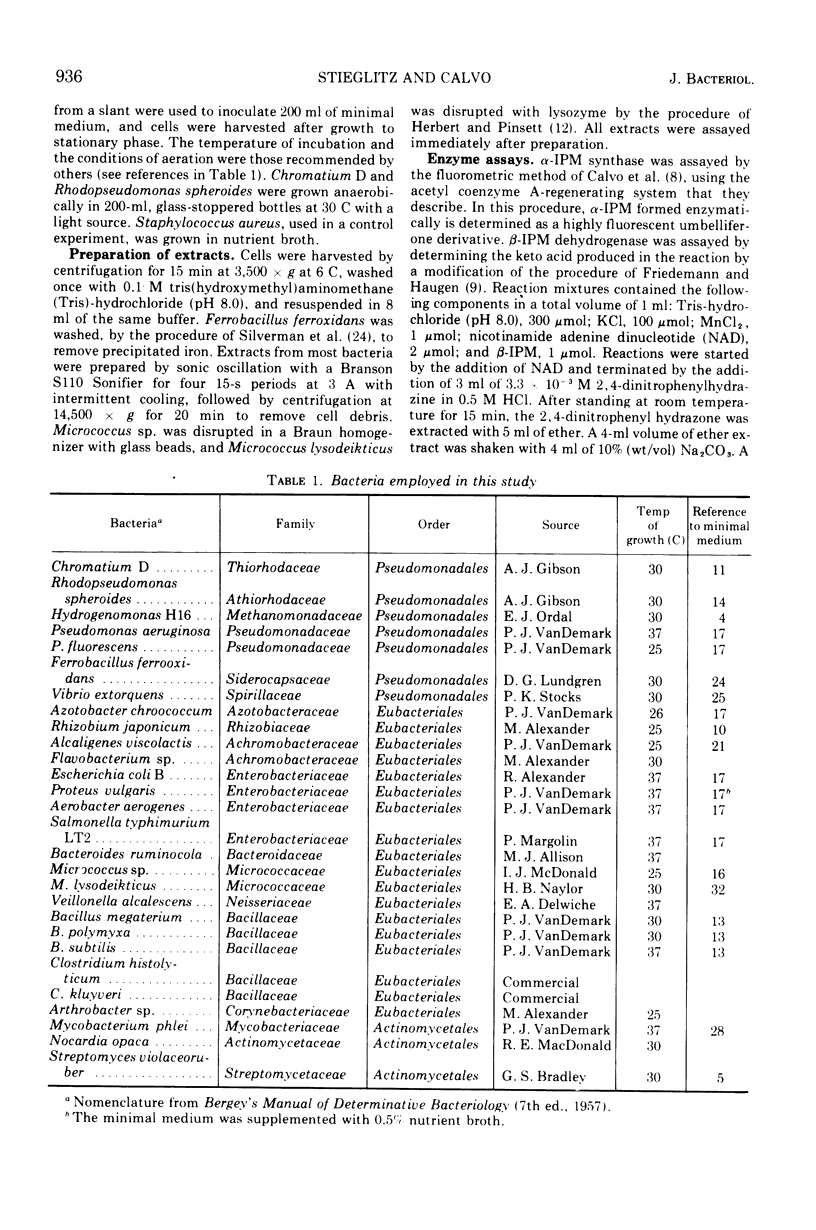
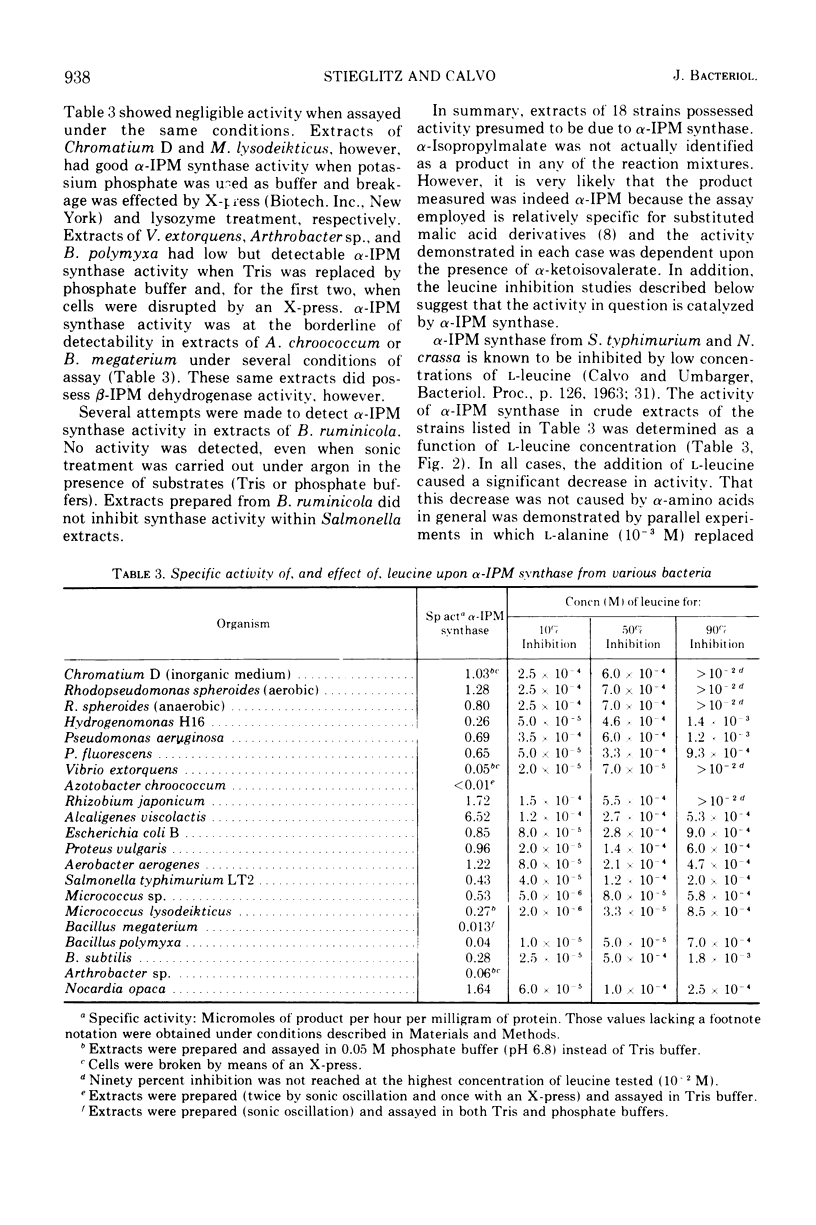
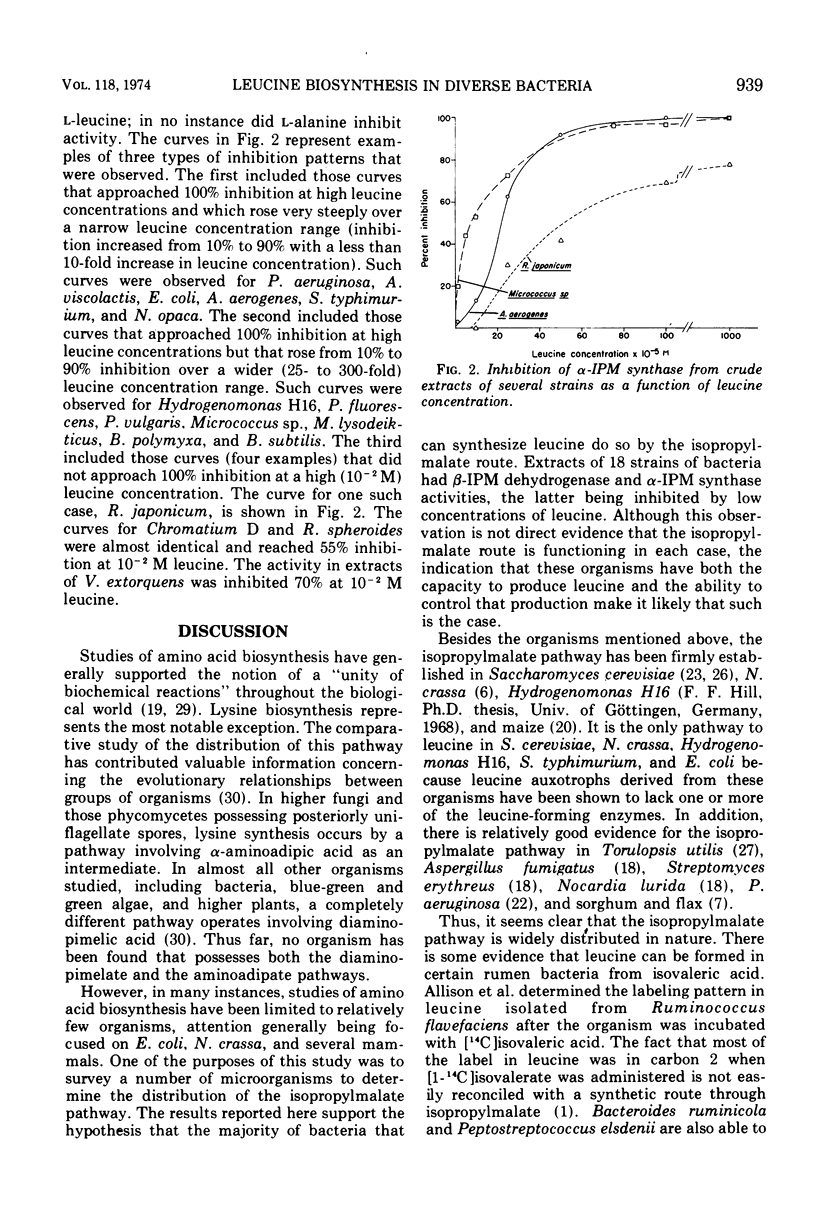
α-Isopropylmalate synthase and β-isopropylmalate dehydrogenase activities were detected in extracts of the following organisms: Chromatium D, Rhodopseudomonas spheroides, Hydrogenomonas H16, Pseudomonas aeruginosa, Pseudomonas fluorescens, Vibrio extorquens, Rhizobium japonicum, Alcaligenes viscolactis, Escherichia coli B, Proteus vulgaris, Aerobacter aerogenes, Salmonella typhimurium, Micrococcus sp., Micrococcus lysodeikticus, Bacillus polymyxa, Bacillus subtilis, and Nocardia opaca. The α-isopropylmalate synthase activity in these extracts was inhibited by low concentrations of l-leucine. Taken together with other data, these results suggest that the isopropylmalate pathway is widespread among organisms that can synthesize leucine.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON M. J., BRYANT M. P., DOETSCH R. N. Studies on the metabolic function of branched-chain volatile fatty acids, growth factors for ruminococci. I. Incorporation of isovalerate into leucine. J Bacteriol. 1962 Mar;83:523–532. doi: 10.1128/jb.83.3.523-532.