Abstract
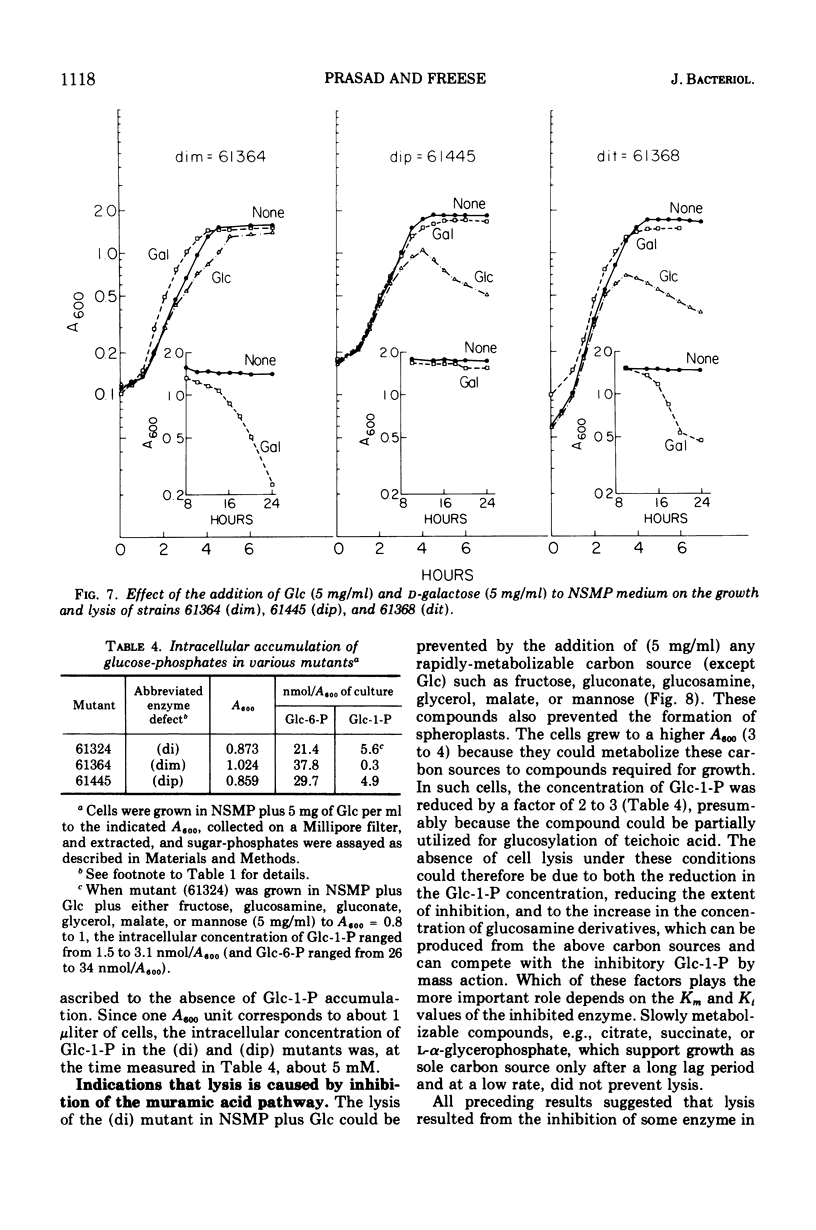
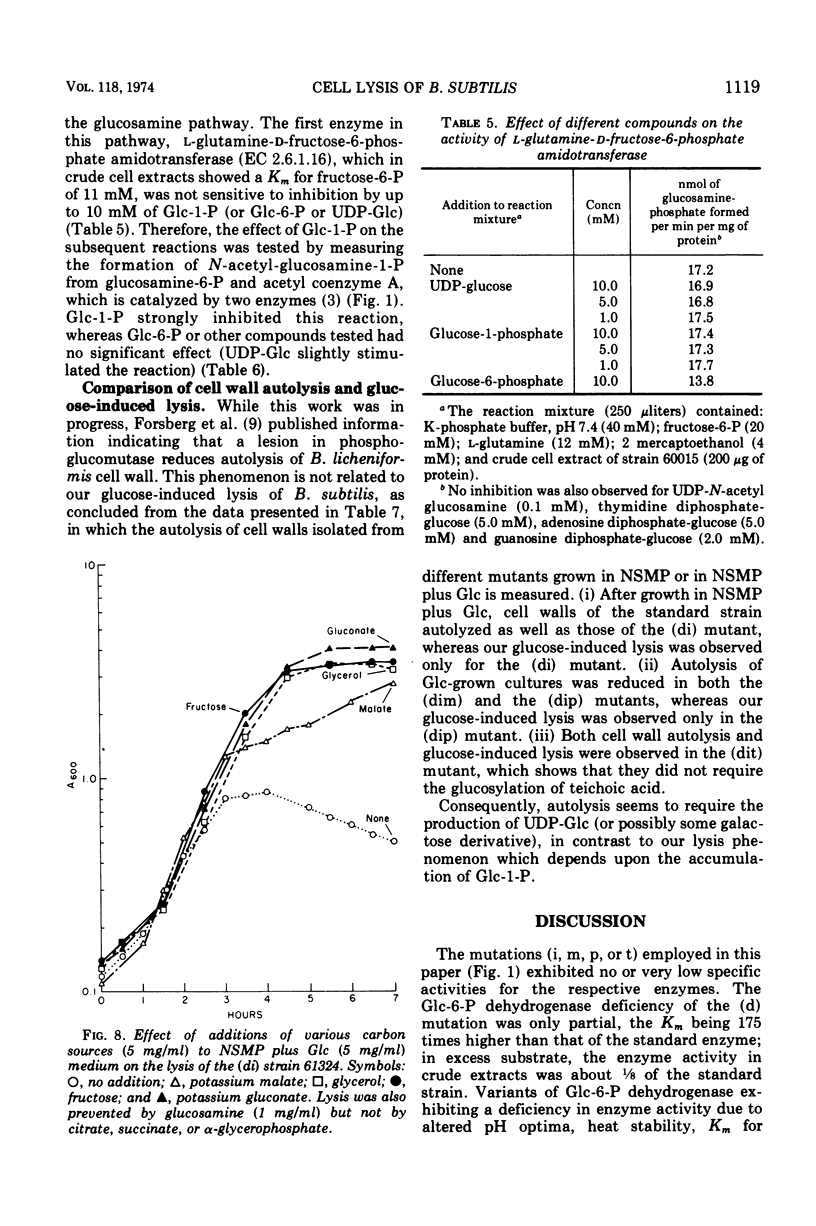
Mutants deficient in both glucose-6-phosphate dehydrogenase and phosphoglucose isomerase lysed 4 to 5 h after growth in nutrient medium containing glucose, or after prolonged incubation if the medium contained galactose. The lysis could be prevented by the addition of any other rapidly metabolizable carbon source such as fructose, glucosamine, or glycerol. The glucose-induced lysis was also abolished by introduction of a third mutation lacking phospho-glucose mutase activity but not by a third mutation lacking uridine diphosphate-glucose pyrophosphorylase or teichoic acid glucosyl transferase activity. Galactose-induced lysis was prevented only if the additional mutation abolished the uridine diphosphate-glucose pyrophosphorylase activity. The results showed that lysis was caused by the intracellular accumulation of glucose-1-phosphate, which in turn inhibited at least one of the two enzymes that convert glucosamine-6-phosphate to N-acetyl glucosamine-6-phosphate.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BATES C. J., PASTERNAK C. A. FURTHER STUDIES ON THE REGULATION OF AMINO SUGAR METABOLISM IN BACILLUS SUBTILIS. Biochem J. 1965 Jul;96:147–154. doi: 10.1042/bj0960147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEST G. K., DURHAM N. N. EFFECT OF VANCOMYCIN ON BACILLUS SUBTILIS. Arch Biochem Biophys. 1964 Apr;105:120–125. doi: 10.1016/0003-9861(64)90242-5. [DOI] [PubMed] [Google Scholar]
- BOYER S. H., PORTER I. H., WEILBACHER R. G. Electrophoretic heterogeneity of glucose-6-phosphate dehydrogenase and its relationship to enzyme deficiency in man. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1868–1876. doi: 10.1073/pnas.48.10.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkulis I. L. GROWTH INHIBITION OF EBERTHELLA TYPHOSA BY CERTAIN CARBOHYDRATES AND ITS RELEASE BY MUTATION. J Bacteriol. 1949 Jul;58(1):103–109. doi: 10.1128/jb.58.1.103-109.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzarelli N. R., Koch J. P., Hayashi S., Lin E. C. Growth stasis by accumulated L-alpha-glycerophosphate in Escherichia coli. J Bacteriol. 1965 Nov;90(5):1325–1329. doi: 10.1128/jb.90.5.1325-1329.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENGLESBERG E., ANDERSON R. L., WEINBERG R., LEE N., HOFFEE P., HUTTENHAUER G., BOYER H. L-Arabinose-sensitive, L-ribulose 5-phosphate 4-epimerase-deficient mutants of Escherichia coli. J Bacteriol. 1962 Jul;84:137–146. doi: 10.1128/jb.84.1.137-146.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENGLESBERG E., BARON L. S. Mutation to L-rhamnose resistance and transduction to L-rhamnose utilization in Salmonella typhosa. J Bacteriol. 1959 Nov;78:675–686. doi: 10.1128/jb.78.5.675-686.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOSTER J. W. Morphogenesis in bacteria: some aspects of spore formation. Q Rev Biol. 1956 Jun;31(2):102–118. doi: 10.1086/401259. [DOI] [PubMed] [Google Scholar]
