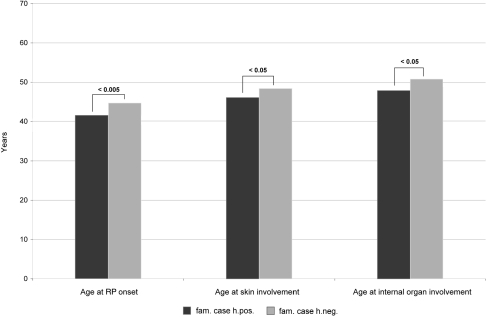
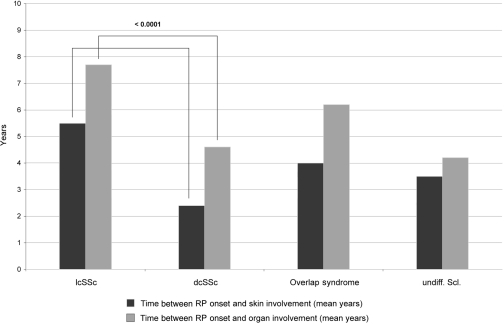
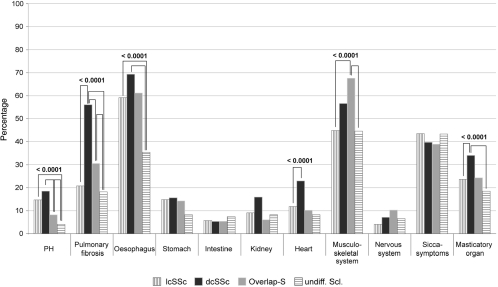
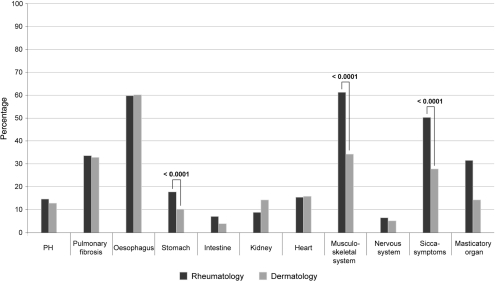
N Hunzelmann
N Hunzelmann
1Department of Dermatology and Venerology, University of Cologne, Cologne, 2Department of Rheumatology, University of Aachen, Aachen, 3Institute of Biostatistics, Informatics and Epidemiology, University of Cologne, Cologne, 4Clinical Research Unit for Rheumatology, University Medical Center Freiburg, Freiburg ,5Department of Dermatology, Dresden University Hospital, Dresden, 6Department of Rheumatology and Clinical Immunology, Kerckhoff Clinic, Bad Nauheim, 7Rheumatology and Clinical Immunology, Charité, Berlin, 8Hospital Cologne-Merheim, Medical Clinic I, 9Department of Dermatology, University of Münster, Münster, 10Department of Dermatology, Venerology and Allergology, University Hospital Charité, Berlin, 11Department of Rheumatology, University of Cologne, Cologne, 12Department of Dermatology, University of Mainz, Mainz, 13Department of Dermatology and Allergology, University of Munich, Munich, 14Department of Dermatology and Allergology, University of Ulm, Ulm, 15Department of Dermatology, 16Department of Internal Medicine, University of Regensburg, Regensburg, 17Department of Internal Medicine, University of Regensburg, Regensburg, 18Department of Dermatology, Allergology and Environmental Medicine, Private University Witten-Herdecke, HELIOS Klinikum Wuppertal, Wuppertal, 19Hospital Eilbek, Hamburg, 20Department of Internal Medicine, University of Giessen, Giessen, 21Clinic for Rheumatology, Bad Bramstedt, 22Department of Dermatology, University of Düsseldorf, Düsseldorf, 23Department of Dermatology and Venerology,Georg-August-University of Göttingen, Göttingen, 24Department of Dermatology, University of Tübingen, Tübingen, 25Department of Internal Medicine, University of Tübingen, Tübingen, 26Department of Internal Medicine, University of Heidelberg, Heidelberg, 27Johanniter Hospital in Fläming gGmbH, Center of Rheumatology of Brandenburg, Germany, 28Department of Dermatology and Venerology, University of Graz, Graz, Austria, 29Department of Internal Medicine, Center of Rheumatology, Baden-Baden and 30Department of Dermatology, Clinic of Minden, Minden, Germany.
1,✉,
E Genth
E Genth
1Department of Dermatology and Venerology, University of Cologne, Cologne, 2Department of Rheumatology, University of Aachen, Aachen, 3Institute of Biostatistics, Informatics and Epidemiology, University of Cologne, Cologne, 4Clinical Research Unit for Rheumatology, University Medical Center Freiburg, Freiburg ,5Department of Dermatology, Dresden University Hospital, Dresden, 6Department of Rheumatology and Clinical Immunology, Kerckhoff Clinic, Bad Nauheim, 7Rheumatology and Clinical Immunology, Charité, Berlin, 8Hospital Cologne-Merheim, Medical Clinic I, 9Department of Dermatology, University of Münster, Münster, 10Department of Dermatology, Venerology and Allergology, University Hospital Charité, Berlin, 11Department of Rheumatology, University of Cologne, Cologne, 12Department of Dermatology, University of Mainz, Mainz, 13Department of Dermatology and Allergology, University of Munich, Munich, 14Department of Dermatology and Allergology, University of Ulm, Ulm, 15Department of Dermatology, 16Department of Internal Medicine, University of Regensburg, Regensburg, 17Department of Internal Medicine, University of Regensburg, Regensburg, 18Department of Dermatology, Allergology and Environmental Medicine, Private University Witten-Herdecke, HELIOS Klinikum Wuppertal, Wuppertal, 19Hospital Eilbek, Hamburg, 20Department of Internal Medicine, University of Giessen, Giessen, 21Clinic for Rheumatology, Bad Bramstedt, 22Department of Dermatology, University of Düsseldorf, Düsseldorf, 23Department of Dermatology and Venerology,Georg-August-University of Göttingen, Göttingen, 24Department of Dermatology, University of Tübingen, Tübingen, 25Department of Internal Medicine, University of Tübingen, Tübingen, 26Department of Internal Medicine, University of Heidelberg, Heidelberg, 27Johanniter Hospital in Fläming gGmbH, Center of Rheumatology of Brandenburg, Germany, 28Department of Dermatology and Venerology, University of Graz, Graz, Austria, 29Department of Internal Medicine, Center of Rheumatology, Baden-Baden and 30Department of Dermatology, Clinic of Minden, Minden, Germany.
2,
T Krieg
T Krieg
1Department of Dermatology and Venerology, University of Cologne, Cologne, 2Department of Rheumatology, University of Aachen, Aachen, 3Institute of Biostatistics, Informatics and Epidemiology, University of Cologne, Cologne, 4Clinical Research Unit for Rheumatology, University Medical Center Freiburg, Freiburg ,5Department of Dermatology, Dresden University Hospital, Dresden, 6Department of Rheumatology and Clinical Immunology, Kerckhoff Clinic, Bad Nauheim, 7Rheumatology and Clinical Immunology, Charité, Berlin, 8Hospital Cologne-Merheim, Medical Clinic I, 9Department of Dermatology, University of Münster, Münster, 10Department of Dermatology, Venerology and Allergology, University Hospital Charité, Berlin, 11Department of Rheumatology, University of Cologne, Cologne, 12Department of Dermatology, University of Mainz, Mainz, 13Department of Dermatology and Allergology, University of Munich, Munich, 14Department of Dermatology and Allergology, University of Ulm, Ulm, 15Department of Dermatology, 16Department of Internal Medicine, University of Regensburg, Regensburg, 17Department of Internal Medicine, University of Regensburg, Regensburg, 18Department of Dermatology, Allergology and Environmental Medicine, Private University Witten-Herdecke, HELIOS Klinikum Wuppertal, Wuppertal, 19Hospital Eilbek, Hamburg, 20Department of Internal Medicine, University of Giessen, Giessen, 21Clinic for Rheumatology, Bad Bramstedt, 22Department of Dermatology, University of Düsseldorf, Düsseldorf, 23Department of Dermatology and Venerology,Georg-August-University of Göttingen, Göttingen, 24Department of Dermatology, University of Tübingen, Tübingen, 25Department of Internal Medicine, University of Tübingen, Tübingen, 26Department of Internal Medicine, University of Heidelberg, Heidelberg, 27Johanniter Hospital in Fläming gGmbH, Center of Rheumatology of Brandenburg, Germany, 28Department of Dermatology and Venerology, University of Graz, Graz, Austria, 29Department of Internal Medicine, Center of Rheumatology, Baden-Baden and 30Department of Dermatology, Clinic of Minden, Minden, Germany.
1,
W Lehmacher
W Lehmacher
1Department of Dermatology and Venerology, University of Cologne, Cologne, 2Department of Rheumatology, University of Aachen, Aachen, 3Institute of Biostatistics, Informatics and Epidemiology, University of Cologne, Cologne, 4Clinical Research Unit for Rheumatology, University Medical Center Freiburg, Freiburg ,5Department of Dermatology, Dresden University Hospital, Dresden, 6Department of Rheumatology and Clinical Immunology, Kerckhoff Clinic, Bad Nauheim, 7Rheumatology and Clinical Immunology, Charité, Berlin, 8Hospital Cologne-Merheim, Medical Clinic I, 9Department of Dermatology, University of Münster, Münster, 10Department of Dermatology, Venerology and Allergology, University Hospital Charité, Berlin, 11Department of Rheumatology, University of Cologne, Cologne, 12Department of Dermatology, University of Mainz, Mainz, 13Department of Dermatology and Allergology, University of Munich, Munich, 14Department of Dermatology and Allergology, University of Ulm, Ulm, 15Department of Dermatology, 16Department of Internal Medicine, University of Regensburg, Regensburg, 17Department of Internal Medicine, University of Regensburg, Regensburg, 18Department of Dermatology, Allergology and Environmental Medicine, Private University Witten-Herdecke, HELIOS Klinikum Wuppertal, Wuppertal, 19Hospital Eilbek, Hamburg, 20Department of Internal Medicine, University of Giessen, Giessen, 21Clinic for Rheumatology, Bad Bramstedt, 22Department of Dermatology, University of Düsseldorf, Düsseldorf, 23Department of Dermatology and Venerology,Georg-August-University of Göttingen, Göttingen, 24Department of Dermatology, University of Tübingen, Tübingen, 25Department of Internal Medicine, University of Tübingen, Tübingen, 26Department of Internal Medicine, University of Heidelberg, Heidelberg, 27Johanniter Hospital in Fläming gGmbH, Center of Rheumatology of Brandenburg, Germany, 28Department of Dermatology and Venerology, University of Graz, Graz, Austria, 29Department of Internal Medicine, Center of Rheumatology, Baden-Baden and 30Department of Dermatology, Clinic of Minden, Minden, Germany.
3,
I Melchers
I Melchers
1Department of Dermatology and Venerology, University of Cologne, Cologne, 2Department of Rheumatology, University of Aachen, Aachen, 3Institute of Biostatistics, Informatics and Epidemiology, University of Cologne, Cologne, 4Clinical Research Unit for Rheumatology, University Medical Center Freiburg, Freiburg ,5Department of Dermatology, Dresden University Hospital, Dresden, 6Department of Rheumatology and Clinical Immunology, Kerckhoff Clinic, Bad Nauheim, 7Rheumatology and Clinical Immunology, Charité, Berlin, 8Hospital Cologne-Merheim, Medical Clinic I, 9Department of Dermatology, University of Münster, Münster, 10Department of Dermatology, Venerology and Allergology, University Hospital Charité, Berlin, 11Department of Rheumatology, University of Cologne, Cologne, 12Department of Dermatology, University of Mainz, Mainz, 13Department of Dermatology and Allergology, University of Munich, Munich, 14Department of Dermatology and Allergology, University of Ulm, Ulm, 15Department of Dermatology, 16Department of Internal Medicine, University of Regensburg, Regensburg, 17Department of Internal Medicine, University of Regensburg, Regensburg, 18Department of Dermatology, Allergology and Environmental Medicine, Private University Witten-Herdecke, HELIOS Klinikum Wuppertal, Wuppertal, 19Hospital Eilbek, Hamburg, 20Department of Internal Medicine, University of Giessen, Giessen, 21Clinic for Rheumatology, Bad Bramstedt, 22Department of Dermatology, University of Düsseldorf, Düsseldorf, 23Department of Dermatology and Venerology,Georg-August-University of Göttingen, Göttingen, 24Department of Dermatology, University of Tübingen, Tübingen, 25Department of Internal Medicine, University of Tübingen, Tübingen, 26Department of Internal Medicine, University of Heidelberg, Heidelberg, 27Johanniter Hospital in Fläming gGmbH, Center of Rheumatology of Brandenburg, Germany, 28Department of Dermatology and Venerology, University of Graz, Graz, Austria, 29Department of Internal Medicine, Center of Rheumatology, Baden-Baden and 30Department of Dermatology, Clinic of Minden, Minden, Germany.
