Abstract
Objective. IA therapy with hyaluronan (HA) is reported to provide symptomatic relief and disease modification in OA. This study assessed the pathological changes in the synovium of an ovine model of OA and evaluated the effects of two HA preparations on this pathology.
Methods. Eighteen sheep had bilateral lateral meniscectomy to induce OA. Four months post-surgery animals received IA saline or HA (Hyalgan®) weekly for 5 weeks or three injections of an amide derivative of HA (HYADD®4-G) every 2 weeks (n = 6 per group). Six months after meniscectomy, sheep were killed, knee joint synovium processed, scored for pathological change and compared with synovium from non-operated animals. Sections of synovium from normal and treated joints were also immunostained for TNF-α, HSP-47, TGF-β, CD44, connective tissue growth factor (CTGF) or iNOS. HA synthesis by synovial fibroblasts isolated from each OA joint was quantified.
Results. Aggregate scores of pathological change were higher in OA joint synovia compared with controls, with individual measures of subintimal fibrosis and vascularity predominantly affected. Depth of intimal fibrosis was also significantly higher in meniscectomized joints. IA treatment with Hyalgan® decreased aggregate score, vascularity and depth of fibrosis. HYADD®4-G treatment decreased vascularity, intimal hyperplasia and increased high-molecular weight HA synthesis by synovial fibroblasts. CD44, CTGF or iNOS expression was increased in the synovial lining of OA joints compared with normal, but there was no significant modulation of this increase by either HA preparation.
Conclusion. Increased fibrosis and vascularity are hallmarks of pathological change in synovium in this meniscectomy model of OA. Both the IA HA and an amide derivative of HA reduced aspects of this pathology thus providing a potential mechanism for improving joint mobility and function in OA.
Keywords: Osteoarthritis, Animal model, Synovium, Hyaluronan
Introduction
OA is a multifactorial joint disorder in which ageing, genetic, hormonal and mechanical factors are all major contributors to its onset and progression. All of the tissues that constitute the synovial joint—cartilage, bone ligaments, menisci, joint capsule and synovial lining—undergo change in OA and contribute to the degenerative process. While the initial site of injury in OA is still the subject of debate, degradation of articular cartilage (AC) is the defining pathological event. The release of fragmented matrix components from cartilage into SF initiates an inflammatory response in the synovium and underlying joint capsule [1, 2]. This synovitis promotes the production of pro-inflammatory mediators by the resident lining cells, particularly the tissue macrophages. These cells are an abundant source of cytokines, prostanoids, pro-coagulant factors, proteinases and oxygen-derived free radicals including nitric oxide (NO) [3]. These products have profound effects on the metabolism of joint connective tissues leading to progression of the AC breakdown and synovial fibrosis, as well as contributing to joint pain and disability characteristic of OA. Moreover, recent MRI studies have demonstrated a strong and unique association between synovitis, capsular thickening and the severity of knee pain [4, 5], further highlighting the important role of the synovium in the pathogenesis of OA [1]. Fibrosis of the synovium occurs in most arthropathies and may be indicative of epithelial to mesenchymal transitions in resident fibroblasts leading to increased extracellular matrix (ECM) deposition [6]. Potential key modulators in the fibrotic process are heat shock protein-47 (HSP-47), a collagen chaperone required for collagen synthesis, TGF-β and connective tissue growth factor (CTGF), both regulators of collagen metabolism [7]. On the other hand, TNF-α is implicated in prevention of ECM accumulation [8].
Hyaluronan (HA) confers to SF its unique rheological properties, which together with lubricin, provides exceptionally efficient biomechanical protection of AC and peri-articular tissues [9, 10]. In OA joints, SF HA concentration and molecular weight (MW) are decreased [11], compromising its viscoelasticity and lubricating ability. In an attempt to restore normality to the SF of OA joints, Balazs [12] introduced the concept of ‘viscosupplementation’, whereby a highly purified high-MW HA (HMW-HA) is injected into the joints of OA patients. Since the introduction of this therapeutic approach, a large number of HA preparations of varying MWs and origins have been developed and have been reported to provide symptomatic relief in the treatment of OA [13–17]. A recent Cochrane review found that the clinical effects of IA HA therapy are slower at onset, but more sustained than with IA corticosteroids [18].