- FUKASAWA T., JOKURA K., KURAHASHI K. MUTATIONS IN ESCHERICHIA COLI THAT AFFECT URIDINE DIPHOSPHATE GLUCOSE PYROPHOSPHORYLASE ACTIVITY AND GALACTOSE FERMENTATION. Biochim Biophys Acta. 1963 Sep 10;74:608–620. doi: 10.1016/0006-3002(63)91412-4. [DOI] [PubMed] [Google Scholar]
- FUKASAWA T., NIKAIDO H. Galactose-sensitive mutants of Salmonella. II. Bacteriolysis induced by galactose. Biochim Biophys Acta. 1961 Apr 15;48:470–483. doi: 10.1016/0006-3002(61)90045-2. [DOI] [PubMed] [Google Scholar]
- Forsberg C. W., Wyrick P. B., Ward J. B., Rogers H. J. Effect of phosphate limitation on the morphology and wall composition of Bacillus licheniformis and its phosphoglucomutase-deficient mutants. J Bacteriol. 1973 Feb;113(2):969–984. doi: 10.1128/jb.113.2.969-984.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortnagel P., Freese E. Analysis of sporulation mutants. II. Mutants blocked in the citric acid cycle. J Bacteriol. 1968 Apr;95(4):1431–1438. doi: 10.1128/jb.95.4.1431-1438.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortnagel P. The regulation of aconitase and isocitrate dehydrogenase in sporulation mutants of Bacillus subtilis. Biochim Biophys Acta. 1970 Nov 24;222(2):290–298. doi: 10.1016/0304-4165(70)90116-9. [DOI] [PubMed] [Google Scholar]
- Fraenkel D. G. The accumulation of glucose 6-phosphate from glucose and its effect in an Escherichia coli mutant lacking phosphoglucose isomerase and glucose 6-phosphate dehydrogenase. J Biol Chem. 1968 Dec 25;243(24):6451–6457. [PubMed] [Google Scholar]
- Freese E., Klofat W., Galliers E. Commitment to sporulation and induction of glucose-phosphoenolpyruvate-transferase. Biochim Biophys Acta. 1970 Nov 24;222(2):265–289. doi: 10.1016/0304-4165(70)90115-7. [DOI] [PubMed] [Google Scholar]
- Fréhel C., Ryter A. Réversibilité de la sporulation chez B. subtilis. Ann Inst Pasteur (Paris) 1969 Sep;117(3):297–311. [PubMed] [Google Scholar]
- GOLDBERG E. B., NITOWSKY H. M., COLOWICK S. P. THE ROLE OF GLYCOLYSIS IN THE GROWTH OF TUMOR CELLS. IV. THE BASIS OF GLUCOSE TOXICITY IN OXAMATE-TREATED, CULTURED CELLS. J Biol Chem. 1965 Jul;240:2791–2796. [PubMed] [Google Scholar]
- GRELET N. Le déterminisme de la sporulation de bacillus megatherium II. L'effet de la pénurie des constituants minéraux du milieu synthétique. Ann Inst Pasteur (Paris) 1952 Jan;82(1):66–77. [PubMed] [Google Scholar]
