Abstract
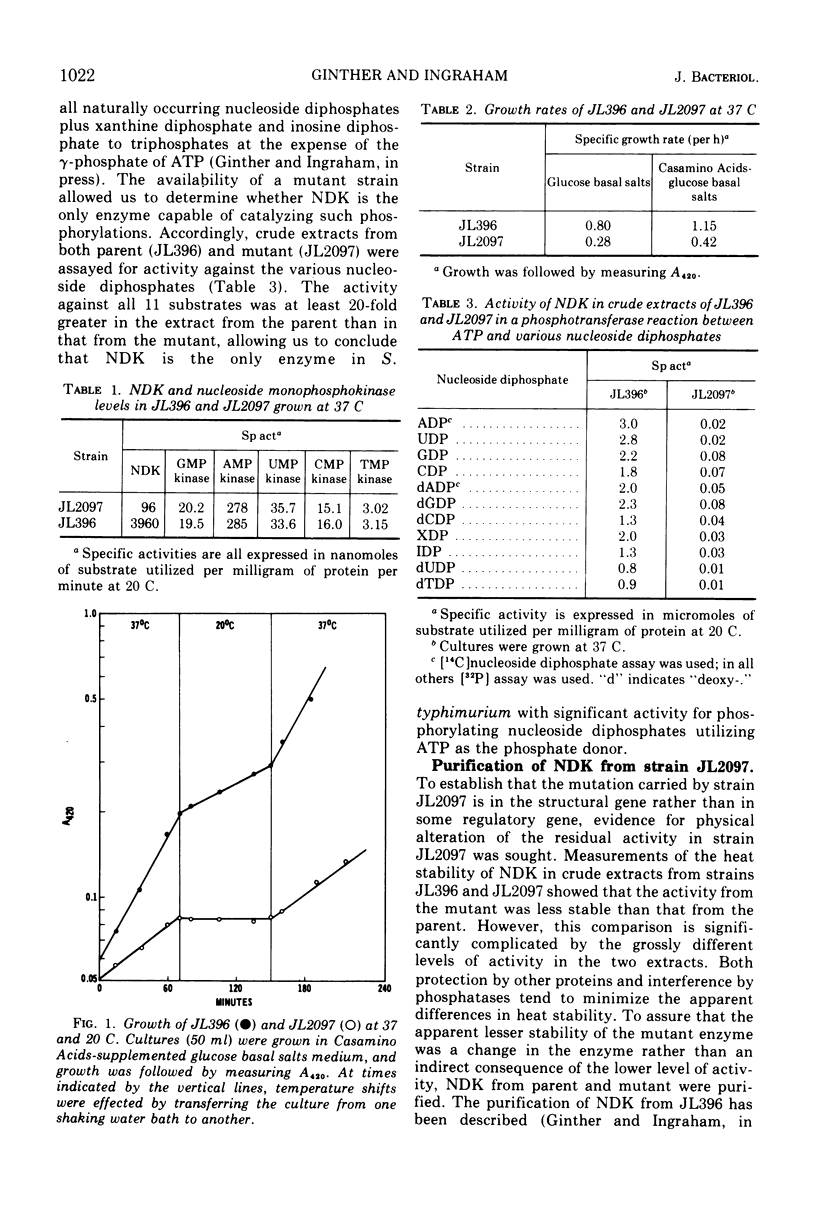
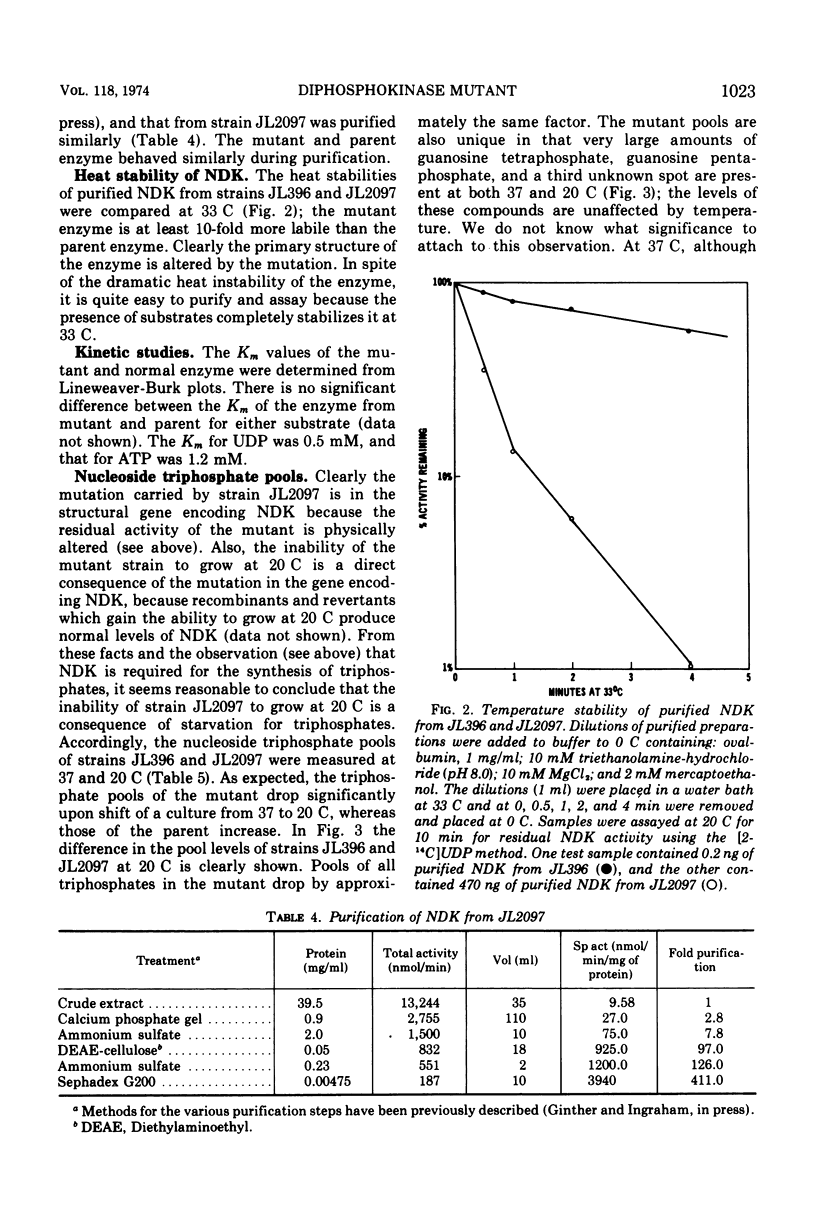
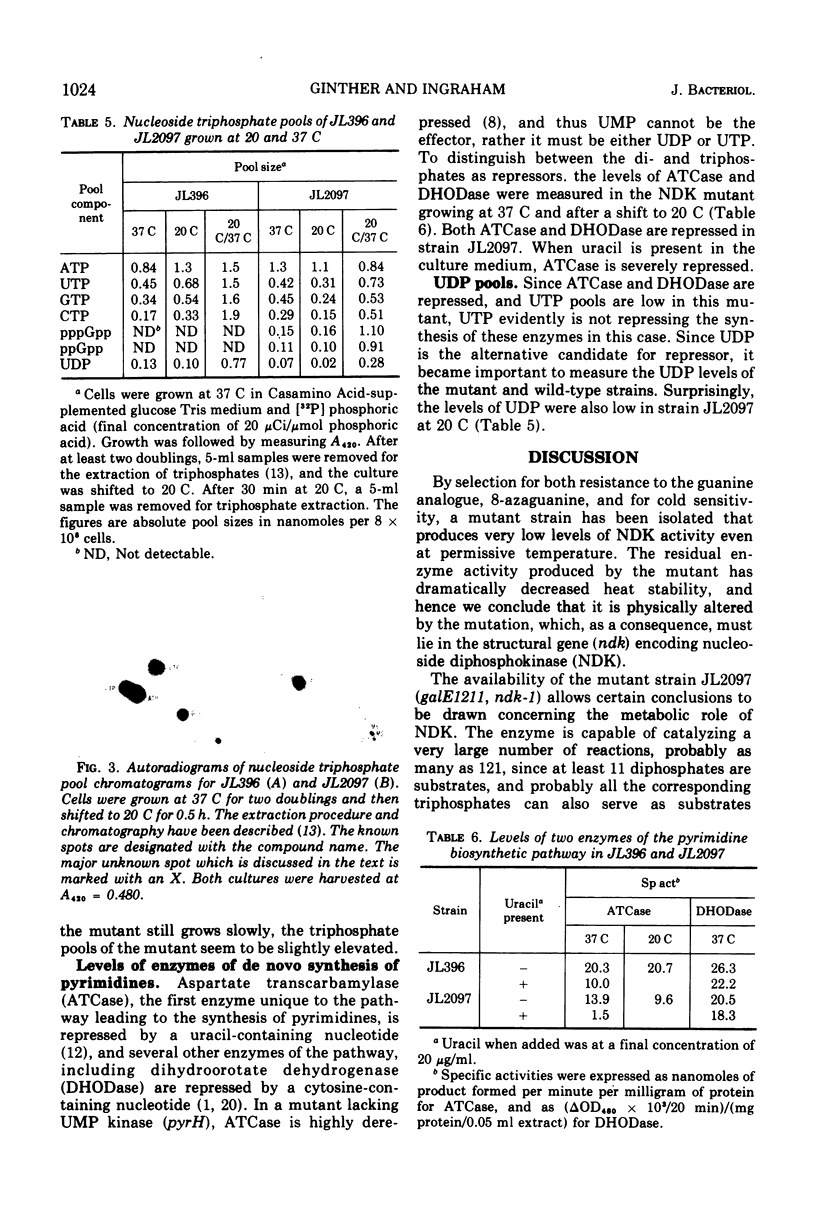
A cold-sensitive mutant of Salmonella typhimurium defective in nucleosidediphosphokinase (ATP:nucleosidediphosphate phosphotransferase, EC 2.7.4.6) has been isolated and characterized. The mutant contains only 2% of the enzyme activity found in the parent, and the heat lability of this activity is 10 times that from the parent at 33 C. Mutant extracts lack the ability to convert any of 11 nucleoside diphosphates tested to the corresponding nucleoside triphosphates, but the nucleosidemonophosphatase activities are normal. Although the nucleoside triphosphate pools of the mutant are depressed significantly at the restrictive temperature (20 C), they are slightly elevated at the permissive temperature (37 C). The levels of guanosine pentaphosphate and guanosine tetraphosphate are dramatically increased. Two representative enzymes of pyrimidine de novo synthesis, aspartic transcarbamylase and dihydroorotate dehydrogenase, are fully repressed at both 37 and 20 C. Intracellular pools of uridine diphosphate are depressed at both permissive and restrictive temperature.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abd-el-Al A., Ingraham J. L. Control of carbamyl phosphate synthesis in Salmonella typhimurium. J Biol Chem. 1969 Aug 10;244(15):4033–4038. [PubMed] [Google Scholar]