4,
M Meurer
M Meurer
1Department of Dermatology and Venerology, University of Cologne, Cologne, 2Department of Rheumatology, University of Aachen, Aachen, 3Institute of Biostatistics, Informatics and Epidemiology, University of Cologne, Cologne, 4Clinical Research Unit for Rheumatology, University Medical Center Freiburg, Freiburg ,5Department of Dermatology, Dresden University Hospital, Dresden, 6Department of Rheumatology and Clinical Immunology, Kerckhoff Clinic, Bad Nauheim, 7Rheumatology and Clinical Immunology, Charité, Berlin, 8Hospital Cologne-Merheim, Medical Clinic I, 9Department of Dermatology, University of Münster, Münster, 10Department of Dermatology, Venerology and Allergology, University Hospital Charité, Berlin, 11Department of Rheumatology, University of Cologne, Cologne, 12Department of Dermatology, University of Mainz, Mainz, 13Department of Dermatology and Allergology, University of Munich, Munich, 14Department of Dermatology and Allergology, University of Ulm, Ulm, 15Department of Dermatology, 16Department of Internal Medicine, University of Regensburg, Regensburg, 17Department of Internal Medicine, University of Regensburg, Regensburg, 18Department of Dermatology, Allergology and Environmental Medicine, Private University Witten-Herdecke, HELIOS Klinikum Wuppertal, Wuppertal, 19Hospital Eilbek, Hamburg, 20Department of Internal Medicine, University of Giessen, Giessen, 21Clinic for Rheumatology, Bad Bramstedt, 22Department of Dermatology, University of Düsseldorf, Düsseldorf, 23Department of Dermatology and Venerology,Georg-August-University of Göttingen, Göttingen, 24Department of Dermatology, University of Tübingen, Tübingen, 25Department of Internal Medicine, University of Tübingen, Tübingen, 26Department of Internal Medicine, University of Heidelberg, Heidelberg, 27Johanniter Hospital in Fläming gGmbH, Center of Rheumatology of Brandenburg, Germany, 28Department of Dermatology and Venerology, University of Graz, Graz, Austria, 29Department of Internal Medicine, Center of Rheumatology, Baden-Baden and 30Department of Dermatology, Clinic of Minden, Minden, Germany.
5,
P Moinzadeh
P Moinzadeh
1Department of Dermatology and Venerology, University of Cologne, Cologne, 2Department of Rheumatology, University of Aachen, Aachen, 3Institute of Biostatistics, Informatics and Epidemiology, University of Cologne, Cologne, 4Clinical Research Unit for Rheumatology, University Medical Center Freiburg, Freiburg ,5Department of Dermatology, Dresden University Hospital, Dresden, 6Department of Rheumatology and Clinical Immunology, Kerckhoff Clinic, Bad Nauheim, 7Rheumatology and Clinical Immunology, Charité, Berlin, 8Hospital Cologne-Merheim, Medical Clinic I, 9Department of Dermatology, University of Münster, Münster, 10Department of Dermatology, Venerology and Allergology, University Hospital Charité, Berlin, 11Department of Rheumatology, University of Cologne, Cologne, 12Department of Dermatology, University of Mainz, Mainz, 13Department of Dermatology and Allergology, University of Munich, Munich, 14Department of Dermatology and Allergology, University of Ulm, Ulm, 15Department of Dermatology, 16Department of Internal Medicine, University of Regensburg, Regensburg, 17Department of Internal Medicine, University of Regensburg, Regensburg, 18Department of Dermatology, Allergology and Environmental Medicine, Private University Witten-Herdecke, HELIOS Klinikum Wuppertal, Wuppertal, 19Hospital Eilbek, Hamburg, 20Department of Internal Medicine, University of Giessen, Giessen, 21Clinic for Rheumatology, Bad Bramstedt, 22Department of Dermatology, University of Düsseldorf, Düsseldorf, 23Department of Dermatology and Venerology,Georg-August-University of Göttingen, Göttingen, 24Department of Dermatology, University of Tübingen, Tübingen, 25Department of Internal Medicine, University of Tübingen, Tübingen, 26Department of Internal Medicine, University of Heidelberg, Heidelberg, 27Johanniter Hospital in Fläming gGmbH, Center of Rheumatology of Brandenburg, Germany, 28Department of Dermatology and Venerology, University of Graz, Graz, Austria, 29Department of Internal Medicine, Center of Rheumatology, Baden-Baden and 30Department of Dermatology, Clinic of Minden, Minden, Germany.
1,
U Müller-Ladner
U Müller-Ladner
1Department of Dermatology and Venerology, University of Cologne, Cologne, 2Department of Rheumatology, University of Aachen, Aachen, 3Institute of Biostatistics, Informatics and Epidemiology, University of Cologne, Cologne, 4Clinical Research Unit for Rheumatology, University Medical Center Freiburg, Freiburg ,5Department of Dermatology, Dresden University Hospital, Dresden, 6Department of Rheumatology and Clinical Immunology, Kerckhoff Clinic, Bad Nauheim, 7Rheumatology and Clinical Immunology, Charité, Berlin, 8Hospital Cologne-Merheim, Medical Clinic I, 9Department of Dermatology, University of Münster, Münster, 10Department of Dermatology, Venerology and Allergology, University Hospital Charité, Berlin, 11Department of Rheumatology, University of Cologne, Cologne, 12Department of Dermatology, University of Mainz, Mainz, 13Department of Dermatology and Allergology, University of Munich, Munich, 14Department of Dermatology and Allergology, University of Ulm, Ulm, 15Department of Dermatology, 16Department of Internal Medicine, University of Regensburg, Regensburg, 17Department of Internal Medicine, University of Regensburg, Regensburg, 18Department of Dermatology, Allergology and Environmental Medicine, Private University Witten-Herdecke, HELIOS Klinikum Wuppertal, Wuppertal, 19Hospital Eilbek, Hamburg, 20Department of Internal Medicine, University of Giessen, Giessen, 21Clinic for Rheumatology, Bad Bramstedt, 22Department of Dermatology, University of Düsseldorf, Düsseldorf, 23Department of Dermatology and Venerology,Georg-August-University of Göttingen, Göttingen, 24Department of Dermatology, University of Tübingen, Tübingen, 25Department of Internal Medicine, University of Tübingen, Tübingen, 26Department of Internal Medicine, University of Heidelberg, Heidelberg, 27Johanniter Hospital in Fläming gGmbH, Center of Rheumatology of Brandenburg, Germany, 28Department of Dermatology and Venerology, University of Graz, Graz, Austria, 29Department of Internal Medicine, Center of Rheumatology, Baden-Baden and 30Department of Dermatology, Clinic of Minden, Minden, Germany.
6,
C Pfeiffer
C Pfeiffer
1Department of Dermatology and Venerology, University of Cologne, Cologne, 2Department of Rheumatology, University of Aachen, Aachen, 3Institute of Biostatistics, Informatics and Epidemiology, University of Cologne, Cologne, 4Clinical Research Unit for Rheumatology, University Medical Center Freiburg, Freiburg ,5Department of Dermatology, Dresden University Hospital, Dresden, 6Department of Rheumatology and Clinical Immunology, Kerckhoff Clinic, Bad Nauheim, 7Rheumatology and Clinical Immunology, Charité, Berlin, 8Hospital Cologne-Merheim, Medical Clinic I, 9Department of Dermatology, University of Münster, Münster, 10Department of Dermatology, Venerology and Allergology, University Hospital Charité, Berlin, 11Department of Rheumatology, University of Cologne, Cologne, 12Department of Dermatology, University of Mainz, Mainz, 13Department of Dermatology and Allergology, University of Munich, Munich, 14Department of Dermatology and Allergology, University of Ulm, Ulm, 15Department of Dermatology, 16Department of Internal Medicine, University of Regensburg, Regensburg, 17Department of Internal Medicine, University of Regensburg, Regensburg, 18Department of Dermatology, Allergology and Environmental Medicine, Private University Witten-Herdecke, HELIOS Klinikum Wuppertal, Wuppertal, 19Hospital Eilbek, Hamburg, 20Department of Internal Medicine, University of Giessen, Giessen, 21Clinic for Rheumatology, Bad Bramstedt, 22Department of Dermatology, University of Düsseldorf, Düsseldorf, 23Department of Dermatology and Venerology,Georg-August-University of Göttingen, Göttingen, 24Department of Dermatology, University of Tübingen, Tübingen, 25Department of Internal Medicine, University of Tübingen, Tübingen, 26Department of Internal Medicine, University of Heidelberg, Heidelberg, 27Johanniter Hospital in Fläming gGmbH, Center of Rheumatology of Brandenburg, Germany, 28Department of Dermatology and Venerology, University of Graz, Graz, Austria, 29Department of Internal Medicine, Center of Rheumatology, Baden-Baden and 30Department of Dermatology, Clinic of Minden, Minden, Germany.
5,
G Riemekasten
G Riemekasten
1Department of Dermatology and Venerology, University of Cologne, Cologne, 2Department of Rheumatology, University of Aachen, Aachen, 3Institute of Biostatistics, Informatics and Epidemiology, University of Cologne, Cologne, 4Clinical Research Unit for Rheumatology, University Medical Center Freiburg, Freiburg ,5Department of Dermatology, Dresden University Hospital, Dresden, 6Department of Rheumatology and Clinical Immunology, Kerckhoff Clinic, Bad Nauheim, 7Rheumatology and Clinical Immunology, Charité, Berlin, 8Hospital Cologne-Merheim, Medical Clinic I, 9Department of Dermatology, University of Münster, Münster, 10Department of Dermatology, Venerology and Allergology, University Hospital Charité, Berlin, 11Department of Rheumatology, University of Cologne, Cologne, 12Department of Dermatology, University of Mainz, Mainz, 13Department of Dermatology and Allergology, University of Munich, Munich, 14Department of Dermatology and Allergology, University of Ulm, Ulm, 15Department of Dermatology, 16Department of Internal Medicine, University of Regensburg, Regensburg, 17Department of Internal Medicine, University of Regensburg, Regensburg, 18Department of Dermatology, Allergology and Environmental Medicine, Private University Witten-Herdecke, HELIOS Klinikum Wuppertal, Wuppertal, 19Hospital Eilbek, Hamburg, 20Department of Internal Medicine, University of Giessen, Giessen, 21Clinic for Rheumatology, Bad Bramstedt, 22Department of Dermatology, University of Düsseldorf, Düsseldorf, 23Department of Dermatology and Venerology,Georg-August-University of Göttingen, Göttingen, 24Department of Dermatology, University of Tübingen, Tübingen, 25Department of Internal Medicine, University of Tübingen, Tübingen, 26Department of Internal Medicine, University of Heidelberg, Heidelberg, 27Johanniter Hospital in Fläming gGmbH, Center of Rheumatology of Brandenburg, Germany, 28Department of Dermatology and Venerology, University of Graz, Graz, Austria, 29Department of Internal Medicine, Center of Rheumatology, Baden-Baden and 30Department of Dermatology, Clinic of Minden, Minden, Germany.
7,
E Schulze-Lohoff
E Schulze-Lohoff
1Department of Dermatology and Venerology, University of Cologne, Cologne, 2Department of Rheumatology, University of Aachen, Aachen, 3Institute of Biostatistics, Informatics and Epidemiology, University of Cologne, Cologne, 4Clinical Research Unit for Rheumatology, University Medical Center Freiburg, Freiburg ,5Department of Dermatology, Dresden University Hospital, Dresden, 6Department of Rheumatology and Clinical Immunology, Kerckhoff Clinic, Bad Nauheim, 7Rheumatology and Clinical Immunology, Charité, Berlin, 8Hospital Cologne-Merheim, Medical Clinic I, 9Department of Dermatology, University of Münster, Münster, 10Department of Dermatology, Venerology and Allergology, University Hospital Charité, Berlin, 11Department of Rheumatology, University of Cologne, Cologne, 12Department of Dermatology, University of Mainz, Mainz, 13Department of Dermatology and Allergology, University of Munich, Munich, 14Department of Dermatology and Allergology, University of Ulm, Ulm, 15Department of Dermatology, 16Department of Internal Medicine, University of Regensburg, Regensburg, 17Department of Internal Medicine, University of Regensburg, Regensburg, 18Department of Dermatology, Allergology and Environmental Medicine, Private University Witten-Herdecke, HELIOS Klinikum Wuppertal, Wuppertal, 19Hospital Eilbek, Hamburg, 20Department of Internal Medicine, University of Giessen, Giessen, 21Clinic for Rheumatology, Bad Bramstedt, 22Department of Dermatology, University of Düsseldorf, Düsseldorf, 23Department of Dermatology and Venerology,Georg-August-University of Göttingen, Göttingen, 24Department of Dermatology, University of Tübingen, Tübingen, 25Department of Internal Medicine, University of Tübingen, Tübingen, 26Department of Internal Medicine, University of Heidelberg, Heidelberg, 27Johanniter Hospital in Fläming gGmbH, Center of Rheumatology of Brandenburg, Germany, 28Department of Dermatology and Venerology, University of Graz, Graz, Austria, 29Department of Internal Medicine, Center of Rheumatology, Baden-Baden and 30Department of Dermatology, Clinic of Minden, Minden, Germany.
