Abstract
Thioredoxin, a ubiquitous 12-kDa regulatory disulfide protein, was found to reduce disulfide bonds of allergens (convert S—S to 2 SH) and thereby mitigate the allergenicity of commercial wheat preparations. Allergenic strength was determined by skin tests with a canine model for food allergy. Statistically significant mitigation was observed with 15 of 16 wheat-sensitive animals. The allergenicity of the protein fractions extracted from wheat flour with the indicated solvent was also assessed: the gliadins (ethanol) were the strongest allergens, followed by glutenins (acetic acid), albumins (water), and globulins (salt water). Of the gliadins, the α and β fractions were most potent, followed by the γ and ω types. Thioredoxin mitigated the allergenicity associated with the major protein fractions—i.e, the gliadins (including the α, β, and γ types) and the glutenins—but gave less consistent results with the minor fractions, the albumins and globulins. In all cases, mitigation was specific to thioredoxin that had been reduced either enzymically by NADPH and NADP–thioredoxin reductase or chemically by dithiothreitol; reduced glutathione was without significant effect. As in previous studies, thioredoxin was particularly effective in the reduction of intramolecular (intrachain) disulfide bonds. The present results demonstrate that the reduction of these disulfide bonds is accompanied by a statistically significant decrease in allergenicity of the active proteins. This decrease occurs alongside the changes identified previously—i.e., increased susceptibility to proteolysis and heat, and altered biochemical activity. The findings open the door to the testing of the thioredoxin system in the production of hypoallergenic, more-digestible foods.
Keywords: food allergy, allergen disulfide, allergenic gliadins
Thioredoxins are a family of 12-kDa proteins functional in the regulation of a spectrum of cellular events throughout the animal, plant, and bacterial kingdoms (1–5). Thioredoxins undergo reversible redox change through a catalytically active disulfide site, [-Cys-Gly-Pro-Cys-]. In the NADP/thioredoxin system characteristic of aerobic heterotrophic cells, the reduction of thioredoxin is linked to NADPH by a flavin enzyme, NADP–thioredoxin reductase (NTR) (1–5) (Eq. 1). Alternatively, thioredoxin can be reduced chemically by the nonphysiological reagent dithiothreitol (DTT).
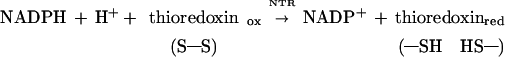 |
1 |
Ongoing studies, initiated more than a decade ago, have demonstrated that thioredoxins linked to NADPH effectively and specifically reduce intramolecular (intrachain) disulfide bonds of an array of proteins, including thionins (6, 7), α-amylase and trypsin inhibitors (8–11), venom neurotoxins (12), oilseed 2S protein (13), and wheat storage proteins such as the gliadins and glutenins (14, 15). Depending on the protein, reduction was accompanied by a pronounced change—either a loss (10–12) or an increase (16) in the associated biochemical activity. In addition, the reduced protein showed an increase in susceptibility to proteolysis and heat (9, 10, 12, 22). Thus, the reduction by thioredoxin brings about a structural change that dramatically alters the function as well as the life expectancy of the protein (Eq. 2).
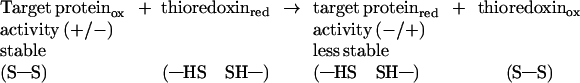 |
2 |
It is well established that many of the principal food allergens are proteins containing intramolecular disulfide bonds (17) and, in fact, certain of the proteins earlier shown to be reduced specifically by thioredoxin are known to cause either asthma—wheat α-amylase inhibitor (18), oilseed 2S protein (19,20)—or food allergy—soy Kunitz trypsin inhibitor (21). The question arises as to whether a change in the allergenic properties of disulfide proteins accompanies previously observed alterations in their biochemical and physical properties on reduction by thioredoxin. Using food allergy elicited by wheat, we have addressed this question in a canine model system and obtained results that prompt an answer in the affirmative.
MATERIALS AND METHODS
Plant Material.
The wheat storage proteins were prepared from semolina flour (Triticum durum Desf. cv. Monroe). The commercial allergen preparation, consisting of an extract of whole hard red and soft white wheat grain mix (food) in 50% glycerol and 50% saline buffer (1:10, wt/vol) was purchased from Miles.
Animals.
A colony of inbred, high IgE-producing, atopic dogs was sensitized and maintained at the Animal Resources Service, School of Veterinary Medicine, University of California, Davis (23, 24). The dogs have been selected for a genetic predisposition to allergy and have a 15-year history of food and pollen hypersensitivity. As described below, they have been regularly immunized with specific food extracts and periodically characterized for food hypersensitivity since birth.
Chemicals and Enzymes.
Reagents for sodium dodecyl sulfate/polyacrylamide gel electrophoresis (SDS/PAGE) and acidic native gel electrophoresis (acidic-PAGE) were obtained from Sigma and Bio-Rad. DTT was purchased from Boehringer Mannheim and monobromobimane (mBBr), from Calbiochem. Thioredoxin and NTR were purified from Escherichia coli strains overexpressing the proteins (9, 16). Glutathione reductase was purified from spinach leaves by the same procedure used for spinach NTR (25). NADPH, other biochemicals, reagents, and enzymes were purchased from Sigma.
