Abstract
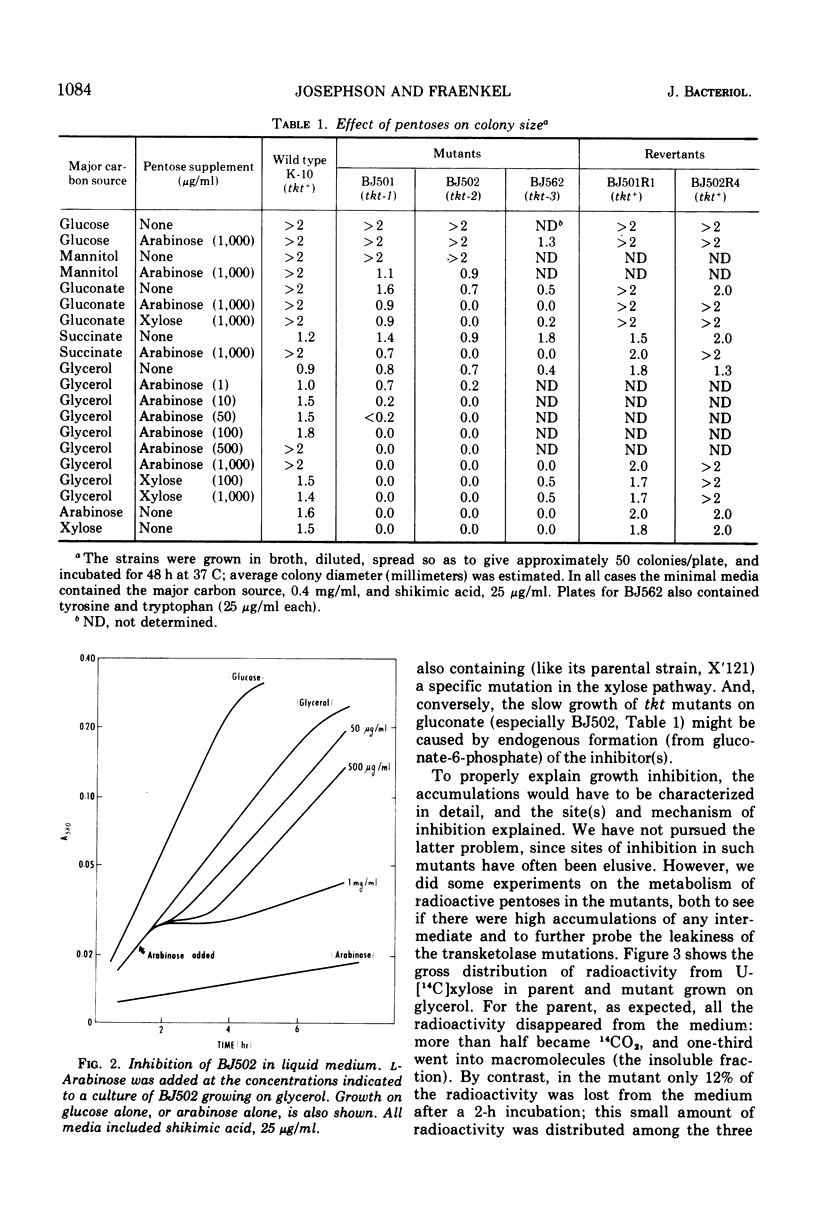
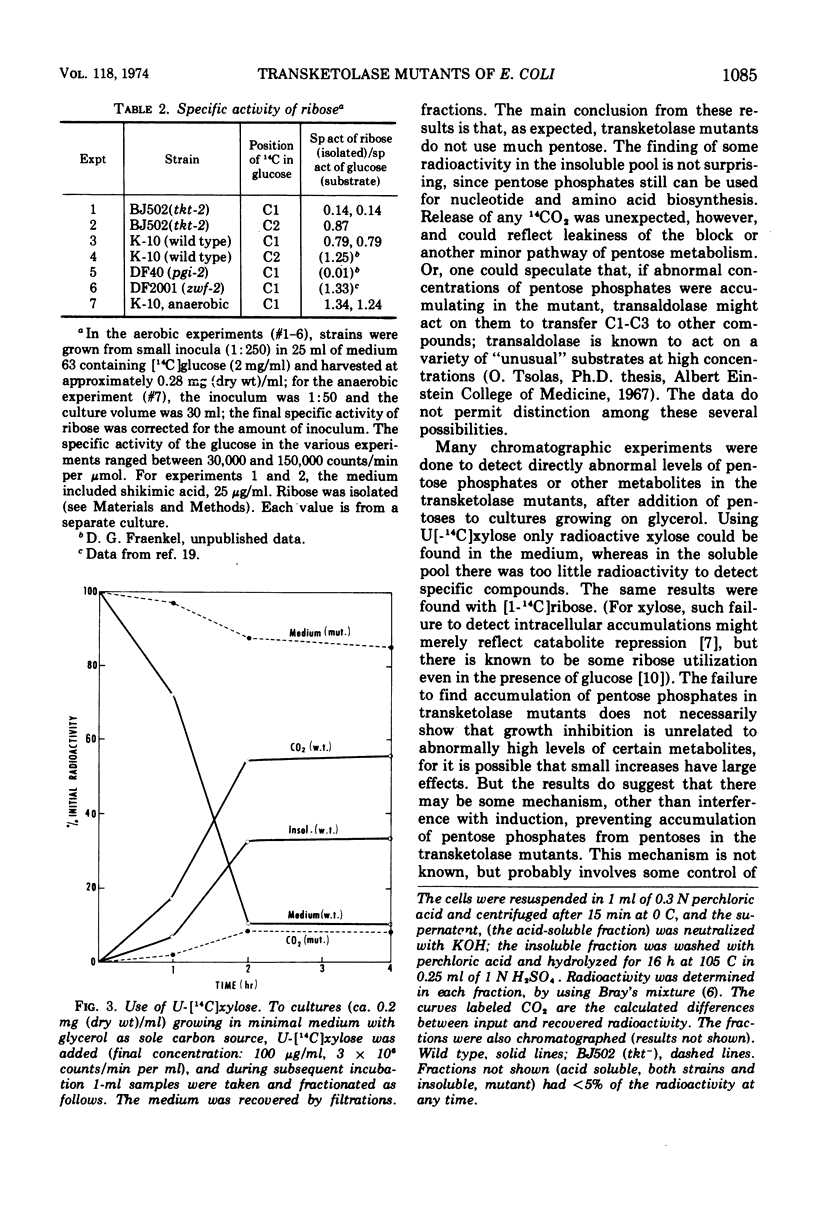
This paper continues the description of transketolase mutants of Escherichia coli; they are absolutely unable to grow on pentoses, but slightly “leaky” with respect to their aromatic requirement (B. L. Josephson and D. G. Fraenkel, 1969). Several experiments have explored the degree of leakiness and shown it to be low. There is little conversion of radioactive xylose to carbon dioxide. The labeling of ribose in cells grown on [1-14C]glucose and [2-14C]glucose accords with its origin being chiefly by the oxidative pathway. A mutant lacking both transketolase and gluconate-6-phosphate dehydrogenase has been constructed; it requires supplementation with pentose. Pentoses are inhibitory to growth of transketolase mutants, but high levels of pentose phosphates do not accumulate in this situation. Several experimental results are suggestive of regulation of metabolic flow in the oxidative branch of the hexose monophosphate shunt.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown A. T., Wittenberger C. L. Mechanism for regulating the distribution of glucose carbon between the Embden-Meyerhof and hexose-monophosphate pathways in Streptococcus faecalis. J Bacteriol. 1971 May;106(2):456–467. doi: 10.1128/jb.106.2.456-467.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri R. L., Sable H. Z. Enzymes of pentose biosynthesis. II. Evidence that the proposed control of glucose 6-phosphate dehydrogenase by reduced diphosphopyridine nucleotide is an instrumental artifact. J Biol Chem. 1973 Apr 25;248(8):2815–2817. [PubMed] [Google Scholar]
- Clark M. G., Williams J. F., Blackmore P. F. The transketolase exchange reaction in vitro. Biochem J. 1971 Nov;125(1):381–384. doi: 10.1042/bj1250381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David J. D., Wiesmeyer H. Control of xylose metabolism in Escherichia coli. Biochim Biophys Acta. 1970 Mar 24;201(3):497–499. doi: 10.1016/0304-4165(70)90171-6. [DOI] [PubMed] [Google Scholar]