8,
C Sunderkoetter
C Sunderkoetter
1Department of Dermatology and Venerology, University of Cologne, Cologne, 2Department of Rheumatology, University of Aachen, Aachen, 3Institute of Biostatistics, Informatics and Epidemiology, University of Cologne, Cologne, 4Clinical Research Unit for Rheumatology, University Medical Center Freiburg, Freiburg ,5Department of Dermatology, Dresden University Hospital, Dresden, 6Department of Rheumatology and Clinical Immunology, Kerckhoff Clinic, Bad Nauheim, 7Rheumatology and Clinical Immunology, Charité, Berlin, 8Hospital Cologne-Merheim, Medical Clinic I, 9Department of Dermatology, University of Münster, Münster, 10Department of Dermatology, Venerology and Allergology, University Hospital Charité, Berlin, 11Department of Rheumatology, University of Cologne, Cologne, 12Department of Dermatology, University of Mainz, Mainz, 13Department of Dermatology and Allergology, University of Munich, Munich, 14Department of Dermatology and Allergology, University of Ulm, Ulm, 15Department of Dermatology, 16Department of Internal Medicine, University of Regensburg, Regensburg, 17Department of Internal Medicine, University of Regensburg, Regensburg, 18Department of Dermatology, Allergology and Environmental Medicine, Private University Witten-Herdecke, HELIOS Klinikum Wuppertal, Wuppertal, 19Hospital Eilbek, Hamburg, 20Department of Internal Medicine, University of Giessen, Giessen, 21Clinic for Rheumatology, Bad Bramstedt, 22Department of Dermatology, University of Düsseldorf, Düsseldorf, 23Department of Dermatology and Venerology,Georg-August-University of Göttingen, Göttingen, 24Department of Dermatology, University of Tübingen, Tübingen, 25Department of Internal Medicine, University of Tübingen, Tübingen, 26Department of Internal Medicine, University of Heidelberg, Heidelberg, 27Johanniter Hospital in Fläming gGmbH, Center of Rheumatology of Brandenburg, Germany, 28Department of Dermatology and Venerology, University of Graz, Graz, Austria, 29Department of Internal Medicine, Center of Rheumatology, Baden-Baden and 30Department of Dermatology, Clinic of Minden, Minden, Germany.
9,
M Weber
M Weber
1Department of Dermatology and Venerology, University of Cologne, Cologne, 2Department of Rheumatology, University of Aachen, Aachen, 3Institute of Biostatistics, Informatics and Epidemiology, University of Cologne, Cologne, 4Clinical Research Unit for Rheumatology, University Medical Center Freiburg, Freiburg ,5Department of Dermatology, Dresden University Hospital, Dresden, 6Department of Rheumatology and Clinical Immunology, Kerckhoff Clinic, Bad Nauheim, 7Rheumatology and Clinical Immunology, Charité, Berlin, 8Hospital Cologne-Merheim, Medical Clinic I, 9Department of Dermatology, University of Münster, Münster, 10Department of Dermatology, Venerology and Allergology, University Hospital Charité, Berlin, 11Department of Rheumatology, University of Cologne, Cologne, 12Department of Dermatology, University of Mainz, Mainz, 13Department of Dermatology and Allergology, University of Munich, Munich, 14Department of Dermatology and Allergology, University of Ulm, Ulm, 15Department of Dermatology, 16Department of Internal Medicine, University of Regensburg, Regensburg, 17Department of Internal Medicine, University of Regensburg, Regensburg, 18Department of Dermatology, Allergology and Environmental Medicine, Private University Witten-Herdecke, HELIOS Klinikum Wuppertal, Wuppertal, 19Hospital Eilbek, Hamburg, 20Department of Internal Medicine, University of Giessen, Giessen, 21Clinic for Rheumatology, Bad Bramstedt, 22Department of Dermatology, University of Düsseldorf, Düsseldorf, 23Department of Dermatology and Venerology,Georg-August-University of Göttingen, Göttingen, 24Department of Dermatology, University of Tübingen, Tübingen, 25Department of Internal Medicine, University of Tübingen, Tübingen, 26Department of Internal Medicine, University of Heidelberg, Heidelberg, 27Johanniter Hospital in Fläming gGmbH, Center of Rheumatology of Brandenburg, Germany, 28Department of Dermatology and Venerology, University of Graz, Graz, Austria, 29Department of Internal Medicine, Center of Rheumatology, Baden-Baden and 30Department of Dermatology, Clinic of Minden, Minden, Germany.
8,
M Worm
M Worm
1Department of Dermatology and Venerology, University of Cologne, Cologne, 2Department of Rheumatology, University of Aachen, Aachen, 3Institute of Biostatistics, Informatics and Epidemiology, University of Cologne, Cologne, 4Clinical Research Unit for Rheumatology, University Medical Center Freiburg, Freiburg ,5Department of Dermatology, Dresden University Hospital, Dresden, 6Department of Rheumatology and Clinical Immunology, Kerckhoff Clinic, Bad Nauheim, 7Rheumatology and Clinical Immunology, Charité, Berlin, 8Hospital Cologne-Merheim, Medical Clinic I, 9Department of Dermatology, University of Münster, Münster, 10Department of Dermatology, Venerology and Allergology, University Hospital Charité, Berlin, 11Department of Rheumatology, University of Cologne, Cologne, 12Department of Dermatology, University of Mainz, Mainz, 13Department of Dermatology and Allergology, University of Munich, Munich, 14Department of Dermatology and Allergology, University of Ulm, Ulm, 15Department of Dermatology, 16Department of Internal Medicine, University of Regensburg, Regensburg, 17Department of Internal Medicine, University of Regensburg, Regensburg, 18Department of Dermatology, Allergology and Environmental Medicine, Private University Witten-Herdecke, HELIOS Klinikum Wuppertal, Wuppertal, 19Hospital Eilbek, Hamburg, 20Department of Internal Medicine, University of Giessen, Giessen, 21Clinic for Rheumatology, Bad Bramstedt, 22Department of Dermatology, University of Düsseldorf, Düsseldorf, 23Department of Dermatology and Venerology,Georg-August-University of Göttingen, Göttingen, 24Department of Dermatology, University of Tübingen, Tübingen, 25Department of Internal Medicine, University of Tübingen, Tübingen, 26Department of Internal Medicine, University of Heidelberg, Heidelberg, 27Johanniter Hospital in Fläming gGmbH, Center of Rheumatology of Brandenburg, Germany, 28Department of Dermatology and Venerology, University of Graz, Graz, Austria, 29Department of Internal Medicine, Center of Rheumatology, Baden-Baden and 30Department of Dermatology, Clinic of Minden, Minden, Germany.
10,
P Klaus
P Klaus
1Department of Dermatology and Venerology, University of Cologne, Cologne, 2Department of Rheumatology, University of Aachen, Aachen, 3Institute of Biostatistics, Informatics and Epidemiology, University of Cologne, Cologne, 4Clinical Research Unit for Rheumatology, University Medical Center Freiburg, Freiburg ,5Department of Dermatology, Dresden University Hospital, Dresden, 6Department of Rheumatology and Clinical Immunology, Kerckhoff Clinic, Bad Nauheim, 7Rheumatology and Clinical Immunology, Charité, Berlin, 8Hospital Cologne-Merheim, Medical Clinic I, 9Department of Dermatology, University of Münster, Münster, 10Department of Dermatology, Venerology and Allergology, University Hospital Charité, Berlin, 11Department of Rheumatology, University of Cologne, Cologne, 12Department of Dermatology, University of Mainz, Mainz, 13Department of Dermatology and Allergology, University of Munich, Munich, 14Department of Dermatology and Allergology, University of Ulm, Ulm, 15Department of Dermatology, 16Department of Internal Medicine, University of Regensburg, Regensburg, 17Department of Internal Medicine, University of Regensburg, Regensburg, 18Department of Dermatology, Allergology and Environmental Medicine, Private University Witten-Herdecke, HELIOS Klinikum Wuppertal, Wuppertal, 19Hospital Eilbek, Hamburg, 20Department of Internal Medicine, University of Giessen, Giessen, 21Clinic for Rheumatology, Bad Bramstedt, 22Department of Dermatology, University of Düsseldorf, Düsseldorf, 23Department of Dermatology and Venerology,Georg-August-University of Göttingen, Göttingen, 24Department of Dermatology, University of Tübingen, Tübingen, 25Department of Internal Medicine, University of Tübingen, Tübingen, 26Department of Internal Medicine, University of Heidelberg, Heidelberg, 27Johanniter Hospital in Fläming gGmbH, Center of Rheumatology of Brandenburg, Germany, 28Department of Dermatology and Venerology, University of Graz, Graz, Austria, 29Department of Internal Medicine, Center of Rheumatology, Baden-Baden and 30Department of Dermatology, Clinic of Minden, Minden, Germany.
10,
A Rubbert
A Rubbert
1Department of Dermatology and Venerology, University of Cologne, Cologne, 2Department of Rheumatology, University of Aachen, Aachen, 3Institute of Biostatistics, Informatics and Epidemiology, University of Cologne, Cologne, 4Clinical Research Unit for Rheumatology, University Medical Center Freiburg, Freiburg ,5Department of Dermatology, Dresden University Hospital, Dresden, 6Department of Rheumatology and Clinical Immunology, Kerckhoff Clinic, Bad Nauheim, 7Rheumatology and Clinical Immunology, Charité, Berlin, 8Hospital Cologne-Merheim, Medical Clinic I, 9Department of Dermatology, University of Münster, Münster, 10Department of Dermatology, Venerology and Allergology, University Hospital Charité, Berlin, 11Department of Rheumatology, University of Cologne, Cologne, 12Department of Dermatology, University of Mainz, Mainz, 13Department of Dermatology and Allergology, University of Munich, Munich, 14Department of Dermatology and Allergology, University of Ulm, Ulm, 15Department of Dermatology, 16Department of Internal Medicine, University of Regensburg, Regensburg, 17Department of Internal Medicine, University of Regensburg, Regensburg, 18Department of Dermatology, Allergology and Environmental Medicine, Private University Witten-Herdecke, HELIOS Klinikum Wuppertal, Wuppertal, 19Hospital Eilbek, Hamburg, 20Department of Internal Medicine, University of Giessen, Giessen, 21Clinic for Rheumatology, Bad Bramstedt, 22Department of Dermatology, University of Düsseldorf, Düsseldorf, 23Department of Dermatology and Venerology,Georg-August-University of Göttingen, Göttingen, 24Department of Dermatology, University of Tübingen, Tübingen, 25Department of Internal Medicine, University of Tübingen, Tübingen, 26Department of Internal Medicine, University of Heidelberg, Heidelberg, 27Johanniter Hospital in Fläming gGmbH, Center of Rheumatology of Brandenburg, Germany, 28Department of Dermatology and Venerology, University of Graz, Graz, Austria, 29Department of Internal Medicine, Center of Rheumatology, Baden-Baden and 30Department of Dermatology, Clinic of Minden, Minden, Germany.
11,
K Steinbrink
K Steinbrink
1Department of Dermatology and Venerology, University of Cologne, Cologne, 2Department of Rheumatology, University of Aachen, Aachen, 3Institute of Biostatistics, Informatics and Epidemiology, University of Cologne, Cologne, 4Clinical Research Unit for Rheumatology, University Medical Center Freiburg, Freiburg ,5Department of Dermatology, Dresden University Hospital, Dresden, 6Department of Rheumatology and Clinical Immunology, Kerckhoff Clinic, Bad Nauheim, 7Rheumatology and Clinical Immunology, Charité, Berlin, 8Hospital Cologne-Merheim, Medical Clinic I, 9Department of Dermatology, University of Münster, Münster, 10Department of Dermatology, Venerology and Allergology, University Hospital Charité, Berlin, 11Department of Rheumatology, University of Cologne, Cologne, 12Department of Dermatology, University of Mainz, Mainz, 13Department of Dermatology and Allergology, University of Munich, Munich, 14Department of Dermatology and Allergology, University of Ulm, Ulm, 15Department of Dermatology, 16Department of Internal Medicine, University of Regensburg, Regensburg, 17Department of Internal Medicine, University of Regensburg, Regensburg, 18Department of Dermatology, Allergology and Environmental Medicine, Private University Witten-Herdecke, HELIOS Klinikum Wuppertal, Wuppertal, 19Hospital Eilbek, Hamburg, 20Department of Internal Medicine, University of Giessen, Giessen, 21Clinic for Rheumatology, Bad Bramstedt, 22Department of Dermatology, University of Düsseldorf, Düsseldorf, 23Department of Dermatology and Venerology,Georg-August-University of Göttingen, Göttingen, 24Department of Dermatology, University of Tübingen, Tübingen, 25Department of Internal Medicine, University of Tübingen, Tübingen, 26Department of Internal Medicine, University of Heidelberg, Heidelberg, 27Johanniter Hospital in Fläming gGmbH, Center of Rheumatology of Brandenburg, Germany, 28Department of Dermatology and Venerology, University of Graz, Graz, Austria, 29Department of Internal Medicine, Center of Rheumatology, Baden-Baden and 30Department of Dermatology, Clinic of Minden, Minden, Germany.