Food Sensitization of Atopic Dogs.
Newborn pups from two litters of the atopic dog colony, CGB and GCB, were injected subcutaneously at day 1 with 1 μg each of wheat, cow’s milk, and beef extract (Miles) in 0.2 ml of alum. A third litter, CBB, was injected with soy in addition to the above allergens. Pups received subcutaneously 0.5 μl of distemper–hepatitis vaccine at 21, 49, and 77 days after birth. During this period the pups were boosted with the same food allergy regime used at day 1 (at 22, 29, 50, 57, 78, and 85 days); thereafter pups were boosted at bimonthly intervals. At 3–4 months of age, the pups exhibited positive skin tests with these food allergens and had specific IgE antibodies to the foods determined by anti-canine IgE immunodot assay (26). When fed 200 g of wheat flour gruel or cow’s milk, the sensitized pups at 6 months of age exhibited diarrhea (increased number of loose or watery stools for 2–4 days after feeding challenge) (27). The animals were periodically monitored by IgE enzyme-labeled immunodot, skin, and gastroscopic testing. More complete details of the clinical symptoms have been described (24). All of these tests support the utility of the animals as a model for food allergy.
Skin Tests.
About 3 min prior to skin testing, each dog received 4–5 ml of filtered 0.5% Evans blue dye solution (equivalent to 0.2 ml of 0.5% Evans blue dye per kg of weight) through a cephalic vein to enhance assessment of the cutaneous IgE antibodies (27). Serial dilutions of 100 μl of each sample were injected intradermally on the abdominal skin to establish the titer. After 15–20 min, the allergic response was determined by measuring the size of the blue wheal reaction (maximum length and width). An appropriate negative control (solution of glycerol, sodium myristate, or ethanol diluted in physiological saline buffer) was included for each animal tested. Repeated tests with thioredoxin, NTR, and NADPH alone were included and also found to be consistently negative.
Data Analysis.
The statistical significance of the thioredoxin-linked mitigation of the canine cutaneous response was determined by paired one-tailed t tests. The null hypothesis assuming no difference in wheal area induced by untreated vs. NTS-treated commercial wheat allergen was tested against the alternative hypothesis that thioredoxin treatment resulted in mitigation of allergic response. The paired one-tailed tests were completed for each dilution series at 0.05 level of significance on all sensitive dogs (df = 15).
Extraction of the Wheat Endosperm Proteins.
Albumin, globulin, gliadin, and glutenin fractions were isolated according to their differential solubility. One gram of wheat flour was extracted sequentially with 5 ml of the following solutions: (i) 0.5 M NaCl, (ii) 70% (vol/vol) aqueous ethanol, and (iii) 0.1 M acetic acid, using an electrical rotator at 25°C for 30 min and occasional agitation. The extracted fractions corresponded, respectively, to (i) albumin plus globulin, (ii) gliadin, and (iii) glutenin. Each of the three suspensions was centrifuged at 27,000 × g for 30 min at 4°C. Three successive extractions were performed for each fraction and the resulting supernatant solutions were pooled. The gliadins and glutenins were lyophilized without further treatment. The saline fraction containing albumins plus globulins was dialyzed against 50 mM Tris⋅HCl (pH 7.9). The precipitate, containing the globulins, was collected by centrifugation at 27,000 × g at 4°C for 30 min. The supernatant (albumin) fraction was lyophilized without further treatment; the globulin pellet was resuspended in 50 mM Tris⋅HCl (pH 7.9) containing 0.2 M NaCl and then lyophilized. All lyophilized fractions were stored at −80°C. Prior to skin testing, each fraction was resuspended in 0.85% sodium myristate (pH 7.0), which was added to assist in solubilizing the gliadins and glutenins (28). After estimation of protein concentration, each fraction was serially diluted in physiological buffered saline (PBS) and then used for the skin tests. An appropriate control (minus protein) was included for each animal tested.
Protein Assay.
Protein concentration was determined by the Bradford method (Bio-Rad) using bovine gamma globulin as standard (29). Results were confirmed by the DC protein assay (30).
Protein Reduction.
Reduction of the disulfide bonds of proteins in the commercial allergen extract, wheat Osborne fractions, and the purified gliadin fractions was compared by using one of the following: (i) the NADP/thioredoxin system, consisting of 5 μl of 25 mM NADPH, 8 μl of 0.3 mg/ml E. coli thioredoxin, and 7 μl of 0.3 mg/ml E. coli NTR; (ii) the NADP/glutathione system, composed of 5 μl of 25 mM NADPH, 10 μl of 30 mM glutathione (GSH), and 15 μl of 0.1 mg/ml glutathione reductase; (iii) a combination of 5 μl of 30 mM DTT and 8 μl of 0.3 mg/ml E. coli thioredoxin; or (iv) 10 μl of 30 mM DTT alone (8). Reactions in a final volume of 100 μl containing 30 μg of the commercial allergen or 10 μg of purified protein fractions were carried out for 45 min at 37°C (for skin tests), or 25 min at room temperature (for mBBr labeling and SDS/PAGE analysis). For complete reduction, samples with DTT alone were boiled 5 min before incubation. Seventy micrograms of the commercial allergen was used for mBBr labeling and SDS/PAGE analysis.
mBBr Labeling of Proteins.
