Abstract
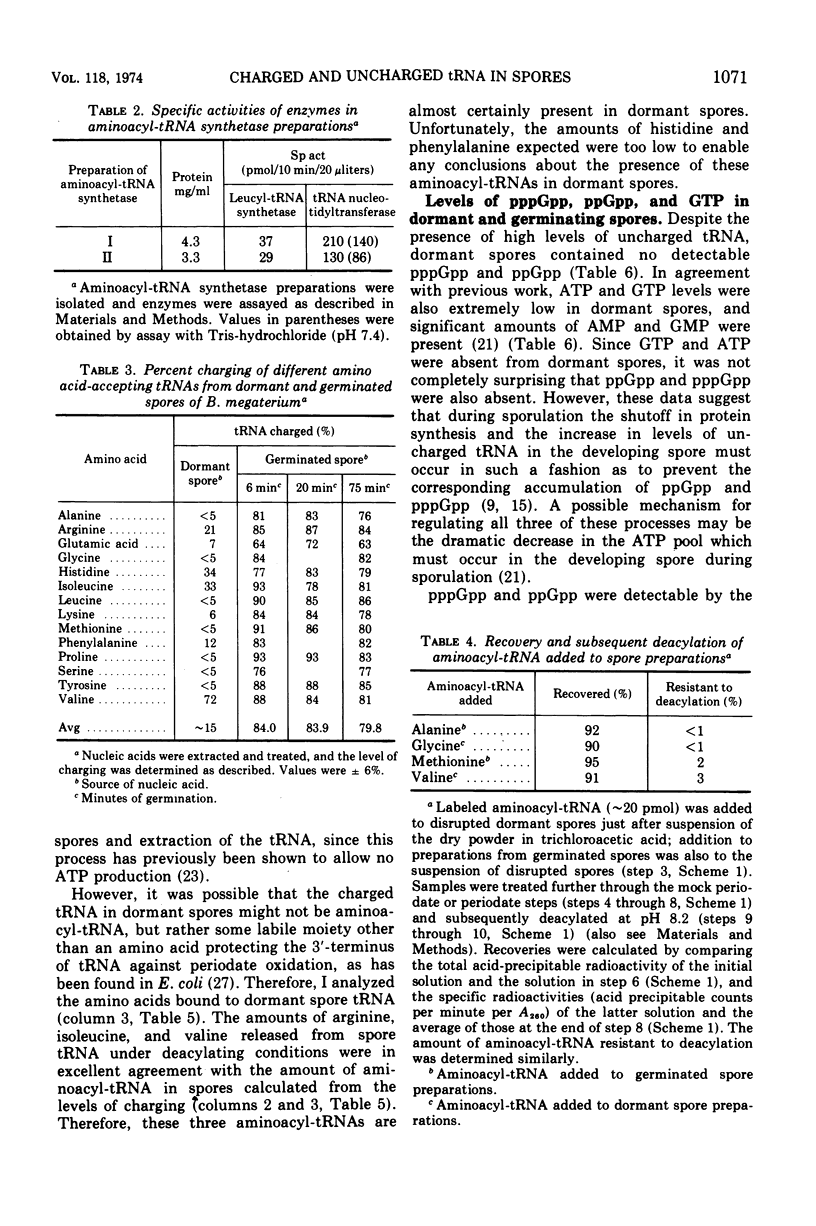
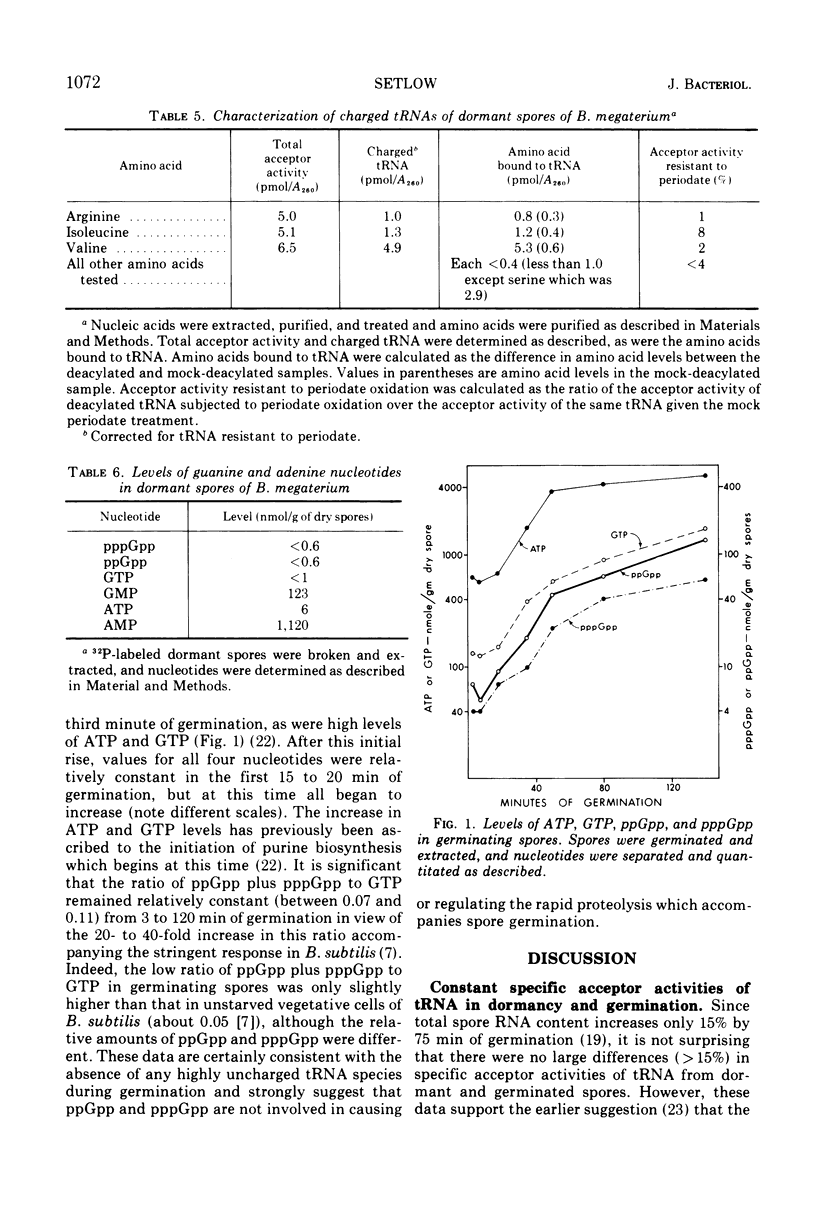
The levels of transfer ribonucleic acids (tRNAs) specific for 14 amino acids were almost identical in dormant spores and in spores germinated from 6 to 75 min. Germinated spore tRNAs specific for all amino acids tested were between 63 and 93% charged, and there was no significant change in this value from 6 to 75 min of germination. In contrast, tRNAs isolated from dormant spores specific for nine different amino acids were almost completely(>93%) uncharged. However, some dormant spore tRNAs, i.e., those for arginine, histidine, isoleucine, and valine, showed significant (21 to 72%) levels of aminoacylation. Dormant spores contained no detectable guanosine penta- (pppGpp), tetra- (ppGpp), or triphosphate (GTP). However, these nucleotides appeared in the first minutes of germination, and thereafter all increased in parallel with a ratio of pppGpp plus ppGpp to GTP of 0.07 to 0.11, which is characteristic of unstarved vegetative cells.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brenner M., De Lorenzo F., Ames B. N. Energy charge and protein synthesis. Control of aminoacyl transfer ribonucleic acid synthetases. J Biol Chem. 1970 Jan 25;245(2):450–452. [PubMed] [Google Scholar]
- Cashel M., Kalbacher B. The control of ribonucleic acid synthesis in Escherichia coli. V. Characterization of a nucleotide associated with the stringent response. J Biol Chem. 1970 May 10;245(9):2309–2318. [PubMed] [Google Scholar]
- Cashel M. The control of ribonucleic acid synthesis in Escherichia coli. IV. Relevance of unusual phosphorylated compounds from amino acid-starved stringent strains. J Biol Chem. 1969 Jun 25;244(12):3133–3141. [PubMed] [Google Scholar]
- Chambon P., Deutscher M. P., Kornberg A. Biochemical studies of bacterial sporulation and germination. X. Ribosomes and nucleic acids of vegetative cells and spores of Bacillus megaterium. J Biol Chem. 1968 Oct 10;243(19):5110–5116. [PubMed] [Google Scholar]
- Deutscher M. P., Chambon P., Konberg A. Biochemical studies of bacterial sporulation and germination. XI. Protein-synthesizing systems from vegetative cells and spores of Bacillus megaterium. J Biol Chem. 1968 Oct 10;243(19):5117–5125. [PubMed] [Google Scholar]