12,
B Grundt
B Grundt
1Department of Dermatology and Venerology, University of Cologne, Cologne, 2Department of Rheumatology, University of Aachen, Aachen, 3Institute of Biostatistics, Informatics and Epidemiology, University of Cologne, Cologne, 4Clinical Research Unit for Rheumatology, University Medical Center Freiburg, Freiburg ,5Department of Dermatology, Dresden University Hospital, Dresden, 6Department of Rheumatology and Clinical Immunology, Kerckhoff Clinic, Bad Nauheim, 7Rheumatology and Clinical Immunology, Charité, Berlin, 8Hospital Cologne-Merheim, Medical Clinic I, 9Department of Dermatology, University of Münster, Münster, 10Department of Dermatology, Venerology and Allergology, University Hospital Charité, Berlin, 11Department of Rheumatology, University of Cologne, Cologne, 12Department of Dermatology, University of Mainz, Mainz, 13Department of Dermatology and Allergology, University of Munich, Munich, 14Department of Dermatology and Allergology, University of Ulm, Ulm, 15Department of Dermatology, 16Department of Internal Medicine, University of Regensburg, Regensburg, 17Department of Internal Medicine, University of Regensburg, Regensburg, 18Department of Dermatology, Allergology and Environmental Medicine, Private University Witten-Herdecke, HELIOS Klinikum Wuppertal, Wuppertal, 19Hospital Eilbek, Hamburg, 20Department of Internal Medicine, University of Giessen, Giessen, 21Clinic for Rheumatology, Bad Bramstedt, 22Department of Dermatology, University of Düsseldorf, Düsseldorf, 23Department of Dermatology and Venerology,Georg-August-University of Göttingen, Göttingen, 24Department of Dermatology, University of Tübingen, Tübingen, 25Department of Internal Medicine, University of Tübingen, Tübingen, 26Department of Internal Medicine, University of Heidelberg, Heidelberg, 27Johanniter Hospital in Fläming gGmbH, Center of Rheumatology of Brandenburg, Germany, 28Department of Dermatology and Venerology, University of Graz, Graz, Austria, 29Department of Internal Medicine, Center of Rheumatology, Baden-Baden and 30Department of Dermatology, Clinic of Minden, Minden, Germany.
12,
R Hein
R Hein
1Department of Dermatology and Venerology, University of Cologne, Cologne, 2Department of Rheumatology, University of Aachen, Aachen, 3Institute of Biostatistics, Informatics and Epidemiology, University of Cologne, Cologne, 4Clinical Research Unit for Rheumatology, University Medical Center Freiburg, Freiburg ,5Department of Dermatology, Dresden University Hospital, Dresden, 6Department of Rheumatology and Clinical Immunology, Kerckhoff Clinic, Bad Nauheim, 7Rheumatology and Clinical Immunology, Charité, Berlin, 8Hospital Cologne-Merheim, Medical Clinic I, 9Department of Dermatology, University of Münster, Münster, 10Department of Dermatology, Venerology and Allergology, University Hospital Charité, Berlin, 11Department of Rheumatology, University of Cologne, Cologne, 12Department of Dermatology, University of Mainz, Mainz, 13Department of Dermatology and Allergology, University of Munich, Munich, 14Department of Dermatology and Allergology, University of Ulm, Ulm, 15Department of Dermatology, 16Department of Internal Medicine, University of Regensburg, Regensburg, 17Department of Internal Medicine, University of Regensburg, Regensburg, 18Department of Dermatology, Allergology and Environmental Medicine, Private University Witten-Herdecke, HELIOS Klinikum Wuppertal, Wuppertal, 19Hospital Eilbek, Hamburg, 20Department of Internal Medicine, University of Giessen, Giessen, 21Clinic for Rheumatology, Bad Bramstedt, 22Department of Dermatology, University of Düsseldorf, Düsseldorf, 23Department of Dermatology and Venerology,Georg-August-University of Göttingen, Göttingen, 24Department of Dermatology, University of Tübingen, Tübingen, 25Department of Internal Medicine, University of Tübingen, Tübingen, 26Department of Internal Medicine, University of Heidelberg, Heidelberg, 27Johanniter Hospital in Fläming gGmbH, Center of Rheumatology of Brandenburg, Germany, 28Department of Dermatology and Venerology, University of Graz, Graz, Austria, 29Department of Internal Medicine, Center of Rheumatology, Baden-Baden and 30Department of Dermatology, Clinic of Minden, Minden, Germany.
13,
K Scharffetter-Kochanek
K Scharffetter-Kochanek
1Department of Dermatology and Venerology, University of Cologne, Cologne, 2Department of Rheumatology, University of Aachen, Aachen, 3Institute of Biostatistics, Informatics and Epidemiology, University of Cologne, Cologne, 4Clinical Research Unit for Rheumatology, University Medical Center Freiburg, Freiburg ,5Department of Dermatology, Dresden University Hospital, Dresden, 6Department of Rheumatology and Clinical Immunology, Kerckhoff Clinic, Bad Nauheim, 7Rheumatology and Clinical Immunology, Charité, Berlin, 8Hospital Cologne-Merheim, Medical Clinic I, 9Department of Dermatology, University of Münster, Münster, 10Department of Dermatology, Venerology and Allergology, University Hospital Charité, Berlin, 11Department of Rheumatology, University of Cologne, Cologne, 12Department of Dermatology, University of Mainz, Mainz, 13Department of Dermatology and Allergology, University of Munich, Munich, 14Department of Dermatology and Allergology, University of Ulm, Ulm, 15Department of Dermatology, 16Department of Internal Medicine, University of Regensburg, Regensburg, 17Department of Internal Medicine, University of Regensburg, Regensburg, 18Department of Dermatology, Allergology and Environmental Medicine, Private University Witten-Herdecke, HELIOS Klinikum Wuppertal, Wuppertal, 19Hospital Eilbek, Hamburg, 20Department of Internal Medicine, University of Giessen, Giessen, 21Clinic for Rheumatology, Bad Bramstedt, 22Department of Dermatology, University of Düsseldorf, Düsseldorf, 23Department of Dermatology and Venerology,Georg-August-University of Göttingen, Göttingen, 24Department of Dermatology, University of Tübingen, Tübingen, 25Department of Internal Medicine, University of Tübingen, Tübingen, 26Department of Internal Medicine, University of Heidelberg, Heidelberg, 27Johanniter Hospital in Fläming gGmbH, Center of Rheumatology of Brandenburg, Germany, 28Department of Dermatology and Venerology, University of Graz, Graz, Austria, 29Department of Internal Medicine, Center of Rheumatology, Baden-Baden and 30Department of Dermatology, Clinic of Minden, Minden, Germany.
14,
R Hinrichs
R Hinrichs
1Department of Dermatology and Venerology, University of Cologne, Cologne, 2Department of Rheumatology, University of Aachen, Aachen, 3Institute of Biostatistics, Informatics and Epidemiology, University of Cologne, Cologne, 4Clinical Research Unit for Rheumatology, University Medical Center Freiburg, Freiburg ,5Department of Dermatology, Dresden University Hospital, Dresden, 6Department of Rheumatology and Clinical Immunology, Kerckhoff Clinic, Bad Nauheim, 7Rheumatology and Clinical Immunology, Charité, Berlin, 8Hospital Cologne-Merheim, Medical Clinic I, 9Department of Dermatology, University of Münster, Münster, 10Department of Dermatology, Venerology and Allergology, University Hospital Charité, Berlin, 11Department of Rheumatology, University of Cologne, Cologne, 12Department of Dermatology, University of Mainz, Mainz, 13Department of Dermatology and Allergology, University of Munich, Munich, 14Department of Dermatology and Allergology, University of Ulm, Ulm, 15Department of Dermatology, 16Department of Internal Medicine, University of Regensburg, Regensburg, 17Department of Internal Medicine, University of Regensburg, Regensburg, 18Department of Dermatology, Allergology and Environmental Medicine, Private University Witten-Herdecke, HELIOS Klinikum Wuppertal, Wuppertal, 19Hospital Eilbek, Hamburg, 20Department of Internal Medicine, University of Giessen, Giessen, 21Clinic for Rheumatology, Bad Bramstedt, 22Department of Dermatology, University of Düsseldorf, Düsseldorf, 23Department of Dermatology and Venerology,Georg-August-University of Göttingen, Göttingen, 24Department of Dermatology, University of Tübingen, Tübingen, 25Department of Internal Medicine, University of Tübingen, Tübingen, 26Department of Internal Medicine, University of Heidelberg, Heidelberg, 27Johanniter Hospital in Fläming gGmbH, Center of Rheumatology of Brandenburg, Germany, 28Department of Dermatology and Venerology, University of Graz, Graz, Austria, 29Department of Internal Medicine, Center of Rheumatology, Baden-Baden and 30Department of Dermatology, Clinic of Minden, Minden, Germany.
14,
K Walker
K Walker
1Department of Dermatology and Venerology, University of Cologne, Cologne, 2Department of Rheumatology, University of Aachen, Aachen, 3Institute of Biostatistics, Informatics and Epidemiology, University of Cologne, Cologne, 4Clinical Research Unit for Rheumatology, University Medical Center Freiburg, Freiburg ,5Department of Dermatology, Dresden University Hospital, Dresden, 6Department of Rheumatology and Clinical Immunology, Kerckhoff Clinic, Bad Nauheim, 7Rheumatology and Clinical Immunology, Charité, Berlin, 8Hospital Cologne-Merheim, Medical Clinic I, 9Department of Dermatology, University of Münster, Münster, 10Department of Dermatology, Venerology and Allergology, University Hospital Charité, Berlin, 11Department of Rheumatology, University of Cologne, Cologne, 12Department of Dermatology, University of Mainz, Mainz, 13Department of Dermatology and Allergology, University of Munich, Munich, 14Department of Dermatology and Allergology, University of Ulm, Ulm, 15Department of Dermatology, 16Department of Internal Medicine, University of Regensburg, Regensburg, 17Department of Internal Medicine, University of Regensburg, Regensburg, 18Department of Dermatology, Allergology and Environmental Medicine, Private University Witten-Herdecke, HELIOS Klinikum Wuppertal, Wuppertal, 19Hospital Eilbek, Hamburg, 20Department of Internal Medicine, University of Giessen, Giessen, 21Clinic for Rheumatology, Bad Bramstedt, 22Department of Dermatology, University of Düsseldorf, Düsseldorf, 23Department of Dermatology and Venerology,Georg-August-University of Göttingen, Göttingen, 24Department of Dermatology, University of Tübingen, Tübingen, 25Department of Internal Medicine, University of Tübingen, Tübingen, 26Department of Internal Medicine, University of Heidelberg, Heidelberg, 27Johanniter Hospital in Fläming gGmbH, Center of Rheumatology of Brandenburg, Germany, 28Department of Dermatology and Venerology, University of Graz, Graz, Austria, 29Department of Internal Medicine, Center of Rheumatology, Baden-Baden and 30Department of Dermatology, Clinic of Minden, Minden, Germany.
14,
R-M Szeimies
R-M Szeimies
1Department of Dermatology and Venerology, University of Cologne, Cologne, 2Department of Rheumatology, University of Aachen, Aachen, 3Institute of Biostatistics, Informatics and Epidemiology, University of Cologne, Cologne, 4Clinical Research Unit for Rheumatology, University Medical Center Freiburg, Freiburg ,5Department of Dermatology, Dresden University Hospital, Dresden, 6Department of Rheumatology and Clinical Immunology, Kerckhoff Clinic, Bad Nauheim, 7Rheumatology and Clinical Immunology, Charité, Berlin, 8Hospital Cologne-Merheim, Medical Clinic I, 9Department of Dermatology, University of Münster, Münster, 10Department of Dermatology, Venerology and Allergology, University Hospital Charité, Berlin, 11Department of Rheumatology, University of Cologne, Cologne, 12Department of Dermatology, University of Mainz, Mainz, 13Department of Dermatology and Allergology, University of Munich, Munich, 14Department of Dermatology and Allergology, University of Ulm, Ulm, 15Department of Dermatology, 16Department of Internal Medicine, University of Regensburg, Regensburg, 17Department of Internal Medicine, University of Regensburg, Regensburg, 18Department of Dermatology, Allergology and Environmental Medicine, Private University Witten-Herdecke, HELIOS Klinikum Wuppertal, Wuppertal, 19Hospital Eilbek, Hamburg, 20Department of Internal Medicine, University of Giessen, Giessen, 21Clinic for Rheumatology, Bad Bramstedt, 22Department of Dermatology, University of Düsseldorf, Düsseldorf, 23Department of Dermatology and Venerology,Georg-August-University of Göttingen, Göttingen, 24Department of Dermatology, University of Tübingen, Tübingen, 25Department of Internal Medicine, University of Tübingen, Tübingen, 26Department of Internal Medicine, University of Heidelberg, Heidelberg, 27Johanniter Hospital in Fläming gGmbH, Center of Rheumatology of Brandenburg, Germany, 28Department of Dermatology and Venerology, University of Graz, Graz, Austria, 29Department of Internal Medicine, Center of Rheumatology, Baden-Baden and 30Department of Dermatology, Clinic of Minden, Minden, Germany.