- Colomb M. G., Chéruy A., Vignais P. V. Nucleoside diphosphokinase from beef heart cytosol. I. Physical and kinetic properties. Biochemistry. 1972 Aug 29;11(18):3370–3378. doi: 10.1021/bi00768a009. [DOI] [PubMed] [Google Scholar]
- Edlund B., Rask L., Olsson P., Wålinder O., Zetterqvist O., Engström L. Preparation of crystalline nucleoside diphosphate kinase from baker's yeast and identification of 1-[32P]phosphohistidine as the main phosphorylated product of an alkaline hydrolysate of enzyme incubated with adenosine [32P]triphosphate. Eur J Biochem. 1969 Jul;9(4):451–455. doi: 10.1111/j.1432-1033.1969.tb00630.x. [DOI] [PubMed] [Google Scholar]
- GERHART J. C., PARDEE A. B. The enzymology of control by feedback inhibition. J Biol Chem. 1962 Mar;237:891–896. [PubMed] [Google Scholar]
- Garces E., Cleland W. W. Kinetic studies of yeast nucleoside diphosphate kinase. Biochemistry. 1969 Feb;8(2):633–640. doi: 10.1021/bi00830a026. [DOI] [PubMed] [Google Scholar]
- Ingraham J. L., Neuhard J. Cold-sensitive mutants of Salmonella typhimurium defective in uridine monophosphate kinase (pyrH). J Biol Chem. 1972 Oct 10;247(19):6259–6265. [PubMed] [Google Scholar]