- David J., Wiesmeyer H. Regulation of ribose metabolism in Escherichia coli. 3. Regulation of ribose utilization in vivo. Biochim Biophys Acta. 1970 Apr 14;208(1):68–76. doi: 10.1016/0304-4165(70)90049-8. [DOI] [PubMed] [Google Scholar]
- David J., Wiesmeyer H. Regulation of ribose metabolism in Escherichia coli. I. The ribose catabolic pathway. Biochim Biophys Acta. 1970 Apr 14;208(1):45–55. doi: 10.1016/0304-4165(70)90047-4. [DOI] [PubMed] [Google Scholar]
- David J., Wiesmeyer H. Regulation of ribose metabolism in Escherichia coli. II. Evidence for two ribose-5-phosphate isomerase activities. Biochim Biophys Acta. 1970 Apr 14;208(1):56–67. doi: 10.1016/0304-4165(70)90048-6. [DOI] [PubMed] [Google Scholar]
- ENGLESBERG E., ANDERSON R. L., WEINBERG R., LEE N., HOFFEE P., HUTTENHAUER G., BOYER H. L-Arabinose-sensitive, L-ribulose 5-phosphate 4-epimerase-deficient mutants of Escherichia coli. J Bacteriol. 1962 Jul;84:137–146. doi: 10.1128/jb.84.1.137-146.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidels L., Osborn M. J. Lipopolysaccharide and aldoheptose biosynthesis in transketolase mutants of Salmonella typhimurium. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1673–1677. doi: 10.1073/pnas.68.8.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraenkel D. G. The accumulation of glucose 6-phosphate from glucose and its effect in an Escherichia coli mutant lacking phosphoglucose isomerase and glucose 6-phosphate dehydrogenase. J Biol Chem. 1968 Dec 25;243(24):6451–6457. [PubMed] [Google Scholar]
- Johnson R., Krasna A. I., Rittenberg D. O studies on the oxidative and nonoxidative pentose phosphate pathways in wild-type and mutant Escherichia coli cells. Biochemistry. 1973 May 8;12(10):1969–1977. doi: 10.1021/bi00734a021. [DOI] [PubMed] [Google Scholar]