15,
S Karrer
S Karrer
1Department of Dermatology and Venerology, University of Cologne, Cologne, 2Department of Rheumatology, University of Aachen, Aachen, 3Institute of Biostatistics, Informatics and Epidemiology, University of Cologne, Cologne, 4Clinical Research Unit for Rheumatology, University Medical Center Freiburg, Freiburg ,5Department of Dermatology, Dresden University Hospital, Dresden, 6Department of Rheumatology and Clinical Immunology, Kerckhoff Clinic, Bad Nauheim, 7Rheumatology and Clinical Immunology, Charité, Berlin, 8Hospital Cologne-Merheim, Medical Clinic I, 9Department of Dermatology, University of Münster, Münster, 10Department of Dermatology, Venerology and Allergology, University Hospital Charité, Berlin, 11Department of Rheumatology, University of Cologne, Cologne, 12Department of Dermatology, University of Mainz, Mainz, 13Department of Dermatology and Allergology, University of Munich, Munich, 14Department of Dermatology and Allergology, University of Ulm, Ulm, 15Department of Dermatology, 16Department of Internal Medicine, University of Regensburg, Regensburg, 17Department of Internal Medicine, University of Regensburg, Regensburg, 18Department of Dermatology, Allergology and Environmental Medicine, Private University Witten-Herdecke, HELIOS Klinikum Wuppertal, Wuppertal, 19Hospital Eilbek, Hamburg, 20Department of Internal Medicine, University of Giessen, Giessen, 21Clinic for Rheumatology, Bad Bramstedt, 22Department of Dermatology, University of Düsseldorf, Düsseldorf, 23Department of Dermatology and Venerology,Georg-August-University of Göttingen, Göttingen, 24Department of Dermatology, University of Tübingen, Tübingen, 25Department of Internal Medicine, University of Tübingen, Tübingen, 26Department of Internal Medicine, University of Heidelberg, Heidelberg, 27Johanniter Hospital in Fläming gGmbH, Center of Rheumatology of Brandenburg, Germany, 28Department of Dermatology and Venerology, University of Graz, Graz, Austria, 29Department of Internal Medicine, Center of Rheumatology, Baden-Baden and 30Department of Dermatology, Clinic of Minden, Minden, Germany.
15,
A Müller
A Müller
1Department of Dermatology and Venerology, University of Cologne, Cologne, 2Department of Rheumatology, University of Aachen, Aachen, 3Institute of Biostatistics, Informatics and Epidemiology, University of Cologne, Cologne, 4Clinical Research Unit for Rheumatology, University Medical Center Freiburg, Freiburg ,5Department of Dermatology, Dresden University Hospital, Dresden, 6Department of Rheumatology and Clinical Immunology, Kerckhoff Clinic, Bad Nauheim, 7Rheumatology and Clinical Immunology, Charité, Berlin, 8Hospital Cologne-Merheim, Medical Clinic I, 9Department of Dermatology, University of Münster, Münster, 10Department of Dermatology, Venerology and Allergology, University Hospital Charité, Berlin, 11Department of Rheumatology, University of Cologne, Cologne, 12Department of Dermatology, University of Mainz, Mainz, 13Department of Dermatology and Allergology, University of Munich, Munich, 14Department of Dermatology and Allergology, University of Ulm, Ulm, 15Department of Dermatology, 16Department of Internal Medicine, University of Regensburg, Regensburg, 17Department of Internal Medicine, University of Regensburg, Regensburg, 18Department of Dermatology, Allergology and Environmental Medicine, Private University Witten-Herdecke, HELIOS Klinikum Wuppertal, Wuppertal, 19Hospital Eilbek, Hamburg, 20Department of Internal Medicine, University of Giessen, Giessen, 21Clinic for Rheumatology, Bad Bramstedt, 22Department of Dermatology, University of Düsseldorf, Düsseldorf, 23Department of Dermatology and Venerology,Georg-August-University of Göttingen, Göttingen, 24Department of Dermatology, University of Tübingen, Tübingen, 25Department of Internal Medicine, University of Tübingen, Tübingen, 26Department of Internal Medicine, University of Heidelberg, Heidelberg, 27Johanniter Hospital in Fläming gGmbH, Center of Rheumatology of Brandenburg, Germany, 28Department of Dermatology and Venerology, University of Graz, Graz, Austria, 29Department of Internal Medicine, Center of Rheumatology, Baden-Baden and 30Department of Dermatology, Clinic of Minden, Minden, Germany.
16,
C Seitz
C Seitz
1Department of Dermatology and Venerology, University of Cologne, Cologne, 2Department of Rheumatology, University of Aachen, Aachen, 3Institute of Biostatistics, Informatics and Epidemiology, University of Cologne, Cologne, 4Clinical Research Unit for Rheumatology, University Medical Center Freiburg, Freiburg ,5Department of Dermatology, Dresden University Hospital, Dresden, 6Department of Rheumatology and Clinical Immunology, Kerckhoff Clinic, Bad Nauheim, 7Rheumatology and Clinical Immunology, Charité, Berlin, 8Hospital Cologne-Merheim, Medical Clinic I, 9Department of Dermatology, University of Münster, Münster, 10Department of Dermatology, Venerology and Allergology, University Hospital Charité, Berlin, 11Department of Rheumatology, University of Cologne, Cologne, 12Department of Dermatology, University of Mainz, Mainz, 13Department of Dermatology and Allergology, University of Munich, Munich, 14Department of Dermatology and Allergology, University of Ulm, Ulm, 15Department of Dermatology, 16Department of Internal Medicine, University of Regensburg, Regensburg, 17Department of Internal Medicine, University of Regensburg, Regensburg, 18Department of Dermatology, Allergology and Environmental Medicine, Private University Witten-Herdecke, HELIOS Klinikum Wuppertal, Wuppertal, 19Hospital Eilbek, Hamburg, 20Department of Internal Medicine, University of Giessen, Giessen, 21Clinic for Rheumatology, Bad Bramstedt, 22Department of Dermatology, University of Düsseldorf, Düsseldorf, 23Department of Dermatology and Venerology,Georg-August-University of Göttingen, Göttingen, 24Department of Dermatology, University of Tübingen, Tübingen, 25Department of Internal Medicine, University of Tübingen, Tübingen, 26Department of Internal Medicine, University of Heidelberg, Heidelberg, 27Johanniter Hospital in Fläming gGmbH, Center of Rheumatology of Brandenburg, Germany, 28Department of Dermatology and Venerology, University of Graz, Graz, Austria, 29Department of Internal Medicine, Center of Rheumatology, Baden-Baden and 30Department of Dermatology, Clinic of Minden, Minden, Germany.
17,
E Schmidt
E Schmidt
1Department of Dermatology and Venerology, University of Cologne, Cologne, 2Department of Rheumatology, University of Aachen, Aachen, 3Institute of Biostatistics, Informatics and Epidemiology, University of Cologne, Cologne, 4Clinical Research Unit for Rheumatology, University Medical Center Freiburg, Freiburg ,5Department of Dermatology, Dresden University Hospital, Dresden, 6Department of Rheumatology and Clinical Immunology, Kerckhoff Clinic, Bad Nauheim, 7Rheumatology and Clinical Immunology, Charité, Berlin, 8Hospital Cologne-Merheim, Medical Clinic I, 9Department of Dermatology, University of Münster, Münster, 10Department of Dermatology, Venerology and Allergology, University Hospital Charité, Berlin, 11Department of Rheumatology, University of Cologne, Cologne, 12Department of Dermatology, University of Mainz, Mainz, 13Department of Dermatology and Allergology, University of Munich, Munich, 14Department of Dermatology and Allergology, University of Ulm, Ulm, 15Department of Dermatology, 16Department of Internal Medicine, University of Regensburg, Regensburg, 17Department of Internal Medicine, University of Regensburg, Regensburg, 18Department of Dermatology, Allergology and Environmental Medicine, Private University Witten-Herdecke, HELIOS Klinikum Wuppertal, Wuppertal, 19Hospital Eilbek, Hamburg, 20Department of Internal Medicine, University of Giessen, Giessen, 21Clinic for Rheumatology, Bad Bramstedt, 22Department of Dermatology, University of Düsseldorf, Düsseldorf, 23Department of Dermatology and Venerology,Georg-August-University of Göttingen, Göttingen, 24Department of Dermatology, University of Tübingen, Tübingen, 25Department of Internal Medicine, University of Tübingen, Tübingen, 26Department of Internal Medicine, University of Heidelberg, Heidelberg, 27Johanniter Hospital in Fläming gGmbH, Center of Rheumatology of Brandenburg, Germany, 28Department of Dermatology and Venerology, University of Graz, Graz, Austria, 29Department of Internal Medicine, Center of Rheumatology, Baden-Baden and 30Department of Dermatology, Clinic of Minden, Minden, Germany.
17,
P Lehmann
P Lehmann
1Department of Dermatology and Venerology, University of Cologne, Cologne, 2Department of Rheumatology, University of Aachen, Aachen, 3Institute of Biostatistics, Informatics and Epidemiology, University of Cologne, Cologne, 4Clinical Research Unit for Rheumatology, University Medical Center Freiburg, Freiburg ,5Department of Dermatology, Dresden University Hospital, Dresden, 6Department of Rheumatology and Clinical Immunology, Kerckhoff Clinic, Bad Nauheim, 7Rheumatology and Clinical Immunology, Charité, Berlin, 8Hospital Cologne-Merheim, Medical Clinic I, 9Department of Dermatology, University of Münster, Münster, 10Department of Dermatology, Venerology and Allergology, University Hospital Charité, Berlin, 11Department of Rheumatology, University of Cologne, Cologne, 12Department of Dermatology, University of Mainz, Mainz, 13Department of Dermatology and Allergology, University of Munich, Munich, 14Department of Dermatology and Allergology, University of Ulm, Ulm, 15Department of Dermatology, 16Department of Internal Medicine, University of Regensburg, Regensburg, 17Department of Internal Medicine, University of Regensburg, Regensburg, 18Department of Dermatology, Allergology and Environmental Medicine, Private University Witten-Herdecke, HELIOS Klinikum Wuppertal, Wuppertal, 19Hospital Eilbek, Hamburg, 20Department of Internal Medicine, University of Giessen, Giessen, 21Clinic for Rheumatology, Bad Bramstedt, 22Department of Dermatology, University of Düsseldorf, Düsseldorf, 23Department of Dermatology and Venerology,Georg-August-University of Göttingen, Göttingen, 24Department of Dermatology, University of Tübingen, Tübingen, 25Department of Internal Medicine, University of Tübingen, Tübingen, 26Department of Internal Medicine, University of Heidelberg, Heidelberg, 27Johanniter Hospital in Fläming gGmbH, Center of Rheumatology of Brandenburg, Germany, 28Department of Dermatology and Venerology, University of Graz, Graz, Austria, 29Department of Internal Medicine, Center of Rheumatology, Baden-Baden and 30Department of Dermatology, Clinic of Minden, Minden, Germany.
18,
I Foeldvári
I Foeldvári
1Department of Dermatology and Venerology, University of Cologne, Cologne, 2Department of Rheumatology, University of Aachen, Aachen, 3Institute of Biostatistics, Informatics and Epidemiology, University of Cologne, Cologne, 4Clinical Research Unit for Rheumatology, University Medical Center Freiburg, Freiburg ,5Department of Dermatology, Dresden University Hospital, Dresden, 6Department of Rheumatology and Clinical Immunology, Kerckhoff Clinic, Bad Nauheim, 7Rheumatology and Clinical Immunology, Charité, Berlin, 8Hospital Cologne-Merheim, Medical Clinic I, 9Department of Dermatology, University of Münster, Münster, 10Department of Dermatology, Venerology and Allergology, University Hospital Charité, Berlin, 11Department of Rheumatology, University of Cologne, Cologne, 12Department of Dermatology, University of Mainz, Mainz, 13Department of Dermatology and Allergology, University of Munich, Munich, 14Department of Dermatology and Allergology, University of Ulm, Ulm, 15Department of Dermatology, 16Department of Internal Medicine, University of Regensburg, Regensburg, 17Department of Internal Medicine, University of Regensburg, Regensburg, 18Department of Dermatology, Allergology and Environmental Medicine, Private University Witten-Herdecke, HELIOS Klinikum Wuppertal, Wuppertal, 19Hospital Eilbek, Hamburg, 20Department of Internal Medicine, University of Giessen, Giessen, 21Clinic for Rheumatology, Bad Bramstedt, 22Department of Dermatology, University of Düsseldorf, Düsseldorf, 23Department of Dermatology and Venerology,Georg-August-University of Göttingen, Göttingen, 24Department of Dermatology, University of Tübingen, Tübingen, 25Department of Internal Medicine, University of Tübingen, Tübingen, 26Department of Internal Medicine, University of Heidelberg, Heidelberg, 27Johanniter Hospital in Fläming gGmbH, Center of Rheumatology of Brandenburg, Germany, 28Department of Dermatology and Venerology, University of Graz, Graz, Austria, 29Department of Internal Medicine, Center of Rheumatology, Baden-Baden and 30Department of Dermatology, Clinic of Minden, Minden, Germany.