- HORECKER B. L., KORNBERG A. The extinction coefficients of the reduced band of pyridine nucleotides. J Biol Chem. 1948 Aug;175(1):385–390. [PubMed] [Google Scholar]
- HYATT M. T., LEVINSON H. S. Conditions affecting Bacillus megaterium spore germination in glucose or various nitrogenous compounds. J Bacteriol. 1962 Jun;83:1231–1237. doi: 10.1128/jb.83.6.1231-1237.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HYATT M. T., LEVINSON H. S. EFFECT OF SUGARS AND OTHER CARBON COMPOUNDS ON GERMINATION AND POSTGERMINATIVE DEVELOPMENT OF BACILLUS MEGATERIUM SPORES. J Bacteriol. 1964 Nov;88:1403–1415. doi: 10.1128/jb.88.5.1403-1415.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOSHI J. G., HANDLER P. PHOSPHOGLUCOMUTASE. I. PURIFICATION AND PROPERTIES OF PHOSPHOGLUCOMUTASE FROM ESCHERICHIA COLI. J Biol Chem. 1964 Sep;239:2741–2751. [PubMed] [Google Scholar]
- Jensen P., Parkes C., Berkowitz D. Mannitol sensitivity. J Bacteriol. 1972 Aug;111(2):351–355. doi: 10.1128/jb.111.2.351-355.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KURAHASHI K., WAHBA A. J. Interference with growth of certain Escherichia coli mutants by galactose. Biochim Biophys Acta. 1958 Nov;30(2):298–302. doi: 10.1016/0006-3002(58)90054-4. [DOI] [PubMed] [Google Scholar]
- Kirkman H. N., Riley H. D., Crowell B. B. DIFFERENT ENZYMIC EXPRESSIONS OF MUTANTS OF HUMAN GLUCOSE-6-PHOSPHATE DEHYDROGENASE. Proc Natl Acad Sci U S A. 1960 Jul;46(7):938–944. doi: 10.1073/pnas.46.7.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klofat W., Picciolo G., Chappelle E. W., Freese E. Production of adenosine triphosphate in normal cells and sporulation mutants of Bacillus subtilis. J Biol Chem. 1969 Jun 25;244(12):3270–3276. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Luzzatto L., Allan N. C. Different properties of glucose 6-phosphate dehydrogenase from human erythrocytes with normal and abnormal enzyme levels. Biochem Biophys Res Commun. 1965 Dec 21;21(6):547–554. doi: 10.1016/0006-291x(65)90520-6. [DOI] [PubMed] [Google Scholar]
- Massie H. R., Zimm B. H. Molecular weight of the DNA in the chromosomes of E. coli and B. subtilis. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1636–1641. doi: 10.1073/pnas.54.6.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIKAIDO H. Galactose-sensitive mutants of Salmonella. I. Metabolism of galactose. Biochim Biophys Acta. 1961 Apr 15;48:460–469. doi: 10.1016/0006-3002(61)90044-0. [DOI] [PubMed] [Google Scholar]