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison M. J., Bucklin J. A., Robinson I. M. Importance of the isovalerate carboxylation pathway of leucine biosynthesis in the rumen. Appl Microbiol. 1966 Sep;14(5):807–814. doi: 10.1128/am.14.5.807-814.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison M. J., Peel J. L. The biosynthesis of valine from isobutyrate by peptostreptococcus elsdenii and Bacteroides ruminicola. Biochem J. 1971 Feb;121(3):431–437. doi: 10.1042/bj1210431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTHA R., ORDAL E. J. NICKEL-DEPENDENT CHEMOLITHOTROPHIC GROWTH OF TWO HYDROGENOMONAS STRAINS. J Bacteriol. 1965 Apr;89:1015–1019. doi: 10.1128/jb.89.4.1015-1019.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo J. M., Bartholomew J. C., Stieglitz B. I. Fluorometric assay of enzymatic reactions involving acetyl Coenzyme A in aldol condensations. Anal Biochem. 1969 Apr 4;28(1):164–181. doi: 10.1016/0003-2697(69)90168-7. [DOI] [PubMed] [Google Scholar]
- HENDLEY D. D. Endogenous fermentation in Thiorhodaceae. J Bacteriol. 1955 Dec;70(6):625–634. doi: 10.1128/jb.70.6.625-634.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNIGHT B. C. J. G., PROOM H. A comparative survey of the nutrition and physiology of mesophilic species in the genus Bacillus. J Gen Microbiol. 1950 Sep;4(3):508–538. doi: 10.1099/00221287-4-3-508. [DOI] [PubMed] [Google Scholar]