19,
F Reichenberger
F Reichenberger
1Department of Dermatology and Venerology, University of Cologne, Cologne, 2Department of Rheumatology, University of Aachen, Aachen, 3Institute of Biostatistics, Informatics and Epidemiology, University of Cologne, Cologne, 4Clinical Research Unit for Rheumatology, University Medical Center Freiburg, Freiburg ,5Department of Dermatology, Dresden University Hospital, Dresden, 6Department of Rheumatology and Clinical Immunology, Kerckhoff Clinic, Bad Nauheim, 7Rheumatology and Clinical Immunology, Charité, Berlin, 8Hospital Cologne-Merheim, Medical Clinic I, 9Department of Dermatology, University of Münster, Münster, 10Department of Dermatology, Venerology and Allergology, University Hospital Charité, Berlin, 11Department of Rheumatology, University of Cologne, Cologne, 12Department of Dermatology, University of Mainz, Mainz, 13Department of Dermatology and Allergology, University of Munich, Munich, 14Department of Dermatology and Allergology, University of Ulm, Ulm, 15Department of Dermatology, 16Department of Internal Medicine, University of Regensburg, Regensburg, 17Department of Internal Medicine, University of Regensburg, Regensburg, 18Department of Dermatology, Allergology and Environmental Medicine, Private University Witten-Herdecke, HELIOS Klinikum Wuppertal, Wuppertal, 19Hospital Eilbek, Hamburg, 20Department of Internal Medicine, University of Giessen, Giessen, 21Clinic for Rheumatology, Bad Bramstedt, 22Department of Dermatology, University of Düsseldorf, Düsseldorf, 23Department of Dermatology and Venerology,Georg-August-University of Göttingen, Göttingen, 24Department of Dermatology, University of Tübingen, Tübingen, 25Department of Internal Medicine, University of Tübingen, Tübingen, 26Department of Internal Medicine, University of Heidelberg, Heidelberg, 27Johanniter Hospital in Fläming gGmbH, Center of Rheumatology of Brandenburg, Germany, 28Department of Dermatology and Venerology, University of Graz, Graz, Austria, 29Department of Internal Medicine, Center of Rheumatology, Baden-Baden and 30Department of Dermatology, Clinic of Minden, Minden, Germany.
20,
WL Gross
WL Gross
1Department of Dermatology and Venerology, University of Cologne, Cologne, 2Department of Rheumatology, University of Aachen, Aachen, 3Institute of Biostatistics, Informatics and Epidemiology, University of Cologne, Cologne, 4Clinical Research Unit for Rheumatology, University Medical Center Freiburg, Freiburg ,5Department of Dermatology, Dresden University Hospital, Dresden, 6Department of Rheumatology and Clinical Immunology, Kerckhoff Clinic, Bad Nauheim, 7Rheumatology and Clinical Immunology, Charité, Berlin, 8Hospital Cologne-Merheim, Medical Clinic I, 9Department of Dermatology, University of Münster, Münster, 10Department of Dermatology, Venerology and Allergology, University Hospital Charité, Berlin, 11Department of Rheumatology, University of Cologne, Cologne, 12Department of Dermatology, University of Mainz, Mainz, 13Department of Dermatology and Allergology, University of Munich, Munich, 14Department of Dermatology and Allergology, University of Ulm, Ulm, 15Department of Dermatology, 16Department of Internal Medicine, University of Regensburg, Regensburg, 17Department of Internal Medicine, University of Regensburg, Regensburg, 18Department of Dermatology, Allergology and Environmental Medicine, Private University Witten-Herdecke, HELIOS Klinikum Wuppertal, Wuppertal, 19Hospital Eilbek, Hamburg, 20Department of Internal Medicine, University of Giessen, Giessen, 21Clinic for Rheumatology, Bad Bramstedt, 22Department of Dermatology, University of Düsseldorf, Düsseldorf, 23Department of Dermatology and Venerology,Georg-August-University of Göttingen, Göttingen, 24Department of Dermatology, University of Tübingen, Tübingen, 25Department of Internal Medicine, University of Tübingen, Tübingen, 26Department of Internal Medicine, University of Heidelberg, Heidelberg, 27Johanniter Hospital in Fläming gGmbH, Center of Rheumatology of Brandenburg, Germany, 28Department of Dermatology and Venerology, University of Graz, Graz, Austria, 29Department of Internal Medicine, Center of Rheumatology, Baden-Baden and 30Department of Dermatology, Clinic of Minden, Minden, Germany.
21,
A Kuhn
A Kuhn
1Department of Dermatology and Venerology, University of Cologne, Cologne, 2Department of Rheumatology, University of Aachen, Aachen, 3Institute of Biostatistics, Informatics and Epidemiology, University of Cologne, Cologne, 4Clinical Research Unit for Rheumatology, University Medical Center Freiburg, Freiburg ,5Department of Dermatology, Dresden University Hospital, Dresden, 6Department of Rheumatology and Clinical Immunology, Kerckhoff Clinic, Bad Nauheim, 7Rheumatology and Clinical Immunology, Charité, Berlin, 8Hospital Cologne-Merheim, Medical Clinic I, 9Department of Dermatology, University of Münster, Münster, 10Department of Dermatology, Venerology and Allergology, University Hospital Charité, Berlin, 11Department of Rheumatology, University of Cologne, Cologne, 12Department of Dermatology, University of Mainz, Mainz, 13Department of Dermatology and Allergology, University of Munich, Munich, 14Department of Dermatology and Allergology, University of Ulm, Ulm, 15Department of Dermatology, 16Department of Internal Medicine, University of Regensburg, Regensburg, 17Department of Internal Medicine, University of Regensburg, Regensburg, 18Department of Dermatology, Allergology and Environmental Medicine, Private University Witten-Herdecke, HELIOS Klinikum Wuppertal, Wuppertal, 19Hospital Eilbek, Hamburg, 20Department of Internal Medicine, University of Giessen, Giessen, 21Clinic for Rheumatology, Bad Bramstedt, 22Department of Dermatology, University of Düsseldorf, Düsseldorf, 23Department of Dermatology and Venerology,Georg-August-University of Göttingen, Göttingen, 24Department of Dermatology, University of Tübingen, Tübingen, 25Department of Internal Medicine, University of Tübingen, Tübingen, 26Department of Internal Medicine, University of Heidelberg, Heidelberg, 27Johanniter Hospital in Fläming gGmbH, Center of Rheumatology of Brandenburg, Germany, 28Department of Dermatology and Venerology, University of Graz, Graz, Austria, 29Department of Internal Medicine, Center of Rheumatology, Baden-Baden and 30Department of Dermatology, Clinic of Minden, Minden, Germany.
22,
M Haust
M Haust
1Department of Dermatology and Venerology, University of Cologne, Cologne, 2Department of Rheumatology, University of Aachen, Aachen, 3Institute of Biostatistics, Informatics and Epidemiology, University of Cologne, Cologne, 4Clinical Research Unit for Rheumatology, University Medical Center Freiburg, Freiburg ,5Department of Dermatology, Dresden University Hospital, Dresden, 6Department of Rheumatology and Clinical Immunology, Kerckhoff Clinic, Bad Nauheim, 7Rheumatology and Clinical Immunology, Charité, Berlin, 8Hospital Cologne-Merheim, Medical Clinic I, 9Department of Dermatology, University of Münster, Münster, 10Department of Dermatology, Venerology and Allergology, University Hospital Charité, Berlin, 11Department of Rheumatology, University of Cologne, Cologne, 12Department of Dermatology, University of Mainz, Mainz, 13Department of Dermatology and Allergology, University of Munich, Munich, 14Department of Dermatology and Allergology, University of Ulm, Ulm, 15Department of Dermatology, 16Department of Internal Medicine, University of Regensburg, Regensburg, 17Department of Internal Medicine, University of Regensburg, Regensburg, 18Department of Dermatology, Allergology and Environmental Medicine, Private University Witten-Herdecke, HELIOS Klinikum Wuppertal, Wuppertal, 19Hospital Eilbek, Hamburg, 20Department of Internal Medicine, University of Giessen, Giessen, 21Clinic for Rheumatology, Bad Bramstedt, 22Department of Dermatology, University of Düsseldorf, Düsseldorf, 23Department of Dermatology and Venerology,Georg-August-University of Göttingen, Göttingen, 24Department of Dermatology, University of Tübingen, Tübingen, 25Department of Internal Medicine, University of Tübingen, Tübingen, 26Department of Internal Medicine, University of Heidelberg, Heidelberg, 27Johanniter Hospital in Fläming gGmbH, Center of Rheumatology of Brandenburg, Germany, 28Department of Dermatology and Venerology, University of Graz, Graz, Austria, 29Department of Internal Medicine, Center of Rheumatology, Baden-Baden and 30Department of Dermatology, Clinic of Minden, Minden, Germany.
22,
K Reich
K Reich
1Department of Dermatology and Venerology, University of Cologne, Cologne, 2Department of Rheumatology, University of Aachen, Aachen, 3Institute of Biostatistics, Informatics and Epidemiology, University of Cologne, Cologne, 4Clinical Research Unit for Rheumatology, University Medical Center Freiburg, Freiburg ,5Department of Dermatology, Dresden University Hospital, Dresden, 6Department of Rheumatology and Clinical Immunology, Kerckhoff Clinic, Bad Nauheim, 7Rheumatology and Clinical Immunology, Charité, Berlin, 8Hospital Cologne-Merheim, Medical Clinic I, 9Department of Dermatology, University of Münster, Münster, 10Department of Dermatology, Venerology and Allergology, University Hospital Charité, Berlin, 11Department of Rheumatology, University of Cologne, Cologne, 12Department of Dermatology, University of Mainz, Mainz, 13Department of Dermatology and Allergology, University of Munich, Munich, 14Department of Dermatology and Allergology, University of Ulm, Ulm, 15Department of Dermatology, 16Department of Internal Medicine, University of Regensburg, Regensburg, 17Department of Internal Medicine, University of Regensburg, Regensburg, 18Department of Dermatology, Allergology and Environmental Medicine, Private University Witten-Herdecke, HELIOS Klinikum Wuppertal, Wuppertal, 19Hospital Eilbek, Hamburg, 20Department of Internal Medicine, University of Giessen, Giessen, 21Clinic for Rheumatology, Bad Bramstedt, 22Department of Dermatology, University of Düsseldorf, Düsseldorf, 23Department of Dermatology and Venerology,Georg-August-University of Göttingen, Göttingen, 24Department of Dermatology, University of Tübingen, Tübingen, 25Department of Internal Medicine, University of Tübingen, Tübingen, 26Department of Internal Medicine, University of Heidelberg, Heidelberg, 27Johanniter Hospital in Fläming gGmbH, Center of Rheumatology of Brandenburg, Germany, 28Department of Dermatology and Venerology, University of Graz, Graz, Austria, 29Department of Internal Medicine, Center of Rheumatology, Baden-Baden and 30Department of Dermatology, Clinic of Minden, Minden, Germany.
23,
M Böhm
M Böhm
1Department of Dermatology and Venerology, University of Cologne, Cologne, 2Department of Rheumatology, University of Aachen, Aachen, 3Institute of Biostatistics, Informatics and Epidemiology, University of Cologne, Cologne, 4Clinical Research Unit for Rheumatology, University Medical Center Freiburg, Freiburg ,5Department of Dermatology, Dresden University Hospital, Dresden, 6Department of Rheumatology and Clinical Immunology, Kerckhoff Clinic, Bad Nauheim, 7Rheumatology and Clinical Immunology, Charité, Berlin, 8Hospital Cologne-Merheim, Medical Clinic I, 9Department of Dermatology, University of Münster, Münster, 10Department of Dermatology, Venerology and Allergology, University Hospital Charité, Berlin, 11Department of Rheumatology, University of Cologne, Cologne, 12Department of Dermatology, University of Mainz, Mainz, 13Department of Dermatology and Allergology, University of Munich, Munich, 14Department of Dermatology and Allergology, University of Ulm, Ulm, 15Department of Dermatology, 16Department of Internal Medicine, University of Regensburg, Regensburg, 17Department of Internal Medicine, University of Regensburg, Regensburg, 18Department of Dermatology, Allergology and Environmental Medicine, Private University Witten-Herdecke, HELIOS Klinikum Wuppertal, Wuppertal, 19Hospital Eilbek, Hamburg, 20Department of Internal Medicine, University of Giessen, Giessen, 21Clinic for Rheumatology, Bad Bramstedt, 22Department of Dermatology, University of Düsseldorf, Düsseldorf, 23Department of Dermatology and Venerology,Georg-August-University of Göttingen, Göttingen, 24Department of Dermatology, University of Tübingen, Tübingen, 25Department of Internal Medicine, University of Tübingen, Tübingen, 26Department of Internal Medicine, University of Heidelberg, Heidelberg, 27Johanniter Hospital in Fläming gGmbH, Center of Rheumatology of Brandenburg, Germany, 28Department of Dermatology and Venerology, University of Graz, Graz, Austria, 29Department of Internal Medicine, Center of Rheumatology, Baden-Baden and 30Department of Dermatology, Clinic of Minden, Minden, Germany.
