Abstract
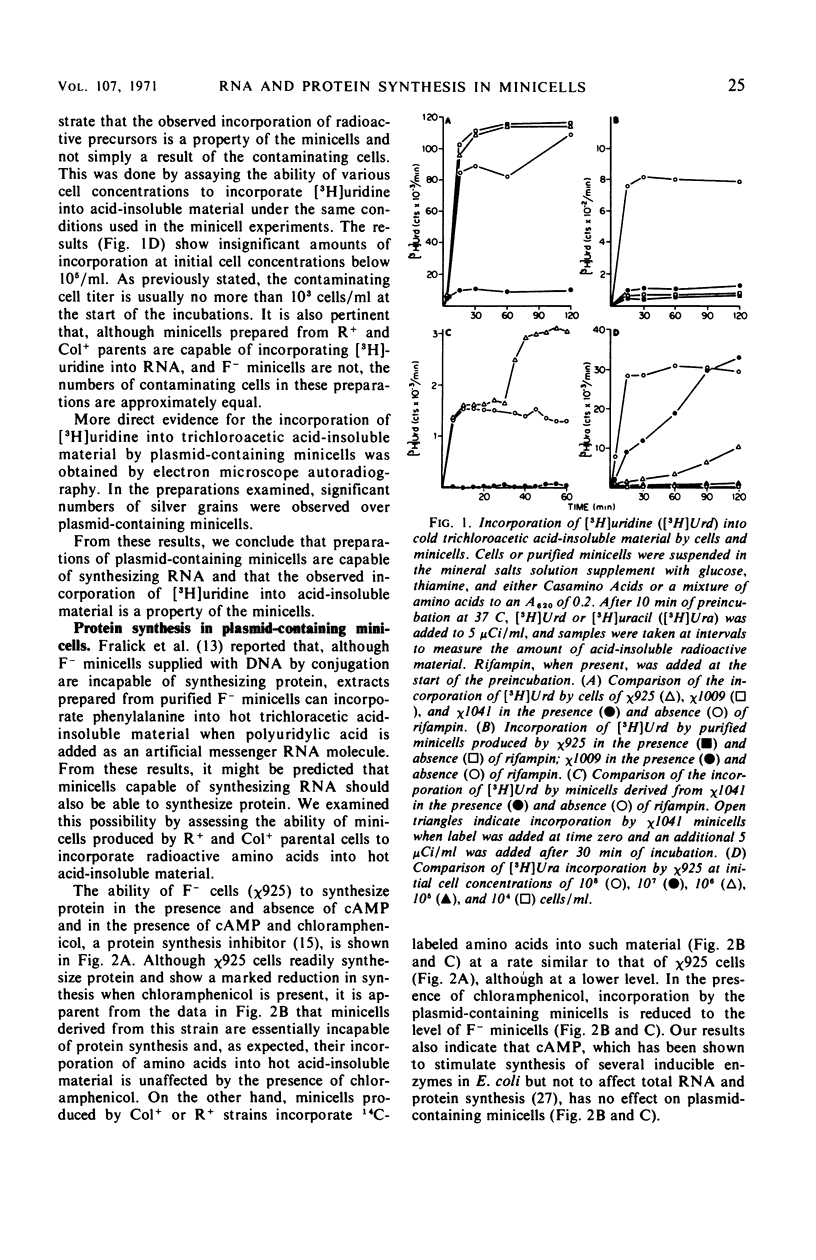
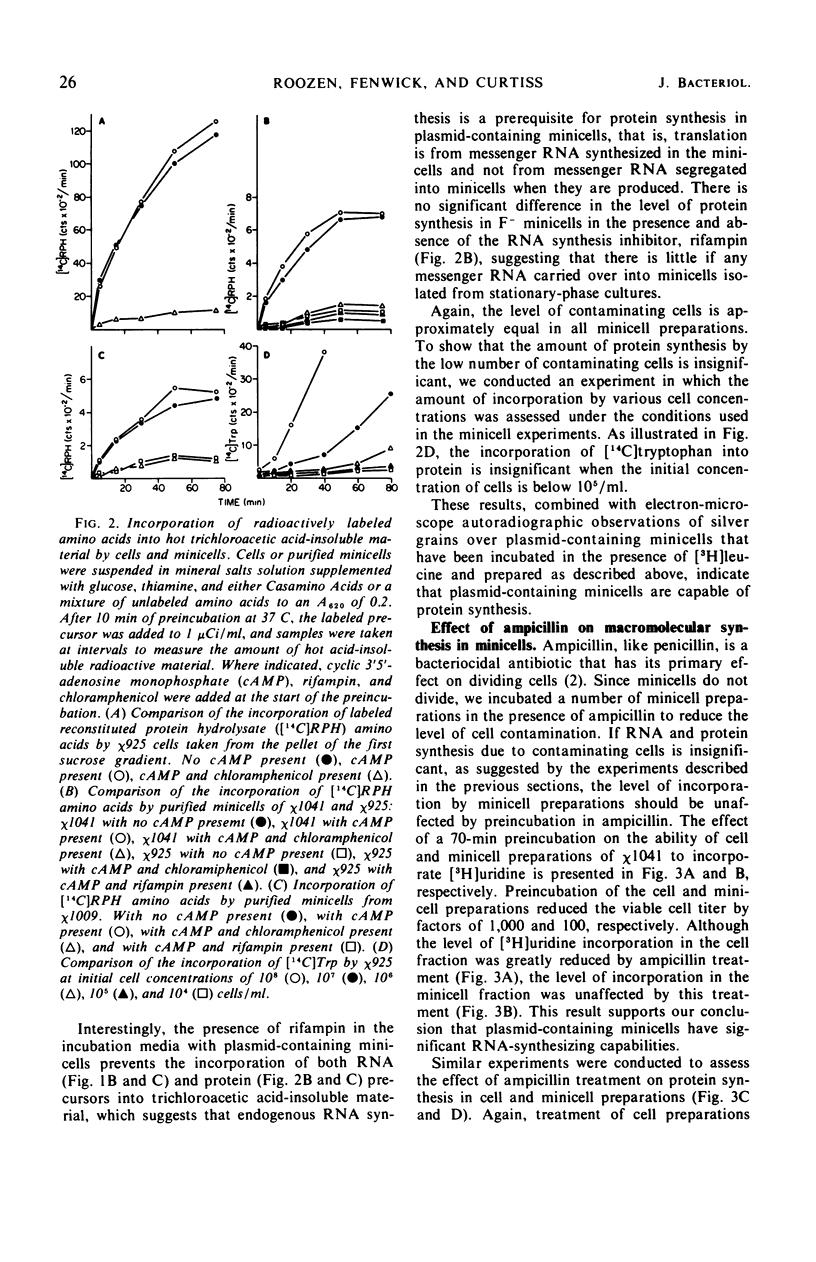
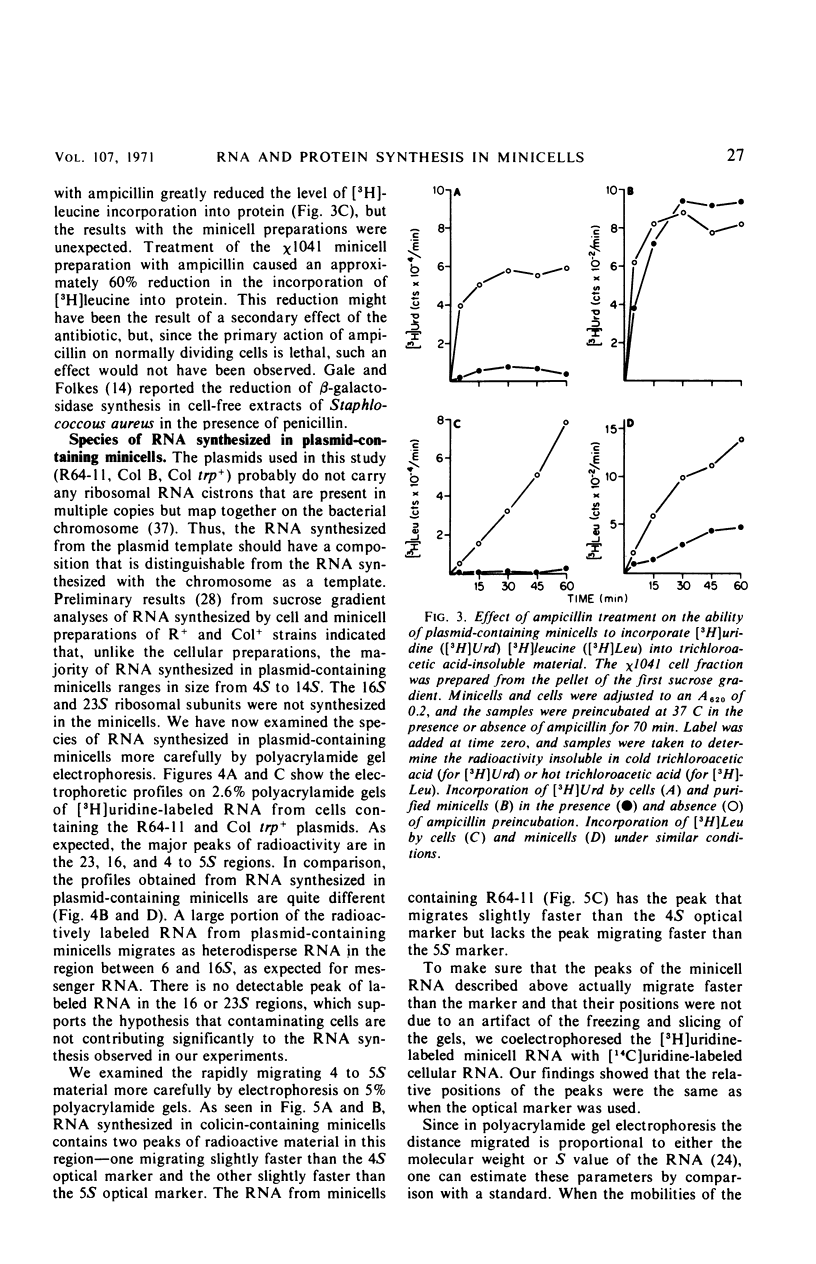
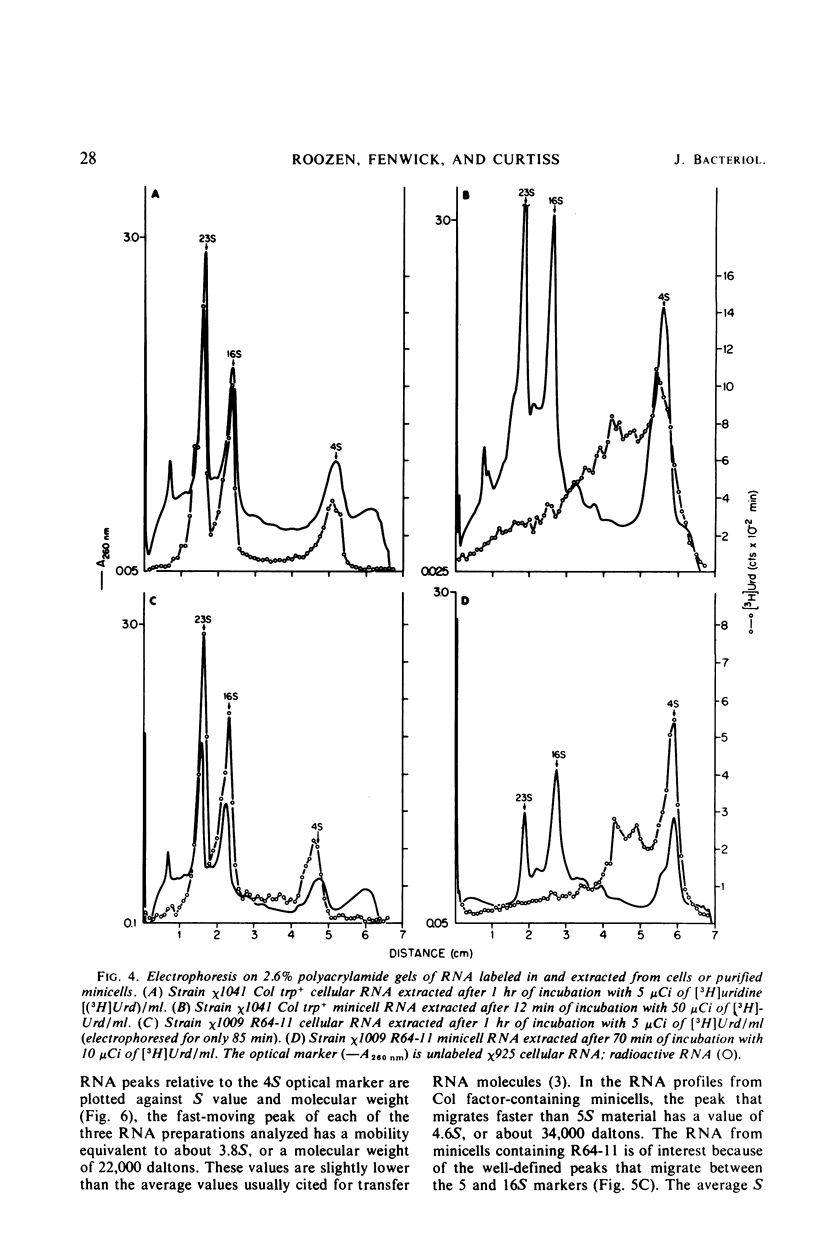
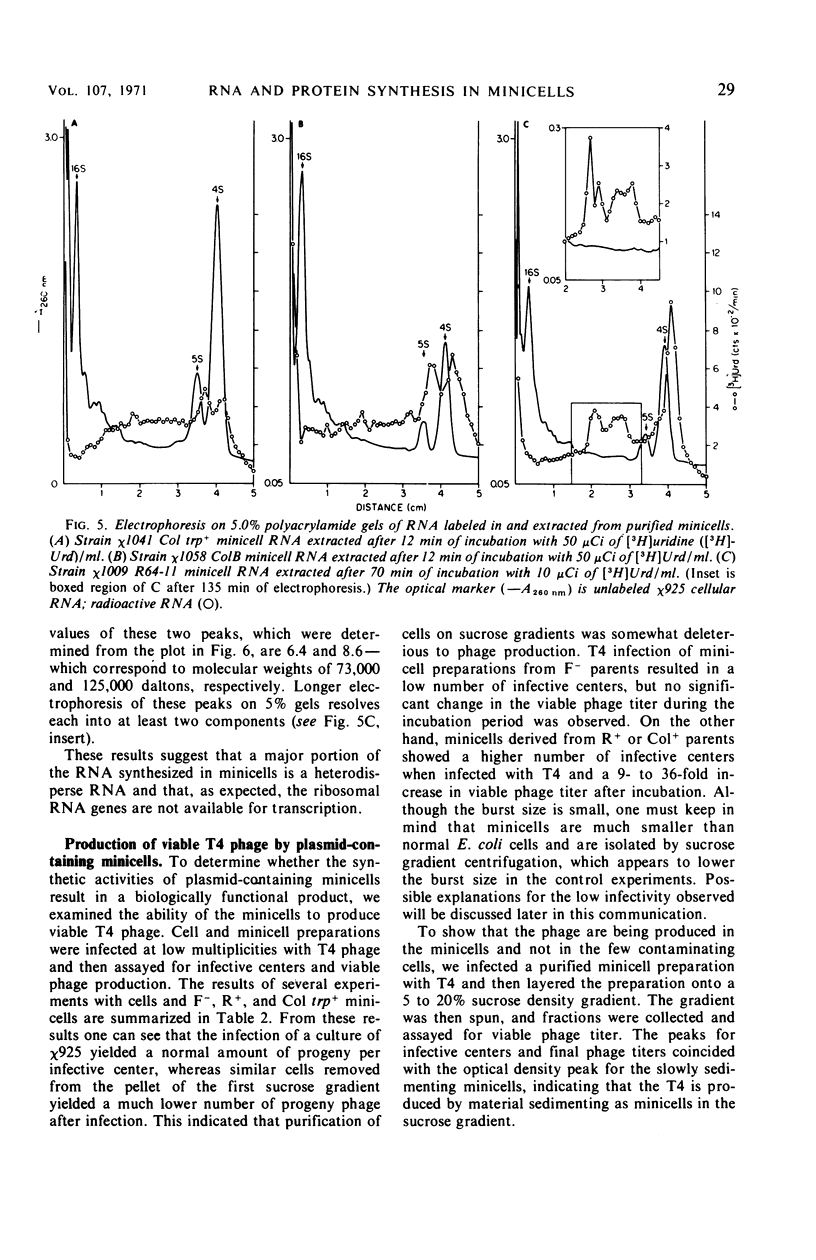
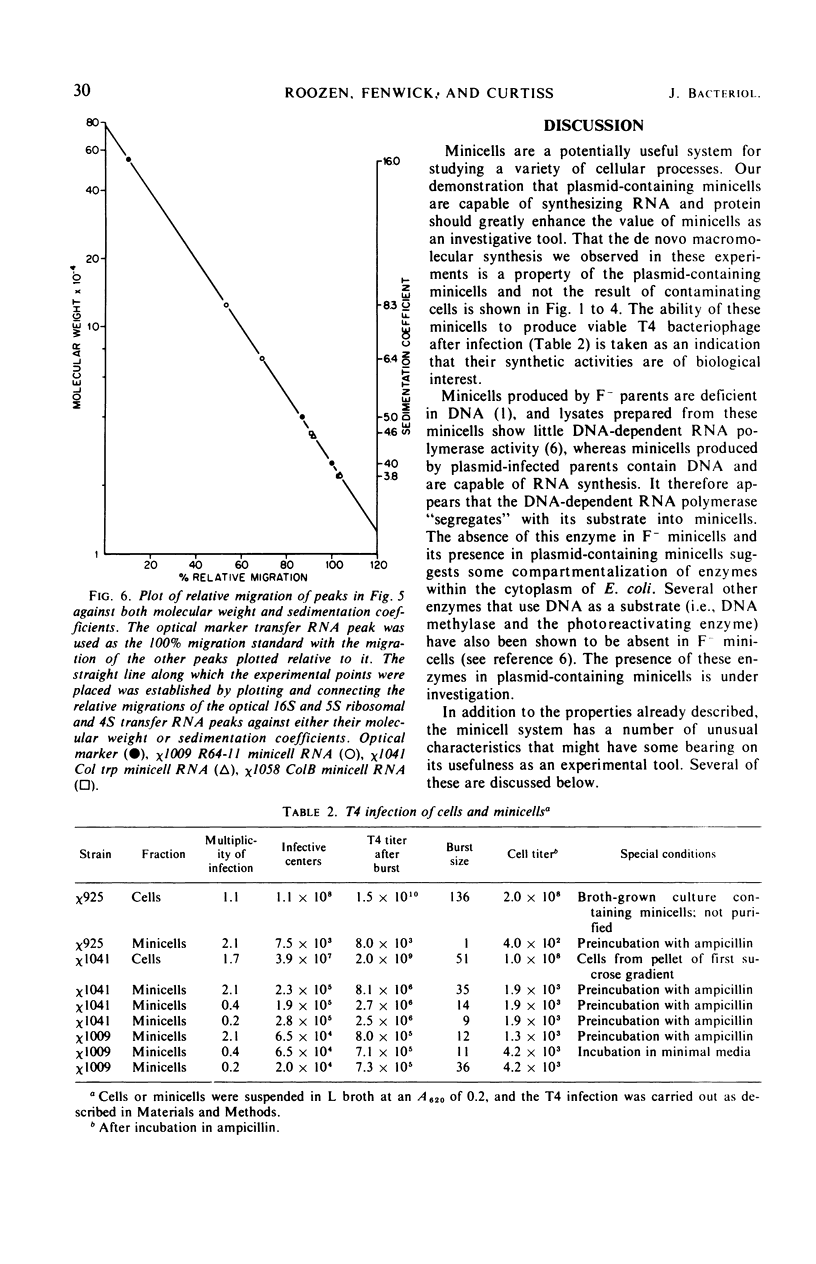
Unlike the deoxyribonucleic acid (DNA)-deficient minicells produced by F− parents, minicells produced by plasmid-containing strains contain significant amounts of plasmid DNA. We examined the ability of plasmid-containing minicells to synthesize ribonucleic acid (RNA) and protein. In vivo, minicells produced by F− parents are unable to incorporate radioactive precursors into acid-insoluble RNA or protein, whereas minicells produced by F′, R+, or Col+ parents are capable of such synthesis. Using a variety of approaches, including polyacrylamide gel analysis of the RNA species produced and electron microscope autoradiography, we demonstrated that the synthesis observed in minicell preparations is a property of the plasmid-containing minicells and not a result of the few cells (approximately 1 per 106 minicells) contaminating the preparations. That the observed synthesis is of biological importance is suggested by the ability of plasmid-containing minicells to yield viable phage upon infection with T4.