Laboratory and animal model studies with HA have identified its potential as a disease-modifying OA drug (DMOAD) [19, 20]. Synovial biopsies taken from joints of OA patients treated with Hyalgan® have supported this view [21, 22]. Various preparations of HA with a range of MWs differentially stimulated synthesis of HMW HA by synovial biopsy cells in culture [23]. Effects of IA administered HA on the in vivo synthesis of synovial HA in ovine joints was also MW dependent, with HMW HA (MW >2000 kDa) preparations being less effective than HA within the MW range of 500–1000 kDa [20]. The possible mechanisms by which HA exerts these effects are still speculative, although CD44 receptors are abundant in synovium and are intimately involved in HA–ECM interactions [24]. A criticism of using HA as a viscosupplement is its relatively short half-life within the joint cavity (<24 h) [25]; therefore, modified HA preparations, such as the amide derivative HYADD®4-G, are being manufactured, which have a longer joint residency but retained SF-like viscoelastic properties [26].
To date there is little data on the effect of HA on synovial pathological changes with OA in animal models. We have used a well-established OA model induced by meniscectomy in sheep to test efficacy of many potential therapies [27], including several trials of IA HA preparations which have variously been shown to reduce cartilage histopathology scores [28, 29], improve gait [30] and increase osteophytosis [31, 32]. However, synovial pathology has not routinely been assessed in these studies.
We have developed and validated a histological scoring system for synovium from OA joints to quantitate pathological changes in this animal model [33]. In the present study, we confirm that there are significant changes to ovine synovium after meniscectomy and have investigated potential mechanisms for the resulting pathology. Furthermore, we evaluate the relative effects of IA Hyalgan® and HYADD®4-G, an amide derivate of Hyalgan®, on the histopathology, immunohistochemistry and ex vivo endogenous HA synthesis of synovium derived from stifle joints of meniscectomized sheep.
Methods
Animal experimentation
Eighteen aged (7- to 8-yrs old) Merino ewes were subjected to bilateral lateral meniscectomy as previously described [34, 35]. Two HA preparations were tested vs saline placebo, Hyalgan® (10 mg/ml) and HYADD®4-G (5 mg/ml), both manufactured and supplied by Fidia Farmaceutici S.p.A., Abano Terme, Italy. Sheep were randomly allocated prior to surgery to one of the three treatment groups (n = 6 per group): OA + saline placebo, OA + Hyalgan® and OA + HYADD®4-G. From weeks 16 to 20 post-meniscectomy, these groups respectively received bilateral IA injections of equivalent volume (2 ml) of sterile normal saline (weekly), Hyalgan® (weekly) or HYADD®4-G (every 2 weeks, at weeks 16, 18 and 20 post-surgery). IA injections were performed under short-acting deep sedation (intravenous diazepam at 0.25 mg/kg with ketamine 5 mg/kg) using a 21-gauge needle and aseptic conditions. Post-operatively, animals were transferred to irrigated pasture (1 hectare paddocks) on the Murdoch University farm, partially supplemented with lucerne chaff and lupins in order to maintain constant body condition. Sheep were monitored daily and killed at 6 months post-meniscectomy. All animal procedures were approved by the Murdoch University Animal Ethics Committee (AEC R779/00).
Tissue collection
After opening the knee joint by severing the cruciate and collateral ligaments, a sample of synovium from the suprapatellar fold was removed and placed in 10% (v/v) neutral buffered formalin. Further samples of synovia were removed from the same area and from adjacent to the femoral condyles, taking care to minimize the amount of fibrous synovium and subsynovium. These samples were processed for cell isolation as described subsequently.