- LEVIN D. H. The incorporation of 8-azaguanine into soluble ribonucleic acid of Bacillus cereus. J Biol Chem. 1963 Mar;238:1098–1104. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MANDEL H. G., SUGARMAN G. I., APTER R. A. Fractionation studies of Bacillus cereus containing 8-azaguanine. J Biol Chem. 1957 Mar;225(1):151–156. [PubMed] [Google Scholar]
- Neuhard J., Ingraham J. Mutants of Salmonella typhimurium requiring cytidine for growth. J Bacteriol. 1968 Jun;95(6):2431–2433. doi: 10.1128/jb.95.6.2431-2433.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhard J., Thomassen E. Turnover of the deoxyribonucleoside triphosphates in Escherichia coli 15 T during thymine starvation. Eur J Biochem. 1971 May 11;20(1):36–43. doi: 10.1111/j.1432-1033.1971.tb01359.x. [DOI] [PubMed] [Google Scholar]
- Norman A. W., Wedding R. T., Black M. K. Detection of phosphohistidine in nucleoside diphosphokinase isolated from Jerusalem artichoke mitochondria. Biochem Biophys Res Commun. 1965 Sep 22;20(6):703–709. doi: 10.1016/0006-291x(65)90073-2. [DOI] [PubMed] [Google Scholar]
- O'Donovan G. A., Gerhart J. C. Isolation and partial characterization of regulatory mutants of the pyrimidine pathway in Salmonella typhimurium. J Bacteriol. 1972 Mar;109(3):1085–1096. doi: 10.1128/jb.109.3.1085-1096.