Sulfhydryl groups were visualized as their fluorescent mBBr derivatives. mBBr, 10 μl of a 100 mM solution, was added to each sample. After 20 min of incubation, the reaction was stopped by adding 10 μl of 100 mM 2-mercaptoethanol, 10 μl of 20% SDS, and 20 μl of SDS/PAGE sample buffer containing 80% (vol/vol) glycerol and 0.005% bromophenol blue. Proteins were then separated by SDS/PAGE as described below.
SDS/PAGE.
Gels (10–20% acrylamide gradient, 1.5-mm
thickness) were prepared according to Laemmli (31) and developed for
16 h at a constant current (7 mV). After electrophoresis, gels
were placed in 12% trichloroacetic acid for 1 h for fixation and
then soaked overnight or longer in 40% (vol/vol) methanol and 10%
(vol/vol) acetic acid with several changes to remove excess mBBr.
Gels were then placed under UV light to visualize fluorescent bands.
Polaroid photographs (Positive/Negative Landfilm, type 55) were taken
through a yellow Wratten gelatin filter no. 8 with an exposure time of
45 s at f4.5. Finally, gels were stained with 0.01%
Coomassie brilliant blue R-250 in 40% methanol and 10% acetic
acid for 1–2 h and destained overnight with a solution of 20% ethanol
and 10% acetic acid. A second Polaroid photograph was taken after
destaining (exposure time of  s at f7).
s at f7).
Separation of Gliadin Fractions.
Reverse-phase chromatography was performed with a BioCAD/Sprint perfusion chromatography workstation (Perseptive Biosystems, Framingham, MA), using a 2 × 10 cm AP2 column (Waters Millipore, Bedford, MA) pressure packed with a Perseptive Biosystems Poros R2M reverse-phase medium. The gliadin fraction was dialyzed vs. 0.5 M NaCl at 4°C for 1 hr to eliminate water-soluble contaminants and centrifuged at 4°C for 60 min at 27,000 × g. The pellet, resuspended in 70% (vol/vol) ethanol, was subjected to reverse-phase chromatography at room temperature. The ethanol solution, 1.5 ml (6 mg of protein), was injected onto the AP2 column, which had been equilibrated with aqueous 0.1% trifluoroacetic acid (2 column volumes). After sample application, the column was washed with 2 column volumes of equilibration buffer and was then eluted at a flow rate of 5 ml/min with 15 column volumes of a 30–40% gradient of acetonitrile containing 0.1% trifluoroacetic acid. Finally, the column was cleaned with 2 column volumes of 100% acetonitrile. The elution time for the entire chromatography was 150 min (includes the equilibration, wash, elution, and cleaning volumes). Protein was monitored by absorbance at 210 nm. Fractions of 5 ml were collected; those containing protein peaks were combined, and the protein was lyophilized and resuspended in 70% ethanol and stored at −80°C. The type of gliadins was determined by acidic polyacrylamide gel electrophoresis (acidic-PAGE) (32, 33). The analysis was performed with 6% acrylamide gels of 3 mm thickness in a 0.25% aluminum lactate running buffer. The gel solution contained 2.5 g of aluminum lactate, 0.24 g of ascorbic acid, and 20 mg of ferrous sulfate heptahydrate per liter. The pH of the gel solution and the running buffer was adjusted to 3.1 with lactic acid. Components were classified according to their electrophoretic mobility into one of the four gliadin classes (α, β, γ, and ω). The gliadins were eluted from the reverse-phase column at the following percentages (vol/vol) of acetonitrile: ω, 32.5%; ω plus β, 33.0%; β, 33.6 and 34.6%; α, 35.8 and 36.5%; γ plus β, 37.4%; β 37.8 and 38.1%; and γ alone, 38.4%. As found previously (14), the α, β, and γ (but not the ω) fractions were effectively and specifically reduced by thioredoxin. The following homogenous fractions were combined, diluted in physiological saline buffer, lyophilized, and used for skin tests: ω, 32.5%; α, 35.8%; β, 38.1%; and γ, 38.4%. A blank sample of 70% ethanol taken through the same separation procedure served as a control.
RESULTS
Commercial Wheat Allergen Extract.