- Prasad C., Diesterhaft M., Freese E. Initiation of spore germination in glycolytic mutants of Bacillus subtilis. J Bacteriol. 1972 Apr;110(1):321–328. doi: 10.1128/jb.110.1.321-328.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana R. S., Halvorson H. O. Method for restricting incorporation of radioactivity from 3 H-thymidine into deoxyribonucleic acid only during outgrowth of spores of Bacillus cereus T. J Bacteriol. 1972 Feb;109(2):599–605. doi: 10.1128/jb.109.2.599-605.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWARZ V., GOLBERG L., KOMROWER G. M., HOLZEL A. Some disturbances of erythrocyte metabolism in galactosaemia. Biochem J. 1956 Jan;62(1):34–40. doi: 10.1042/bj0620034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOLS A., CADENAS E., ALVARADO F. Enzymatic basis of mannose toxicity in honey bees. Science. 1960 Jan 29;131(3396):297–298. doi: 10.1126/science.131.3396.297. [DOI] [PubMed] [Google Scholar]
- SUNDARARAJAN T. A., RAPIN A. M., KALCKAR H. M. Biochemical observations on E. coli mutants defective in uridine diphosphoglucose. Proc Natl Acad Sci U S A. 1962 Dec 15;48:2187–2193. doi: 10.1073/pnas.48.12.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer P., Millet J., Aubert J. P. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci U S A. 1965 Sep;54(3):704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuster C. W., Rundell K. Resistance of Salmonella typhimurium mutants to galactose death. J Bacteriol. 1969 Oct;100(1):103–109. doi: 10.1128/jb.100.1.103-109.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wax R., Freese E., Cashel M. Separation of two functional roles of L-alanine in the initiation of Bacillus subtilis spore germination. J Bacteriol. 1967 Sep;94(3):522–529. doi: 10.1128/jb.94.3.522-529.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willecke K., Pardee A. B. Inducible transport of citrate in a Gram-positive bacterium, Bacillus subtilis. J Biol Chem. 1971 Feb 25;246(4):1032–1040. [PubMed] [Google Scholar]
- YOUNG F. E., SPIZIZEN J., CRAWFORD I. P. BIOCHEMICAL ASPECTS OF COMPETENCE IN THE BACILLUS SUBTILIS TRANSFORMATION SYSTEM. I. CHEMICAL COMPOSITION OF CELL WALLS. J Biol Chem. 1963 Sep;238:3119–3125. [PubMed] [Google Scholar]
- Yarmolinsky M. B., Wiesmeyer H., Kalckar H. M., Jordan E. HEREDITARY DEFECTS IN GALACTOSE METABOLISM IN ESCHERICHIA COLI MUTANTS, II. GALACTOSE-INDUCED SENSITIVITY. Proc Natl Acad Sci U S A. 1959 Dec;45(12):1786–1791. doi: 10.1073/pnas.45.12.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young F. E. Autolytic enzyme associated with cell walls of Bacillus subtilis. J Biol Chem. 1966 Aug 10;241(15):3462–3467. [PubMed] [Google Scholar]
- Young F. E., Smith C., Reilly B. E. Chromosomal location of genes regulating resistance to bacteriophage in Bacillus subtilis. J Bacteriol. 1969 Jun;98(3):1087–1097. doi: 10.1128/jb.98.3.1087-1097.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]