9,
P Saar
P Saar
1Department of Dermatology and Venerology, University of Cologne, Cologne, 2Department of Rheumatology, University of Aachen, Aachen, 3Institute of Biostatistics, Informatics and Epidemiology, University of Cologne, Cologne, 4Clinical Research Unit for Rheumatology, University Medical Center Freiburg, Freiburg ,5Department of Dermatology, Dresden University Hospital, Dresden, 6Department of Rheumatology and Clinical Immunology, Kerckhoff Clinic, Bad Nauheim, 7Rheumatology and Clinical Immunology, Charité, Berlin, 8Hospital Cologne-Merheim, Medical Clinic I, 9Department of Dermatology, University of Münster, Münster, 10Department of Dermatology, Venerology and Allergology, University Hospital Charité, Berlin, 11Department of Rheumatology, University of Cologne, Cologne, 12Department of Dermatology, University of Mainz, Mainz, 13Department of Dermatology and Allergology, University of Munich, Munich, 14Department of Dermatology and Allergology, University of Ulm, Ulm, 15Department of Dermatology, 16Department of Internal Medicine, University of Regensburg, Regensburg, 17Department of Internal Medicine, University of Regensburg, Regensburg, 18Department of Dermatology, Allergology and Environmental Medicine, Private University Witten-Herdecke, HELIOS Klinikum Wuppertal, Wuppertal, 19Hospital Eilbek, Hamburg, 20Department of Internal Medicine, University of Giessen, Giessen, 21Clinic for Rheumatology, Bad Bramstedt, 22Department of Dermatology, University of Düsseldorf, Düsseldorf, 23Department of Dermatology and Venerology,Georg-August-University of Göttingen, Göttingen, 24Department of Dermatology, University of Tübingen, Tübingen, 25Department of Internal Medicine, University of Tübingen, Tübingen, 26Department of Internal Medicine, University of Heidelberg, Heidelberg, 27Johanniter Hospital in Fläming gGmbH, Center of Rheumatology of Brandenburg, Germany, 28Department of Dermatology and Venerology, University of Graz, Graz, Austria, 29Department of Internal Medicine, Center of Rheumatology, Baden-Baden and 30Department of Dermatology, Clinic of Minden, Minden, Germany.
6,
G Fierlbeck
G Fierlbeck
1Department of Dermatology and Venerology, University of Cologne, Cologne, 2Department of Rheumatology, University of Aachen, Aachen, 3Institute of Biostatistics, Informatics and Epidemiology, University of Cologne, Cologne, 4Clinical Research Unit for Rheumatology, University Medical Center Freiburg, Freiburg ,5Department of Dermatology, Dresden University Hospital, Dresden, 6Department of Rheumatology and Clinical Immunology, Kerckhoff Clinic, Bad Nauheim, 7Rheumatology and Clinical Immunology, Charité, Berlin, 8Hospital Cologne-Merheim, Medical Clinic I, 9Department of Dermatology, University of Münster, Münster, 10Department of Dermatology, Venerology and Allergology, University Hospital Charité, Berlin, 11Department of Rheumatology, University of Cologne, Cologne, 12Department of Dermatology, University of Mainz, Mainz, 13Department of Dermatology and Allergology, University of Munich, Munich, 14Department of Dermatology and Allergology, University of Ulm, Ulm, 15Department of Dermatology, 16Department of Internal Medicine, University of Regensburg, Regensburg, 17Department of Internal Medicine, University of Regensburg, Regensburg, 18Department of Dermatology, Allergology and Environmental Medicine, Private University Witten-Herdecke, HELIOS Klinikum Wuppertal, Wuppertal, 19Hospital Eilbek, Hamburg, 20Department of Internal Medicine, University of Giessen, Giessen, 21Clinic for Rheumatology, Bad Bramstedt, 22Department of Dermatology, University of Düsseldorf, Düsseldorf, 23Department of Dermatology and Venerology,Georg-August-University of Göttingen, Göttingen, 24Department of Dermatology, University of Tübingen, Tübingen, 25Department of Internal Medicine, University of Tübingen, Tübingen, 26Department of Internal Medicine, University of Heidelberg, Heidelberg, 27Johanniter Hospital in Fläming gGmbH, Center of Rheumatology of Brandenburg, Germany, 28Department of Dermatology and Venerology, University of Graz, Graz, Austria, 29Department of Internal Medicine, Center of Rheumatology, Baden-Baden and 30Department of Dermatology, Clinic of Minden, Minden, Germany.
24,
I Kötter
I Kötter
1Department of Dermatology and Venerology, University of Cologne, Cologne, 2Department of Rheumatology, University of Aachen, Aachen, 3Institute of Biostatistics, Informatics and Epidemiology, University of Cologne, Cologne, 4Clinical Research Unit for Rheumatology, University Medical Center Freiburg, Freiburg ,5Department of Dermatology, Dresden University Hospital, Dresden, 6Department of Rheumatology and Clinical Immunology, Kerckhoff Clinic, Bad Nauheim, 7Rheumatology and Clinical Immunology, Charité, Berlin, 8Hospital Cologne-Merheim, Medical Clinic I, 9Department of Dermatology, University of Münster, Münster, 10Department of Dermatology, Venerology and Allergology, University Hospital Charité, Berlin, 11Department of Rheumatology, University of Cologne, Cologne, 12Department of Dermatology, University of Mainz, Mainz, 13Department of Dermatology and Allergology, University of Munich, Munich, 14Department of Dermatology and Allergology, University of Ulm, Ulm, 15Department of Dermatology, 16Department of Internal Medicine, University of Regensburg, Regensburg, 17Department of Internal Medicine, University of Regensburg, Regensburg, 18Department of Dermatology, Allergology and Environmental Medicine, Private University Witten-Herdecke, HELIOS Klinikum Wuppertal, Wuppertal, 19Hospital Eilbek, Hamburg, 20Department of Internal Medicine, University of Giessen, Giessen, 21Clinic for Rheumatology, Bad Bramstedt, 22Department of Dermatology, University of Düsseldorf, Düsseldorf, 23Department of Dermatology and Venerology,Georg-August-University of Göttingen, Göttingen, 24Department of Dermatology, University of Tübingen, Tübingen, 25Department of Internal Medicine, University of Tübingen, Tübingen, 26Department of Internal Medicine, University of Heidelberg, Heidelberg, 27Johanniter Hospital in Fläming gGmbH, Center of Rheumatology of Brandenburg, Germany, 28Department of Dermatology and Venerology, University of Graz, Graz, Austria, 29Department of Internal Medicine, Center of Rheumatology, Baden-Baden and 30Department of Dermatology, Clinic of Minden, Minden, Germany.
25,
H-M Lorenz
H-M Lorenz
1Department of Dermatology and Venerology, University of Cologne, Cologne, 2Department of Rheumatology, University of Aachen, Aachen, 3Institute of Biostatistics, Informatics and Epidemiology, University of Cologne, Cologne, 4Clinical Research Unit for Rheumatology, University Medical Center Freiburg, Freiburg ,5Department of Dermatology, Dresden University Hospital, Dresden, 6Department of Rheumatology and Clinical Immunology, Kerckhoff Clinic, Bad Nauheim, 7Rheumatology and Clinical Immunology, Charité, Berlin, 8Hospital Cologne-Merheim, Medical Clinic I, 9Department of Dermatology, University of Münster, Münster, 10Department of Dermatology, Venerology and Allergology, University Hospital Charité, Berlin, 11Department of Rheumatology, University of Cologne, Cologne, 12Department of Dermatology, University of Mainz, Mainz, 13Department of Dermatology and Allergology, University of Munich, Munich, 14Department of Dermatology and Allergology, University of Ulm, Ulm, 15Department of Dermatology, 16Department of Internal Medicine, University of Regensburg, Regensburg, 17Department of Internal Medicine, University of Regensburg, Regensburg, 18Department of Dermatology, Allergology and Environmental Medicine, Private University Witten-Herdecke, HELIOS Klinikum Wuppertal, Wuppertal, 19Hospital Eilbek, Hamburg, 20Department of Internal Medicine, University of Giessen, Giessen, 21Clinic for Rheumatology, Bad Bramstedt, 22Department of Dermatology, University of Düsseldorf, Düsseldorf, 23Department of Dermatology and Venerology,Georg-August-University of Göttingen, Göttingen, 24Department of Dermatology, University of Tübingen, Tübingen, 25Department of Internal Medicine, University of Tübingen, Tübingen, 26Department of Internal Medicine, University of Heidelberg, Heidelberg, 27Johanniter Hospital in Fläming gGmbH, Center of Rheumatology of Brandenburg, Germany, 28Department of Dermatology and Venerology, University of Graz, Graz, Austria, 29Department of Internal Medicine, Center of Rheumatology, Baden-Baden and 30Department of Dermatology, Clinic of Minden, Minden, Germany.
26,
N Blank
N Blank
1Department of Dermatology and Venerology, University of Cologne, Cologne, 2Department of Rheumatology, University of Aachen, Aachen, 3Institute of Biostatistics, Informatics and Epidemiology, University of Cologne, Cologne, 4Clinical Research Unit for Rheumatology, University Medical Center Freiburg, Freiburg ,5Department of Dermatology, Dresden University Hospital, Dresden, 6Department of Rheumatology and Clinical Immunology, Kerckhoff Clinic, Bad Nauheim, 7Rheumatology and Clinical Immunology, Charité, Berlin, 8Hospital Cologne-Merheim, Medical Clinic I, 9Department of Dermatology, University of Münster, Münster, 10Department of Dermatology, Venerology and Allergology, University Hospital Charité, Berlin, 11Department of Rheumatology, University of Cologne, Cologne, 12Department of Dermatology, University of Mainz, Mainz, 13Department of Dermatology and Allergology, University of Munich, Munich, 14Department of Dermatology and Allergology, University of Ulm, Ulm, 15Department of Dermatology, 16Department of Internal Medicine, University of Regensburg, Regensburg, 17Department of Internal Medicine, University of Regensburg, Regensburg, 18Department of Dermatology, Allergology and Environmental Medicine, Private University Witten-Herdecke, HELIOS Klinikum Wuppertal, Wuppertal, 19Hospital Eilbek, Hamburg, 20Department of Internal Medicine, University of Giessen, Giessen, 21Clinic for Rheumatology, Bad Bramstedt, 22Department of Dermatology, University of Düsseldorf, Düsseldorf, 23Department of Dermatology and Venerology,Georg-August-University of Göttingen, Göttingen, 24Department of Dermatology, University of Tübingen, Tübingen, 25Department of Internal Medicine, University of Tübingen, Tübingen, 26Department of Internal Medicine, University of Heidelberg, Heidelberg, 27Johanniter Hospital in Fläming gGmbH, Center of Rheumatology of Brandenburg, Germany, 28Department of Dermatology and Venerology, University of Graz, Graz, Austria, 29Department of Internal Medicine, Center of Rheumatology, Baden-Baden and 30Department of Dermatology, Clinic of Minden, Minden, Germany.
26,
K Gräfenstein
K Gräfenstein
1Department of Dermatology and Venerology, University of Cologne, Cologne, 2Department of Rheumatology, University of Aachen, Aachen, 3Institute of Biostatistics, Informatics and Epidemiology, University of Cologne, Cologne, 4Clinical Research Unit for Rheumatology, University Medical Center Freiburg, Freiburg ,5Department of Dermatology, Dresden University Hospital, Dresden, 6Department of Rheumatology and Clinical Immunology, Kerckhoff Clinic, Bad Nauheim, 7Rheumatology and Clinical Immunology, Charité, Berlin, 8Hospital Cologne-Merheim, Medical Clinic I, 9Department of Dermatology, University of Münster, Münster, 10Department of Dermatology, Venerology and Allergology, University Hospital Charité, Berlin, 11Department of Rheumatology, University of Cologne, Cologne, 12Department of Dermatology, University of Mainz, Mainz, 13Department of Dermatology and Allergology, University of Munich, Munich, 14Department of Dermatology and Allergology, University of Ulm, Ulm, 15Department of Dermatology, 16Department of Internal Medicine, University of Regensburg, Regensburg, 17Department of Internal Medicine, University of Regensburg, Regensburg, 18Department of Dermatology, Allergology and Environmental Medicine, Private University Witten-Herdecke, HELIOS Klinikum Wuppertal, Wuppertal, 19Hospital Eilbek, Hamburg, 20Department of Internal Medicine, University of Giessen, Giessen, 21Clinic for Rheumatology, Bad Bramstedt, 22Department of Dermatology, University of Düsseldorf, Düsseldorf, 23Department of Dermatology and Venerology,Georg-August-University of Göttingen, Göttingen, 24Department of Dermatology, University of Tübingen, Tübingen, 25Department of Internal Medicine, University of Tübingen, Tübingen, 26Department of Internal Medicine, University of Heidelberg, Heidelberg, 27Johanniter Hospital in Fläming gGmbH, Center of Rheumatology of Brandenburg, Germany, 28Department of Dermatology and Venerology, University of Graz, Graz, Austria, 29Department of Internal Medicine, Center of Rheumatology, Baden-Baden and 30Department of Dermatology, Clinic of Minden, Minden, Germany.