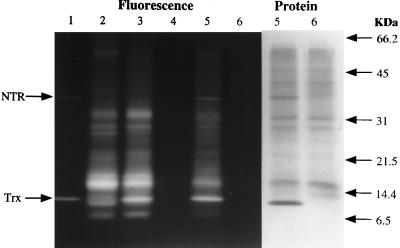
As found previously with other protein preparations from wheat, thioredoxin (lane 1) reduced either enzymically by NADPH (NTS) (lane 5) or chemically by DTT (DTT/Trx) (lane 3) in turn, reduced the proteins of the commercial allergen preparation (Fig. 1). The extent of reduction can be compared with the total reduction obtained with a combination of DTT and heat (DTT, heat) (lane 2) vs. the untreated (control) allergen preparation (lane 6). Glutathione maintained in the reduced form by NADPH and glutathione reductase was without significant effect (lane 4). The specificity of thioredoxin in reducing the proteins, notably one at 16 kDa, raised the question of whether reduction was accompanied by a change in allergenicity. This possibility was tested by performing skin tests on sensitized dogs, as several lines of evidence indicate that skin tests are an accurate indicator of food allergy with the canine model (24).
Figure 1.
Reduction of commercial wheat allergen extract with the NADP/thioredoxin system determined by SDS/PAGE/mBBr labeling procedure. After incubation with the indicated additions, the wheat proteins were derivatized with mBBr, and fluorescence was visualized after SDS/PAGE. Seventy micrograms of commercial wheat allergen extract was applied to all lanes, except lane 1, in 30 mM Tris⋅HCl, pH 7.9. Lane 1, NTS alone: NADPH, NTR, and thioredoxin (both from E. coli). Lane 2, DTT, heat: the sample was heated 5 min in boiling water in the presence of DTT. Lane 3, DTT/Trx: DTT and thioredoxin (from E. coli). Lane 4, NGS: NADPH, glutathione, glutathione reductase (from spinach leaves). Lane 5, NTS: NADPH, NTR, and thioredoxin (both from E. coli). Lane 6, control: no addition. Note that the excess NADPH and DTT maintained NTR, thioredoxin, and target allergen proteins in the reduced state throughout the experiment.
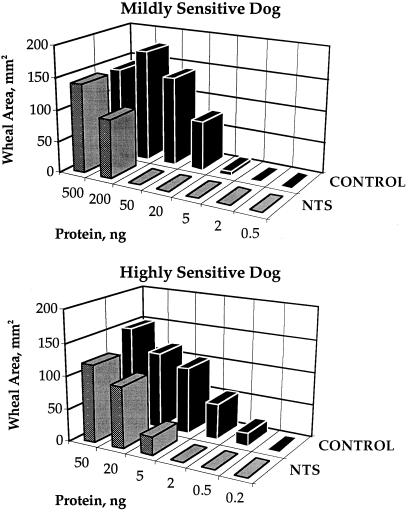
The allergic response to thioredoxin-treated preparations provided a positive answer to this question. The action of thioredoxin consistently lowered the extent of the allergic reaction as monitored by wheal area with both mildly and highly sensitive dogs (Fig. 2). Thus, a 10-fold increase in concentration was required to elicit a reaction after the allergen had been treated by thioredoxin. When tested with all dogs of the colony, thioredoxin mitigated the allergic response with 15 of the 16 animals (94%) showing sensitivity to wheat (Table 1). Mitigation was consistently observed when tested on different days with the same dog (see n values). Furthermore, the effectiveness of thioredoxin was independent of the degree of sensitivity—i.e., whether the animal was mildly or highly sensitive to wheat. Based on t test analysis, the results were statistically significant, with P values ranging from 0.0318 to 0.0050 as the allergen injected increased from 0.5 to 50 ng of protein (Table 2). The higher P values observed at the low allergen concentrations were ascribed to differences in the sensitivity of the highly and mildly sensitive animals to wheat. Although greatly decreased, significant allergenicity always remained after thioredoxin treatment, indicating that accessibility to protein epitopes could not be completely eliminated at high protein concentrations.
Figure 2.
Thioredoxin-linked mitigation of response to commercial wheat allergen extract determined by skin tests in two dogs of differing sensitivity. The type I hypersensitivity reaction determined by the wheal area (mm2) was induced by 100-μl intradermal injections of commercial wheat allergen extract (a 2 × 107 dilution = 1 ng of injected protein) either treated with the NADP/thioredoxin system (NTS) or untreated (Control). The response of two dogs is shown to illustrate the two types of skin reactions, mild sensitivity (dog A) and high sensitivity (dog L) (see Table 1). A PBS/glycerol control was consistently found to be negative for each dog.
Table 1.