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler H. I., Fisher W. D., Cohen A., Hardigree A. A. MINIATURE escherichia coli CELLS DEFICIENT IN DNA. Proc Natl Acad Sci U S A. 1967 Feb;57(2):321–326. doi: 10.1073/pnas.57.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOMAN H. G., ERIKSSON K. G. Penicillin-induced lysis in Escherichia coli. J Gen Microbiol. 1963 Jun;31:339–352. doi: 10.1099/00221287-31-3-339. [DOI] [PubMed] [Google Scholar]
- CARO L. G., VAN TUBERGEN R. P., KOLB J. A. High-resolution autoradiography. I. Methods. J Cell Biol. 1962 Nov;15:173–188. doi: 10.1083/jcb.15.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIS S. R., 3rd CHROMOSOMAL ABERRATIONS ASSOCIATED WITH MUTATIONS TO BACTERIOPHAGE RESISTANCE IN ESCHERICHIA COLI. J Bacteriol. 1965 Jan;89:28–40. doi: 10.1128/jb.89.1.28-40.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro L. Progress in high-resolution autoradiography. Prog Biophys Mol Biol. 1966;16:171–190. doi: 10.1016/0079-6107(66)90006-x. [DOI] [PubMed] [Google Scholar]
- Cohen A., Fisher W. D., Curtiss R., 3rd, Adler H. I. The properties of DNA transferred to minicells during conjugation. Cold Spring Harb Symp Quant Biol. 1968;33:635–641. doi: 10.1101/sqb.1968.033.01.071. [DOI] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Charamella L. J., Stallions D. R., Mays J. A. Parental functions during conjugation in Escherichia coli K-12. Bacteriol Rev. 1968 Dec;32(4 Pt 1):320–348. [PMC free article] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Stallions D. R. Probability of F integration and frequency of stable Hfr donors in F+ populations of Escherichia coli K-12. Genetics. 1969 Sep;63(1):27–38. doi: 10.1093/genetics/63.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtiss R., 3rd Ultraviolet-induced genetic recombination in a partially diploid strain of Escherichia coli. Genetics. 1968 Jan;58(1):9–54. doi: 10.1093/genetics/58.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fralick J. A., Fisher W. D., Adler H. I. Polyuridylic acid-directed phenylalanine incorporation in minicell extracts. J Bacteriol. 1969 Aug;99(2):621–622. doi: 10.1128/jb.99.2.621-622.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALE E. F., FOLKES J. P. Effect of nucleic acids on protein synthesis and amino-acid incorporation in disrupted staphylococcal cells. Nature. 1954 Jun 26;173(4417):1223–1227. doi: 10.1038/1731223a0. [DOI] [PubMed] [Google Scholar]