- Folk W. R., Berg P. Characterization of altered forms of glycyl transfer ribonucleic acid synthetase and the effects of such alterations on aminoacyl transfer ribonucleic acid synthesis in vivo. J Bacteriol. 1970 Apr;102(1):204–212. doi: 10.1128/jb.102.1.204-212.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant J., Margason G. Amino acid control of messenger ribonucleic acid synthesis in Bacillus subtilis. J Biol Chem. 1972 Apr 25;247(8):2289–2294. [PubMed] [Google Scholar]
- Goldberg A. L. A role of aminoacyl-tRNA in the regulation of protein breakdown in Escherichia coli. Proc Natl Acad Sci U S A. 1971 Feb;68(2):362–366. doi: 10.1073/pnas.68.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseltine W. A., Block R. Synthesis of guanosine tetra- and pentaphosphate requires the presence of a codon-specific, uncharged transfer ribonucleic acid in the acceptor site of ribosomes. Proc Natl Acad Sci U S A. 1973 May;70(5):1564–1568. doi: 10.1073/pnas.70.5.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko I., Doi R. H. Alteration of valyl-sRNA during sporulation of bacillus subtilis. Proc Natl Acad Sci U S A. 1966 Mar;55(3):564–571. doi: 10.1073/pnas.55.3.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leibowitz M. J., Soffer R. L. Enzymatic modification of proteins. 3. Purification and properties of a leucyl, phenylalanyl transfer ribonucleic acid protein transferase from Escherichia coli. J Biol Chem. 1970 Apr 25;245(8):2066–2073. [PubMed] [Google Scholar]
- Lewis J. A., Ames B. N. Histidine regulation in Salmonella typhimurium. XI. The percentage of transfer RNA His charged in vivo and its relation to the repression of the histidine operon. J Mol Biol. 1972 Apr 28;66(1):131–142. doi: 10.1016/s0022-2836(72)80011-1. [DOI] [PubMed] [Google Scholar]
- Moskowitz J., Fain J. N. Stimulation by growth hormone and dexamethasone of labeled cyclic adenosine 3',5'-monophosphate accumulation by white fat cells. J Biol Chem. 1970 Mar 10;245(5):1101–1107. [PubMed] [Google Scholar]