- MARGOLIN P. Genetic fine structure of the leucine operon in Salmonella. Genetics. 1963 Mar;48:441–457. doi: 10.1093/genetics/48.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN W. R., COLEMAN W. H., WIDEBURGNE, CANTRELL R., JACKSON M., DENISON F. W., Jr beta-Carboxy-beta-hydroxy-isocaproic acid formation by microorganisms. Biochim Biophys Acta. 1962 Jul 30;62:165–167. doi: 10.1016/0006-3002(62)90503-6. [DOI] [PubMed] [Google Scholar]
- MCDONALD C. E., CHEN L. L. THE LOWRY MODIFICATION OF THE FOLIN REAGENT FOR DETERMINATION OF PROTEINASE ACTIVITY. Anal Biochem. 1965 Jan;10:175–177. doi: 10.1016/0003-2697(65)90255-1. [DOI] [PubMed] [Google Scholar]
- Oaks A. The synthesis of leucine in maize embryos. Biochim Biophys Acta. 1965 Nov 15;111(1):79–89. doi: 10.1016/0304-4165(65)90474-5. [DOI] [PubMed] [Google Scholar]
- PUNCH J. D., OLSON J. C., Jr, SCALETTI J. V. AMINO ACID UTILIZATION BY ALCALIGENES VISCOLACTIS FOR GROWTH AND SLIME PRODUCTION. J Bacteriol. 1965 Jun;89:1521–1525. doi: 10.1128/jb.89.6.1521-1525.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin R., Salamon I. I., Bleiweis A. S., Carlin J., Ajl S. J. Metabolism of ethylmalic acids by Pseudomonas aeruginosa. Biochemistry. 1968 Jan;7(1):377–388. doi: 10.1021/bi00841a048. [DOI] [PubMed] [Google Scholar]