27,
A Juche
A Juche
1Department of Dermatology and Venerology, University of Cologne, Cologne, 2Department of Rheumatology, University of Aachen, Aachen, 3Institute of Biostatistics, Informatics and Epidemiology, University of Cologne, Cologne, 4Clinical Research Unit for Rheumatology, University Medical Center Freiburg, Freiburg ,5Department of Dermatology, Dresden University Hospital, Dresden, 6Department of Rheumatology and Clinical Immunology, Kerckhoff Clinic, Bad Nauheim, 7Rheumatology and Clinical Immunology, Charité, Berlin, 8Hospital Cologne-Merheim, Medical Clinic I, 9Department of Dermatology, University of Münster, Münster, 10Department of Dermatology, Venerology and Allergology, University Hospital Charité, Berlin, 11Department of Rheumatology, University of Cologne, Cologne, 12Department of Dermatology, University of Mainz, Mainz, 13Department of Dermatology and Allergology, University of Munich, Munich, 14Department of Dermatology and Allergology, University of Ulm, Ulm, 15Department of Dermatology, 16Department of Internal Medicine, University of Regensburg, Regensburg, 17Department of Internal Medicine, University of Regensburg, Regensburg, 18Department of Dermatology, Allergology and Environmental Medicine, Private University Witten-Herdecke, HELIOS Klinikum Wuppertal, Wuppertal, 19Hospital Eilbek, Hamburg, 20Department of Internal Medicine, University of Giessen, Giessen, 21Clinic for Rheumatology, Bad Bramstedt, 22Department of Dermatology, University of Düsseldorf, Düsseldorf, 23Department of Dermatology and Venerology,Georg-August-University of Göttingen, Göttingen, 24Department of Dermatology, University of Tübingen, Tübingen, 25Department of Internal Medicine, University of Tübingen, Tübingen, 26Department of Internal Medicine, University of Heidelberg, Heidelberg, 27Johanniter Hospital in Fläming gGmbH, Center of Rheumatology of Brandenburg, Germany, 28Department of Dermatology and Venerology, University of Graz, Graz, Austria, 29Department of Internal Medicine, Center of Rheumatology, Baden-Baden and 30Department of Dermatology, Clinic of Minden, Minden, Germany.
27,
E Aberer
E Aberer
1Department of Dermatology and Venerology, University of Cologne, Cologne, 2Department of Rheumatology, University of Aachen, Aachen, 3Institute of Biostatistics, Informatics and Epidemiology, University of Cologne, Cologne, 4Clinical Research Unit for Rheumatology, University Medical Center Freiburg, Freiburg ,5Department of Dermatology, Dresden University Hospital, Dresden, 6Department of Rheumatology and Clinical Immunology, Kerckhoff Clinic, Bad Nauheim, 7Rheumatology and Clinical Immunology, Charité, Berlin, 8Hospital Cologne-Merheim, Medical Clinic I, 9Department of Dermatology, University of Münster, Münster, 10Department of Dermatology, Venerology and Allergology, University Hospital Charité, Berlin, 11Department of Rheumatology, University of Cologne, Cologne, 12Department of Dermatology, University of Mainz, Mainz, 13Department of Dermatology and Allergology, University of Munich, Munich, 14Department of Dermatology and Allergology, University of Ulm, Ulm, 15Department of Dermatology, 16Department of Internal Medicine, University of Regensburg, Regensburg, 17Department of Internal Medicine, University of Regensburg, Regensburg, 18Department of Dermatology, Allergology and Environmental Medicine, Private University Witten-Herdecke, HELIOS Klinikum Wuppertal, Wuppertal, 19Hospital Eilbek, Hamburg, 20Department of Internal Medicine, University of Giessen, Giessen, 21Clinic for Rheumatology, Bad Bramstedt, 22Department of Dermatology, University of Düsseldorf, Düsseldorf, 23Department of Dermatology and Venerology,Georg-August-University of Göttingen, Göttingen, 24Department of Dermatology, University of Tübingen, Tübingen, 25Department of Internal Medicine, University of Tübingen, Tübingen, 26Department of Internal Medicine, University of Heidelberg, Heidelberg, 27Johanniter Hospital in Fläming gGmbH, Center of Rheumatology of Brandenburg, Germany, 28Department of Dermatology and Venerology, University of Graz, Graz, Austria, 29Department of Internal Medicine, Center of Rheumatology, Baden-Baden and 30Department of Dermatology, Clinic of Minden, Minden, Germany.
28,
G Bali
G Bali
1Department of Dermatology and Venerology, University of Cologne, Cologne, 2Department of Rheumatology, University of Aachen, Aachen, 3Institute of Biostatistics, Informatics and Epidemiology, University of Cologne, Cologne, 4Clinical Research Unit for Rheumatology, University Medical Center Freiburg, Freiburg ,5Department of Dermatology, Dresden University Hospital, Dresden, 6Department of Rheumatology and Clinical Immunology, Kerckhoff Clinic, Bad Nauheim, 7Rheumatology and Clinical Immunology, Charité, Berlin, 8Hospital Cologne-Merheim, Medical Clinic I, 9Department of Dermatology, University of Münster, Münster, 10Department of Dermatology, Venerology and Allergology, University Hospital Charité, Berlin, 11Department of Rheumatology, University of Cologne, Cologne, 12Department of Dermatology, University of Mainz, Mainz, 13Department of Dermatology and Allergology, University of Munich, Munich, 14Department of Dermatology and Allergology, University of Ulm, Ulm, 15Department of Dermatology, 16Department of Internal Medicine, University of Regensburg, Regensburg, 17Department of Internal Medicine, University of Regensburg, Regensburg, 18Department of Dermatology, Allergology and Environmental Medicine, Private University Witten-Herdecke, HELIOS Klinikum Wuppertal, Wuppertal, 19Hospital Eilbek, Hamburg, 20Department of Internal Medicine, University of Giessen, Giessen, 21Clinic for Rheumatology, Bad Bramstedt, 22Department of Dermatology, University of Düsseldorf, Düsseldorf, 23Department of Dermatology and Venerology,Georg-August-University of Göttingen, Göttingen, 24Department of Dermatology, University of Tübingen, Tübingen, 25Department of Internal Medicine, University of Tübingen, Tübingen, 26Department of Internal Medicine, University of Heidelberg, Heidelberg, 27Johanniter Hospital in Fläming gGmbH, Center of Rheumatology of Brandenburg, Germany, 28Department of Dermatology and Venerology, University of Graz, Graz, Austria, 29Department of Internal Medicine, Center of Rheumatology, Baden-Baden and 30Department of Dermatology, Clinic of Minden, Minden, Germany.
28,
C Fiehn
C Fiehn
1Department of Dermatology and Venerology, University of Cologne, Cologne, 2Department of Rheumatology, University of Aachen, Aachen, 3Institute of Biostatistics, Informatics and Epidemiology, University of Cologne, Cologne, 4Clinical Research Unit for Rheumatology, University Medical Center Freiburg, Freiburg ,5Department of Dermatology, Dresden University Hospital, Dresden, 6Department of Rheumatology and Clinical Immunology, Kerckhoff Clinic, Bad Nauheim, 7Rheumatology and Clinical Immunology, Charité, Berlin, 8Hospital Cologne-Merheim, Medical Clinic I, 9Department of Dermatology, University of Münster, Münster, 10Department of Dermatology, Venerology and Allergology, University Hospital Charité, Berlin, 11Department of Rheumatology, University of Cologne, Cologne, 12Department of Dermatology, University of Mainz, Mainz, 13Department of Dermatology and Allergology, University of Munich, Munich, 14Department of Dermatology and Allergology, University of Ulm, Ulm, 15Department of Dermatology, 16Department of Internal Medicine, University of Regensburg, Regensburg, 17Department of Internal Medicine, University of Regensburg, Regensburg, 18Department of Dermatology, Allergology and Environmental Medicine, Private University Witten-Herdecke, HELIOS Klinikum Wuppertal, Wuppertal, 19Hospital Eilbek, Hamburg, 20Department of Internal Medicine, University of Giessen, Giessen, 21Clinic for Rheumatology, Bad Bramstedt, 22Department of Dermatology, University of Düsseldorf, Düsseldorf, 23Department of Dermatology and Venerology,Georg-August-University of Göttingen, Göttingen, 24Department of Dermatology, University of Tübingen, Tübingen, 25Department of Internal Medicine, University of Tübingen, Tübingen, 26Department of Internal Medicine, University of Heidelberg, Heidelberg, 27Johanniter Hospital in Fläming gGmbH, Center of Rheumatology of Brandenburg, Germany, 28Department of Dermatology and Venerology, University of Graz, Graz, Austria, 29Department of Internal Medicine, Center of Rheumatology, Baden-Baden and 30Department of Dermatology, Clinic of Minden, Minden, Germany.
29,
R Stadler
R Stadler
1Department of Dermatology and Venerology, University of Cologne, Cologne, 2Department of Rheumatology, University of Aachen, Aachen, 3Institute of Biostatistics, Informatics and Epidemiology, University of Cologne, Cologne, 4Clinical Research Unit for Rheumatology, University Medical Center Freiburg, Freiburg ,5Department of Dermatology, Dresden University Hospital, Dresden, 6Department of Rheumatology and Clinical Immunology, Kerckhoff Clinic, Bad Nauheim, 7Rheumatology and Clinical Immunology, Charité, Berlin, 8Hospital Cologne-Merheim, Medical Clinic I, 9Department of Dermatology, University of Münster, Münster, 10Department of Dermatology, Venerology and Allergology, University Hospital Charité, Berlin, 11Department of Rheumatology, University of Cologne, Cologne, 12Department of Dermatology, University of Mainz, Mainz, 13Department of Dermatology and Allergology, University of Munich, Munich, 14Department of Dermatology and Allergology, University of Ulm, Ulm, 15Department of Dermatology, 16Department of Internal Medicine, University of Regensburg, Regensburg, 17Department of Internal Medicine, University of Regensburg, Regensburg, 18Department of Dermatology, Allergology and Environmental Medicine, Private University Witten-Herdecke, HELIOS Klinikum Wuppertal, Wuppertal, 19Hospital Eilbek, Hamburg, 20Department of Internal Medicine, University of Giessen, Giessen, 21Clinic for Rheumatology, Bad Bramstedt, 22Department of Dermatology, University of Düsseldorf, Düsseldorf, 23Department of Dermatology and Venerology,Georg-August-University of Göttingen, Göttingen, 24Department of Dermatology, University of Tübingen, Tübingen, 25Department of Internal Medicine, University of Tübingen, Tübingen, 26Department of Internal Medicine, University of Heidelberg, Heidelberg, 27Johanniter Hospital in Fläming gGmbH, Center of Rheumatology of Brandenburg, Germany, 28Department of Dermatology and Venerology, University of Graz, Graz, Austria, 29Department of Internal Medicine, Center of Rheumatology, Baden-Baden and 30Department of Dermatology, Clinic of Minden, Minden, Germany.
30,
V Bartels
V Bartels
1Department of Dermatology and Venerology, University of Cologne, Cologne, 2Department of Rheumatology, University of Aachen, Aachen, 3Institute of Biostatistics, Informatics and Epidemiology, University of Cologne, Cologne, 4Clinical Research Unit for Rheumatology, University Medical Center Freiburg, Freiburg ,5Department of Dermatology, Dresden University Hospital, Dresden, 6Department of Rheumatology and Clinical Immunology, Kerckhoff Clinic, Bad Nauheim, 7Rheumatology and Clinical Immunology, Charité, Berlin, 8Hospital Cologne-Merheim, Medical Clinic I, 9Department of Dermatology, University of Münster, Münster, 10Department of Dermatology, Venerology and Allergology, University Hospital Charité, Berlin, 11Department of Rheumatology, University of Cologne, Cologne, 12Department of Dermatology, University of Mainz, Mainz, 13Department of Dermatology and Allergology, University of Munich, Munich, 14Department of Dermatology and Allergology, University of Ulm, Ulm, 15Department of Dermatology, 16Department of Internal Medicine, University of Regensburg, Regensburg, 17Department of Internal Medicine, University of Regensburg, Regensburg, 18Department of Dermatology, Allergology and Environmental Medicine, Private University Witten-Herdecke, HELIOS Klinikum Wuppertal, Wuppertal, 19Hospital Eilbek, Hamburg, 20Department of Internal Medicine, University of Giessen, Giessen, 21Clinic for Rheumatology, Bad Bramstedt, 22Department of Dermatology, University of Düsseldorf, Düsseldorf, 23Department of Dermatology and Venerology,Georg-August-University of Göttingen, Göttingen, 24Department of Dermatology, University of Tübingen, Tübingen, 25Department of Internal Medicine, University of Tübingen, Tübingen, 26Department of Internal Medicine, University of Heidelberg, Heidelberg, 27Johanniter Hospital in Fläming gGmbH, Center of Rheumatology of Brandenburg, Germany, 28Department of Dermatology and Venerology, University of Graz, Graz, Austria, 29Department of Internal Medicine, Center of Rheumatology, Baden-Baden and 30Department of Dermatology, Clinic of Minden, Minden, Germany.
30