Thioredoxin-linked mitigation of response to commercial wheat allergen in individual dogs of the colony
| Dog | Allergic to wheat | Mitigation of allergenicity | n* |
|---|---|---|---|
| A | + | Yes | 1 |
| B | ++ | Yes | 3 |
| C | + | Yes | 3 |
| D | + | Slight | 2 |
| E | + | Yes | 1 |
| F | + | Yes | 2 |
| G | + | Yes | 1 |
| H | + | Yes | 2 |
| I | + | Yes | 2 |
| J | ++ | Yes | 1 |
| K | + | Yes | 1 |
| L | ++ | Yes | 2 |
| M | + | No | 1 |
| N | ++ | Yes | 2 |
| O | + | Yes | 1 |
| P | + | Yes | 1 |
| Q | − | NA | NA |
| R | − | NA | NA |
| S | − | NA | NA |
| T | − | NA | NA |
| U | − | NA | NA |
| Total dogs tested21 | 16 | ||
| Total positive16 | 15 | ||
| % mitigation | 94 | ||
Mild sensitivity (+) indicates a positive skin test with an allergen dilution of 3 × 105; 1 ng = 2 × 107 dilution. High sensitivity (++) indicates a positive reaction with an allergen dilution of 106 or more. NA, not applicable. Dogs A through U correspond, respectively, to the following dogs in the colony: 6GCB2 (A), 6GCB3 (B), 6CGB1 (C), 6CGB6 (D), 6CGB5 (E), 6GCB4 (F), 6GCB8 (G), 6GCB11 (H), 6GCB7 (I), 6CGB9 (J), 6CGB7 (K), 6CGB8 (L), 6CGB4 (M), 5CBB1 (N), 6CBB3 (O), 5CBB5 (P), 5CBB6 (Q), 6GCB1 (R), 6GCB5 (S), 6GCB9 (T) and 6GCB10 (U).
n = number of wheat commercial allergen mitigation skin tests.
Table 2.
Statistical significance of thioredoxin-linked mitigation of response to commercial wheat allergen in all sensitive dogs of the colony
| Allergen, ng* | t test value | P value | Mitigation |
|---|---|---|---|
| 50 | 4.08 | 0.0005 | Significant |
| 20 | 4.58 | 0.0001 | Significant |
| 5 | 2.68 | 0.0086 | Significant |
| 2 | 2.02 | 0.0309 | Significant |
| 0.5 | 2.00 | 0.0318 | Significant |
A P value of less than 0.05 indicates statistically significant mitigation.
One nanogram of injected protein = 2 × 107 dilution of commercial wheat allergen extract.
Osborne Fractions.
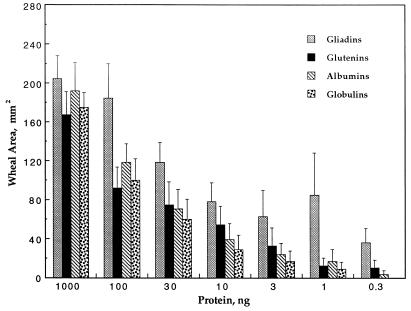
The proteins of wheat flour have classically been divided into four classes on the basis of solubility in the indicated solvent: albumins (distilled water) account for 15% of the total protein, globulins (salt water) for 5%, gliadins (ethanol) for 40%, and glutenins (acetic acid) also for 40% (34). Each of these fractions, whose abundance varies somewhat among different wheat cultivars, contains intramolecular disulfide bonds that are known to be effectively and in some cases specifically reduced by thioredoxin (14, 15). The results of Fig. 3 show that, while each fraction elicited an immunological reaction, there was a difference in response. In animals showing a range of sensitivity to wheat, gliadins were consistently the strongest allergens, glutenins were second, albumins third, and globulins fourth (Fig. 3). On the basis of their abundance noted above, the relative importance of the four flour fractions as food allergens in dogs would follow the order gliadin > glutenin > albumin > globulin. It remains to be seen whether the activity observed with the glutenins as well as the albumins and globulins is due to contaminating gliadins or to sequence homologies between gliadins and authentic members of other families (35).
Figure 3.
Relative allergenicity of wheat Osborne fractions. The full range of concentrations (1,000 to 0.3 ng) was tested with four dogs, three mildly and one highly sensitive. The concentration range from 100 to 3 ng of protein was tested with an additional four dogs, one mildly and three highly sensitive; response to this same concentration range was repeated with two mildly and one highly sensitive dogs. The data from all of these trials are incorporated. The error bars refer to standard error.
As observed with the commercial allergen preparations, thioredoxin treatment decreased the allergic response elicited by the flour fractions, most notably by the gliadins and glutenins (Table 3). The mitigating effect of thioredoxin, which was similar for the highly and mildly sensitive dogs, was consistently observed with gliadins and glutenins tested in six dogs over a 2-year period (8 and 12 trials for the protein fractions, respectively). The albumins and globulins also responded to thioredoxin, but, as seen in Table 3, in a less consistent manner. Reasons for this tendency toward inconsistency with the water-soluble proteins are not known.
Table 3.
Thioredoxin-linked mitigation of the allergic response to the Osborne fractions
| Protein, ng | %
mitigation
|
|||
|---|---|---|---|---|
| Gliadins | Glutenins | Albumins | Globulins | |
| Highly sensitive dog | ||||
| 30 | 53 | 17 | 33 | 27 |
| 10 | 66 | 20 | 44 | 0 |
| 3 | 100 | 62 | 69 | 66 |
| 1 | 100 | 100 | 46 | 100 |
| 0.3 | 100 | 100 | — | — |
| Mildly sensitive dog | ||||
| 300 | 50 | 100 | 33 | 75 |
| 100 | 10 | 100 | 0 | 100 |
| 30 | 78 | 100 | — | — |
| 10 | 100 | — | — | — |
Percent mitigation is defined as the wheal area of the control minus that observed after treatment divided by that of the control times 100. Dogs B and C, were used for the highly and mildly sensitive dogs, respectively (see Table 1).