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri R., Yue R. H., Jacobs H. K., Maland L., Wu L., Kuby S. A. Nucleoside triphosphate-nucleoside diphosphate transphosphorylase (nucleoside diphosphokinase). 3. Subunit structure of the crystalline enzyme from brewers' yeast. J Biol Chem. 1973 Jun 25;248(12):4486–4499. [PubMed] [Google Scholar]
- RATLIFF R. L., WEAVER R. H., LARDY H. A., KUBY S. A. NUCLEOSIDE TRIPHOSPHATE-NUCLEOSIDE DIPHOSPHATE TRANSPHOSPHORYLASE (NUCLEOSIDE DIPHOSPHOKINASE). I. ISOLATION OF THE CRYSTALLINE ENZYME FROM BREWERS' YEAST. J Biol Chem. 1964 Jan;239:301–309. [PubMed] [Google Scholar]
- Sedmak J., Ramaley R. Purification and properties of Bacillus subtilis nucleoside diphosphokinase. J Biol Chem. 1971 Sep 10;246(17):5365–5372. [PubMed] [Google Scholar]
- Williams J. C., O'Donovan G. A. Repression of enzyme synthesis of the pyrimidine pathway in Salmonella typhimurium. J Bacteriol. 1973 Sep;115(3):1071–1076. doi: 10.1128/jb.115.3.1071-1076.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue R. H., Ratliff R. L., Kuby S. A. Nucleoside triphosphate-nucleoside diphosphate transphosphorylase (nucleoside diphosphokinase). II. Physical properties of the crystalline enzyme from brewers' yeast. Biochemistry. 1967 Sep;6(9):2923–2932. doi: 10.1021/bi00861a037. [DOI] [PubMed] [Google Scholar]