- GALE E. F., PAINE T. F. The assimilation of amino-acids by bacteria; the action of inhibitors and antibiotics on the accumulation of free glutamic acid and the formation of combined of combined glutamate in Staphylococcus aureus. Biochem J. 1951 Mar;48(3):298–301. doi: 10.1042/bj0480298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros F., Gallant J., Weisberg R., Cashel M. Decryptification of RNA polymerase in whole cells of Escherichia coli. J Mol Biol. 1967 May 14;25(3):555–557. doi: 10.1016/0022-2836(67)90206-9. [DOI] [PubMed] [Google Scholar]
- Inselburg J., Fuke M. Replicating DNA: structure of colicin factor E1. Science. 1970 Aug 7;169(3945):590–592. doi: 10.1126/science.169.3945.590. [DOI] [PubMed] [Google Scholar]
- Inselburg J. Segregation into and replication of plasmid deoxyribonucleic acid in chromosomeless segregants of Escherichia coli. J Bacteriol. 1970 Jun;102(3):642–647. doi: 10.1128/jb.102.3.642-647.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass L. R., Yarmolinsky M. B. Segregation of functional sex factor into minicells. Proc Natl Acad Sci U S A. 1970 Jul;66(3):815–822. doi: 10.1073/pnas.66.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontomichalou P., Mitani M., Clowes R. C. Circular R-factor molecules controlling penicillinase synthesis, replicating in Escherichia coli under either relaxed or stringent control. J Bacteriol. 1970 Oct;104(1):34–44. doi: 10.1128/jb.104.1.34-44.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Levy S. B., Norman P. Segregation of transferable R factors into Escherichia coli minicells. Nature. 1970 Aug 8;227(5258):606–607. doi: 10.1038/227606a0. [DOI] [PubMed] [Google Scholar]
- Levy S. B. Resistance of minicells to penicillin lysis: a method of obtaining large quantities of purified minicells. J Bacteriol. 1970 Sep;103(3):836–839. doi: 10.1128/jb.103.3.836-839.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewicki P. P., Sinskey A. J. Precision of RNA separation by polyacrylamide gel electrophoresis. Anal Biochem. 1970 Feb;33(2):273–278. doi: 10.1016/0003-2697(70)90297-6. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meynell G. G., Lawn A. M. Filamentous phages specific for the I sex factor. Nature. 1968 Mar 23;217(5134):1184–1186. doi: 10.1038/2171184a0. [DOI] [PubMed] [Google Scholar]