Gliadin Fractions.
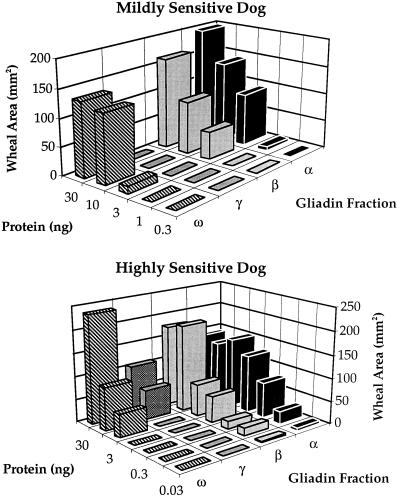
As the gliadin fraction of flour proved to be most active, it was of interest to determine the relative allergenicity of the purified components, which are made up of monomeric proteins of well defined types. The principal types, α, β, and γ, contain intramolecular disulfide groups (35), that, among physiological reductants, are most effectively reduced by thioredoxin (14). The evidence available indicates that the α- and β-gliadin group contains three intrachain disulfide bonds per molecule and that the corresponding number for the γ-gliadins is four. The molecular mass of these proteins, which are devoid of interchain disulfides, ranges from 30 to 50 kDa. The less abundant ω-type lacks disulfides. Two of the fractions, α and β, originally believed to be different, are now considered to fall into a single group (35, 36). On the basis of scanning acidic-PAGE gels, the relative abundance of the gliadins in our preparation was found to be 60% α + β, 30% γ, and 10% ω. These figures fall within the range reported by others (37).
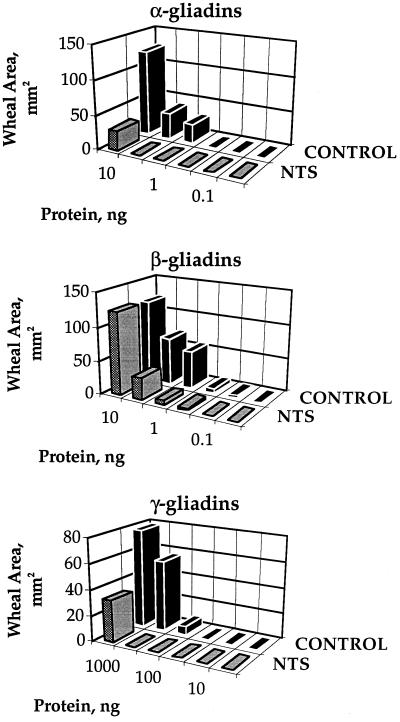
Highly and mildly sensitive dogs showed similar responses to the different gliadins: α similar to β > ω > γ (Fig. 4). Furthermore, of the total of 16 dogs showing sensitivity to wheat, 12 showed the strongest response to the α- and β-gliadins, 1 showed an equal response to all gliadin fractions, and 3 were not sensitive to gliadins (data not shown). Thus, 12 of 13 gliadin-sensitive animals showed the greatest response to α- and β-gliadins. On this basis, it appears that α- plus β-type gliadins represent the major allergenic forms. Significantly, their allergenicity, like the weaker γ-type, was consistently decreased on reduction by thioredoxin (Fig. 5). The results suggest that the ability of thioredoxin to mitigate effects of the commercial wheat allergen preparation was linked to gliadin sensitivity. Thus, the two dogs that showed either no or slight response to treatment of the commercial preparation with thioredoxin were insensitive to gliadins (D and M, Table 1).
Figure 4.
Thioredoxin-linked mitigation of skin test response to different Osborne fractions determined in two dogs of different sensitivity. Dogs A and L were used for the highly and mildly sensitive dogs, respectively (see Table 1).
Figure 5.
Thioredoxin-linked mitigation of skin test response to S-rich gliadins determined in a mildly sensitive dog. Dog C was used (see Table 1).
DISCUSSION
The present findings add a new dimension to our understanding of the consequences of the reduction of intramolecular disulfide bonds of proteins by thioredoxin. Thus, the ability to mitigate an allergic response in a canine model system can now be added to the changes identified earlier—i.e., a pronounced shift in biochemical activity and an increase in susceptibility to proteolysis and heat. Apparently, with most of the dogs, a decrease in accessibility or an actual obliteration of specific IgE epitopes accompanies the structural changes effected by thioredoxin. It is noteworthy that the reduction of intramolecular disulfide bonds—typically a facile, specific reaction for thioredoxin—has such significant consequences on immunological activity. Even more extensive decreases in allergenicity have recently been reported for the asthma house dust mite allergen in which cysteine residues were replaced by site-directed mutagenesis with amino acids incapable of forming disulfide bonds (38, 39). Collectively these studies emphasize the importance of the disulfide bond within protein allergen molecules and in so doing complement similar findings with enzyme inhibitors (8–11) and venom neurotoxins (12). In this as in the earlier work from our laboratory, thioredoxin could not be replaced by other thiol reagents: monothiols such as reduced glutathione were inactive and dithiols such as DTT were less specific and in some cases slow to react.