- Neidhardt F. C. Roles of amino acid activating enzymes in cellular physiology. Bacteriol Rev. 1966 Dec;30(4):701–719. doi: 10.1128/br.30.4.701-719.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana R. S., Halvorson H. O. Nature of deoxyribonucleic acid synthesis and its relationship to protein synthesis during outgrowth of Bacillus cereus T. J Bacteriol. 1972 Feb;109(2):606–615. doi: 10.1128/jb.109.2.606-615.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SACKS L. E., BAILEY G. F. DRY RUPTURE OF BACTERIAL SPORES. J Bacteriol. 1963 Mar;85:720–721. doi: 10.1128/jb.85.3.720-721.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P., Kornberg A. Biochemical studies of bacterial sporulation and germination. 23. Nucleotide metabolism during spore germination. J Biol Chem. 1970 Jul 25;245(14):3645–3652. [PubMed] [Google Scholar]
- Setlow P., Kornberg A. Biochemical studies of bacterial sporulation and germination. XVII. Sulfhydryl and disulfide levels in dormancy and germination. J Bacteriol. 1969 Dec;100(3):1155–1160. doi: 10.1128/jb.100.3.1155-1160.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P., Kornberg A. Biochemical studies of bacterial sporulation and germination. XXII. Energy metabolism in early stages of germination of Bacillus megaterium spores. J Biol Chem. 1970 Jul 25;245(14):3637–3644. [PubMed] [Google Scholar]
- Setlow P. Polyamine levels during growth, sporulation, and spore germination of Bacillus megaterium. J Bacteriol. 1974 Mar;117(3):1171–1177. doi: 10.1128/jb.117.3.1171-1177.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P., Primus G., Deutscher M. P. Absence of 3'-terminal residues from transfer ribonucleic acid of dormant spores of Bacillus megaterium. J Bacteriol. 1974 Jan;117(1):126–132. doi: 10.1128/jb.117.1.126-132.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vold B. S. Variations in activity of aminoacyl-tRNA synthetases as a function of development in Bacillus subtilis. Arch Biochem Biophys. 1973 Feb;154(2):691–695. doi: 10.1016/0003-9861(73)90024-6. [DOI] [PubMed] [Google Scholar]
- Yegian C. D., Stent G. S., Martin E. M. Intracellular condition of Escherichia coli transfer RNA. Proc Natl Acad Sci U S A. 1966 Apr;55(4):839–846. doi: 10.1073/pnas.55.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]