- Josephson B. L., Fraenkel D. G. Transketolase mutants of Escherichia coli. J Bacteriol. 1969 Dec;100(3):1289–1295. doi: 10.1128/jb.100.3.1289-1295.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J., Rognstad R. The labeling of pentose phosphate from glucose-14C and estimation of the rates of transaldolase, transketolase, the contribution of the pentose cycle, and ribose phosphate synthesis. Biochemistry. 1967 Jul;6(7):2227–2247. doi: 10.1021/bi00859a046. [DOI] [PubMed] [Google Scholar]
- Kelly B. L., Sunshine M. G. Association of temperate phage P2 with the production of histidine negative segregants by Escherichia coli. Biochem Biophys Res Commun. 1967 Jul 21;28(2):237–243. doi: 10.1016/0006-291x(67)90435-4. [DOI] [PubMed] [Google Scholar]
- Kupor S. R., Fraenkel D. G. Glucose metabolism in 6 phosphogluconolactonase mutants of Escherichia coli. J Biol Chem. 1972 Mar 25;247(6):1904–1910. [PubMed] [Google Scholar]
- LJUNGDAHL L., WOOD H. G., RACKER E., COURI D. Formation of unequally labeled fructose 6-phosphate by an exchange reaction catalyzed by transaldolase. J Biol Chem. 1961 Jun;236:1622–1625. [PubMed] [Google Scholar]
- Model P., Rittenberg D. Measurement of the activity of the hexose monophosphate pathway of glucose metabolism with the use of [18O]glucose. Variations in its activity in Escherichia coli with growth conditions. Biochemistry. 1967 Jan;6(1):69–80. doi: 10.1021/bi00853a013. [DOI] [PubMed] [Google Scholar]
- Osborn M. J. Structure and biosynthesis of the bacterial cell wall. Annu Rev Biochem. 1969;38:501–538. doi: 10.1146/annurev.bi.38.070169.002441. [DOI] [PubMed] [Google Scholar]
- Sanwal B. D. Regulatory mechanisms involving nicotinamide adenine nucleotides as allosteric effectors. 3. Control of glucose 6-phosphate dehydrogenase. J Biol Chem. 1970 Apr 10;245(7):1626–1631. [PubMed] [Google Scholar]
- Skinner A. J., Cooper R. A. The regulation of ribose-5-phosphate isomerisation in Escherichia coli K12. FEBS Lett. 1971 Jan 30;12(5):293–296. doi: 10.1016/0014-5793(71)80202-8. [DOI] [PubMed] [Google Scholar]