In addition to giving new insight into the effect of thioredoxin on target proteins, the present investigation adds information on the food allergens of wheat. Most of the work on wheat allergy has been done with occupationally sensitized asthma patients (baker’s asthma). Although it is generally concluded that the albumin fraction, notably the α-amylase-inhibitor proteins, is the main cause (40), some studies conclude that gliadins and glutenins are also major allergens in baker’s asthma (41, 42). Similarly, our studies support the importance of gliadins and glutenins based on the canine food allergy model. It will be of interest to perform epitope analyses of the most active allergens to determine if they are structurally related.
As determined by skin tests with our model, the α- and β-gliadins evoked the strongest response. This finding is of particular interest because it suggests a possible parallel between food allergy and celiac disease—a condition in which these same proteins appear to play a primary role (43, 44). It will be of interest to determine whether thioredoxin alleviates the effect of the disulfide proteins specifically identified for celiac disease as well as baker’s asthma. Significantly, the allergenicity of such disulfide proteins may be related to their heat stability, resistance to proteolytic digestion (45), and, for glycoproteins, carbohydrate content (46).
A final question concerns the clinical relevance of this work—that is, whether the ability of thioredoxin to mitigate the allergic response to wheat has potential application. While this question can be answered only after additional experiments with sera of wheat-sensitive patients, we may say at this point that wheat is being considered a model for other foods. Accordingly, given the appropriate food, the thioredoxin system could possibly be used as a treatment to provide hypoallergenic as well as more digestible diets for infants and young animals. With this in mind, we have initiated work on soy and milk to determine if the associated allergenicity can also be decreased by thioredoxin either alone or in combination with other treatments.
Acknowledgments
We thank Dr. I. Besse and Mr. K. Habben for their help with the gliadin separation procedure and Drs. K. Ishizaka and J. Yodoi for their comments on the manuscript. The biochemical aspects of this study were supported by National Science Foundation Grant MCB-9316496. We also acknowledge a gift from Cargill Incorporated.
ABBREVIATIONS
- NTR
NADP–thioredoxin reductase
- mBBr
monobromobimane
- DTT/Trx
dithiothreitol/thioredoxin
- NTS
NADP/thioredoxin system
- NGS
NADP/glutathione system
References
- 1.Holmgren A. Annu Rev Biochem. 1985;54:237–271. doi: 10.1146/annurev.bi.54.070185.001321. [DOI] [PubMed] [Google Scholar]
- 2.Holmgren A. J Biol Chem. 1989;264:13963–13966. [PubMed] [Google Scholar]
- 3.Buchanan B B. Arch Biochem Biophys. 1991;288:1–9. doi: 10.1016/0003-9861(91)90157-e. [DOI] [PubMed] [Google Scholar]
- 4.Buchanan B B, Schürmann P, Decottignies P, Lozano R M. Arch Biochem Biophys. 1994;314:257–260. doi: 10.1006/abbi.1994.1439. [DOI] [PubMed] [Google Scholar]
- 5.Williams C H., Jr FASEB J. 1995;9:1267–1276. doi: 10.1096/fasebj.9.13.7557016. [DOI] [PubMed] [Google Scholar]
- 6.Wada K, Buchanan B B. FEBS Lett. 1981;124:237–240. [Google Scholar]
- 7.Johnson T C, Wada K, Buchanan B B, Holmgren A. Plant Physiol. 1987;85:446–451. doi: 10.1104/pp.85.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kobrehel K, Yee B C, Buchanan B B. J Biol Chem. 1991;266:16135–16140. [PubMed] [Google Scholar]
- 9.Jiao J, Yee B C, Kobrehel K, Buchanan B B. J Agric Food Chem. 1992;40:2333–2336. [Google Scholar]
- 10.Jiao J, Yee B C, Wong J H, Kobrehel K, Buchanan B B. Plant Physiol Biochem. 1993;31:799–804. [Google Scholar]
- 11.Wong J H, Jiao J-A, Kobrehel K, Buchanan B B. Plant Physiol. 1995;108:67. [Google Scholar]
- 12.Lozano R M, Yee B C, Buchanan B B. Arch Biochem Biophys. 1994;309:356–362. doi: 10.1006/abbi.1994.1124. [DOI] [PubMed] [Google Scholar]
- 13.Shin S, Wong J H, Kobrehel K, Buchanan B B. Planta. 1993;189:557–560. [Google Scholar]