- Perlman R., Pastan I. Cyclic 3'5-AMP: stimulation of beta-galactosidase and tryptophanase induction in E. coli. Biochem Biophys Res Commun. 1968 Mar 27;30(6):656–664. doi: 10.1016/0006-291x(68)90563-9. [DOI] [PubMed] [Google Scholar]
- Rownd R. Replication of a bacterial episome under relaxed control. J Mol Biol. 1969 Sep 28;44(3):387–402. doi: 10.1016/0022-2836(69)90368-4. [DOI] [PubMed] [Google Scholar]
- Rubenstein K. E., Nass M. M., Cohen S. S. Synthetic capabilities of plasmolyzed cells and spheroplasts of Escherichia coli. J Bacteriol. 1970 Oct;104(1):443–452. doi: 10.1128/jb.104.1.443-452.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherberg N. H., Weiss S. B. Detection of bacteriophage T4- and T5-coded transfer RNAs. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1164–1171. doi: 10.1073/pnas.67.3.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sippel A., Hartmann G. Mode of action of rafamycin on the RNA polymerase reaction. Biochim Biophys Acta. 1968 Mar 18;157(1):218–219. doi: 10.1016/0005-2787(68)90286-4. [DOI] [PubMed] [Google Scholar]
- Subak-Sharpe H., Shepherd W. M., Hay J. Studies on sRNA coded by herpes virus. Cold Spring Harb Symp Quant Biol. 1966;31:583–594. doi: 10.1101/sqb.1966.031.01.076. [DOI] [PubMed] [Google Scholar]
- Trávnícek M. RNA with amino acid-acceptor activity isolated from an oncogenic virus. Biochim Biophys Acta. 1968 Oct 29;166(3):757–759. [PubMed] [Google Scholar]
- Weinberg R. A., Penman S. Small molecular weight monodisperse nuclear RNA. J Mol Biol. 1968 Dec;38(3):289–304. doi: 10.1016/0022-2836(68)90387-2. [DOI] [PubMed] [Google Scholar]
- Weiss S. B., Hsu W. T., Foft J. W., Scherberg N. H. Transfer RNA coded by the T4 bacteriophage genome. Proc Natl Acad Sci U S A. 1968 Sep;61(1):114–121. doi: 10.1073/pnas.61.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M. T., Vermeulen C. W., Atwood K. C. Location of the genes for 16S and 23S ribosomal RNA in the genetic map of Escherichia coli. Proc Natl Acad Sci U S A. 1970 Sep;67(1):26–31. doi: 10.1073/pnas.67.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinder N. D. RNA phages. Annu Rev Microbiol. 1965;19:455–472. doi: 10.1146/annurev.mi.19.100165.002323. [DOI] [PubMed] [Google Scholar]