- SILVERMAN M. P., LUNDGREN D. G. Studies on the chemoautotrophic iron bacterium Ferrobacillus ferrooxidans. I. An improved medium and a harvesting procedure for securing high cell yields. J Bacteriol. 1959 May;77(5):642–647. doi: 10.1128/jb.77.5.642-647.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOCKS P. K., MCCLESKEY C. S. IDENTITY OF THE PINK-PIGMENTED METHANOL-OXIDIZING BACTERIA AS VIBRIO EXTORQUENS. J Bacteriol. 1964 Oct;88:1065–1070. doi: 10.1128/jb.88.4.1065-1070.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRASSMAN M., CECI L. N. Enzymatic formation of alpha-isopropylmalic acid, an intermediate in leucine biosynthesis. J Biol Chem. 1963 Jul;238:2445–2452. [PubMed] [Google Scholar]
- TAKEYA K., HISATSUNE K. Mycobacterial cell walls. I. Methods of preparation and treatment with various chemicals. J Bacteriol. 1963 Jan;85:16–23. doi: 10.1128/jb.85.1.16-23.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLIN H. L., NAYLOR H. B. Basic nutritional requirements of Micrococcus lysodeikticus. J Bacteriol. 1957 Aug;74(2):163–167. doi: 10.1128/jb.74.2.163-167.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]