- 14.Kobrehel K, Wong J H, Balogh A, Kiss F, Yee B C, Buchanan B B. Plant Physiol. 1992;99:919–924. doi: 10.1104/pp.99.3.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong J H, Kobrehel K, Nimbona C, Yee B C, Balogh A, Kiss F, Buchanan B B. Cereal Chem. 1993;70:113–114. [Google Scholar]
- 16.Besse I, Wong J H, Kobrehel K, Buchanan B B. Proc Natl Acad Sci USA. 1996;93:3169–3175. doi: 10.1073/pnas.93.8.3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lehrer S B, Horner W E, Reese G. Crit Rev Food Sci Nutr. 1996;36:553–564. doi: 10.1080/10408399609527739. [DOI] [PubMed] [Google Scholar]
- 18.Gomez L, Martin E, Hernandez D, Sanchez-Monge R, Barber D, Del Pozo V, De Andres B, Armentia A, Lahoz C, Salcedo G, Palomino P. FEBS Lett. 1990;261:85–88. doi: 10.1016/0014-5793(90)80642-v. [DOI] [PubMed] [Google Scholar]
- 19.Youle R J, Huang A H C. Plant Physiol. 1978;61:1040–1042. doi: 10.1104/pp.61.6.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teuber, S. S., Dandekar, A. M. & Peterson, W. R. (1997) J. Allergy Clin. Immunol., in press. [DOI] [PubMed]
- 21.Ogawa T, Bando N, Tsuji H, Okajima H, Nishikawa K, Sasoka K. J Nutr Sci Vitaminol. 1991;37:555–565. doi: 10.3177/jnsv.37.555. [DOI] [PubMed] [Google Scholar]
- 22.Thorpe S C, Kemeny D M, Panzani R C, McGurl B, Lord M. J Allergy Clin Immunol. 1988;82:67–72. doi: 10.1016/0091-6749(88)90053-x. [DOI] [PubMed] [Google Scholar]
- 23.Frick O L, Brooks D L. Am J Vet Res. 1983;44:440–445. [PubMed] [Google Scholar]
- 24.Ermel R W, Koch M, Griffey S M, Reinhart G A, Frick O L. Lab Animal Sci. 1997;47:40–49. [PubMed] [Google Scholar]
- 25.Florencio F J, Yee B C, Johnson T C, Buchanan B B. Arch Biochem Biophys. 1988;266:496–507. doi: 10.1016/0003-9861(88)90282-2. [DOI] [PubMed] [Google Scholar]
- 26.Frick O L, Derer M, Bigler B, De Week A L. J Allergy Clin Immunol. 1993;91:316. (abstr.). [Google Scholar]
- 27.Barker S, Frick O L. J Allergy Clin Immunol. 1991;87:176. (abstr.). [Google Scholar]
- 28.Kobrehel K, Bushuk W. Cereal Chem. 1977;54:833–839. [Google Scholar]
- 29.Bradford M. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 30.Anonymous . Bio-Rad DC Protein Assay Instruction Manual. Hercules, CA: Bio-Rad; 1992. [Google Scholar]
- 31.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 32.Bushuk W, Zillman R R. Can J Plant Sci. 1978;58:505–515. [Google Scholar]
- 33.Sapirstein H D, Bushuk W. Cereal Chem. 1985;62:372–377. [Google Scholar]
- 34.Payne P I, Rhodes A P. Encyclopaedia of Plant Physiology, New Series. 14A. Berlin: Springer; 1982. pp. 346–369. [Google Scholar]
- 35.Shewry P R, Napier J A, Tatham A S. Plant Cell. 1995;7:945–956. doi: 10.1105/tpc.7.7.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shewry P R, Tatham A S. Biochem J. 1990;267:1–12. doi: 10.1042/bj2670001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wieser H, Seilmeier W, Belitz H-D. J Cereal Sci. 1994;19:149–155. [Google Scholar]
- 38.Nishiyama C, Fukada M, Usui Y, Iwamoto N, Yuuki T, Okumura Y, Okudaira H. Molec Immunol. 1995;32:1021–1029. doi: 10.1016/0161-5890(95)00091-7. [DOI] [PubMed] [Google Scholar]
- 39.Smith A M, Chapman M D. Molec Immunol. 1996;33:399–405. doi: 10.1016/0161-5890(95)00150-6. [DOI] [PubMed] [Google Scholar]
- 40.Fraenken J, Koenig W. Allergologie. 1996;19:21–28. [Google Scholar]
- 41.Walsh B J, Wrigley C W, Musk A W, Baldo B A. J Allergy Clin Immunol. 1985;76:23–28. doi: 10.1016/0091-6749(85)90799-7. [DOI] [PubMed] [Google Scholar]
- 42.Sandiford C P, Tatham A, Jones M G, Fido R, Tee R D, Shewry P R, Taylor A J N. J Allergy Clin Immunol. 1996;97:202. [Google Scholar]
- 43.Kagnoff M F, Austin R K, Johnson H C L, Bernardin J E, Dietler M D, Kasarda D D. J Immunol. 1982;129:2693–2697. [PubMed] [Google Scholar]
- 44.Howdle P D, Blair G E. Gut. 1992;33:573–575. doi: 10.1136/gut.33.5.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Astwood J D, Leach J N, Fuchs R L. Nat Biotechnol. 1996;14:1269–1273. doi: 10.1038/nbt1096-1269. [DOI] [PubMed] [Google Scholar]
- 46.Garcia-Casado G, Sanchez-Monge R, Chrispeels M J, Armentia A, Salcedo G, Gomez L. Glycobiol. 1996;6:471–477. doi: 10.1093/glycob/6.4.471. [DOI] [PubMed] [Google Scholar]