Histological processing of synovium
Synovial specimens were fixed in 10% (v/v) neutral buffered formalin for 24 h. Samples were then transferred to 70% (v/v) ethanol and routinely processed through ascending grades of ethanol [70–100% (v/v)], cleared in chloroform, then infiltrated and embedded in paraffin wax. Sections (4 µm) were cut on a rotary microtome and stained with haematoxylin and eosin (H&E).
Histopathology of synovium
H&E stained sections of synovium from both joints of all sheep, as well as freshly-stained synovium sections from a previous group of age-matched non-operated control (NOC) sheep, were coded and scored blind by two observers. The scores ranged from 0 to 3 on the tissue criteria outlined in Table 1. All sections were assessed by both observers to avoid bias from interobserver variability, which has been assessed for this scoring protocol and found to be acceptable [33].
Table 1.
Non-parametric scoring of ovine synovial histopathology
| Criteria | Score | Observation |
|---|---|---|
| Intimal hyperplasia | 0 | 1–2 layers only |
| 1 | 3–4 layers, focal | |
| 2 | ≥5 layers, focal | |
| 3 | ≥5 layers, diffuse | |
| Lymphocytic/plasmocytic infiltration | 0 | None |
| 1 | One focus of infiltration | |
| 2 | 2–5 foci of infiltration | |
| 3 | Diffuse infiltration or >5 foci | |
| Subintimal fibrosis | 0 | None |
| (loose connective tissue areas only) | 1 | Light focal collagenous staining up to 30% |
| 2 | Heavy focal staining or total light diffuse collagenous staining | |
| 3 | Heavy diffuse collagenous staining | |
| Vascularity | 0 | 0–2 vascular elements per 100× field |
| 1 | 3–4 vascular elements per 100× field | |
| 2 | 5–8 vascular elements per 100× field | |
| 3 | >8 vascular elements per 100× field | |
| Aggregate score (joint) | 0–12 | Sum of the scores obtained for the four criteria above |
| Aggregate score (sheep) | 0–24 | Sum of the scores obtained for the four criteria above for both left and right joints. |
Depth of intimal fibrosis was determined using an eyepiece graticule of 1 cm2 that, when viewed using a 40× objective, projected a 250 µm2 grid on each slide. Areas assessed were required to have a fairly straight intimal edge for at least 250 µm, to be at least 250 µm deep and not cover any normal fibrous synovial tissue. Five randomly selected areas were counted per section at each observation.
Immunohistology
Immunostaining for CD44, TGF-β, CTGF, TNF-α, inducible NO synthase (iNOS) and HSP-47 was performed on sections from one joint of each sheep as well as age-matched identically processed NOC synovial specimens. Endogenous peroxidase activity was initially blocked by incubating the tissue sections with 3% (v/v) H2O2 for 5 min.
Some sections (for TGF-β, TNF-α and iNOS immunostaining) were pre-digested with bovine testicular bovine hyaluronidase (600 U/ml) for 30 min at 37°C in phosphate buffer, pH 7.4; for CD44 immunostaining, heat retrieval was performed in 0.01 M citrate buffer, pH6.0 for 20 min at 99°C (water bath) followed by 10 min cooling at 22°C. Non-specific binding was blocked by incubating the sections in non-serum protein block (DakoCytomation, USA) for 10 min at room temperature. Incubations were performed overnight at 4°C with primary antibodies for CD44 (rat monoclonal; Serotec MCA1449; 1 : 20 dilution), CTGF (rabbit polyclonal; Abcam ab6992; 1 : 1000 dilution), HSP-47 (mouse monoclonal; Stressgen SPA-470; 1 : 130 000 dilution), iNOS (rabbit polyclonal; Cayman Chemicals 160 862; 1 : 1000 dilution), TGF-β (mouse monoclonal; Abcam ab1279; 1 : 100 dilution) and TNF-α (rabbit polyclonal; Chemican AB1842; 1 : 32 000 dilution). Negative controls consisted of omission of primary antibody and addition of species-matched IgG or serum at equivalent concentration to primary antibody. The antibodies were detected using a 30-min incubation with secondary antibody cocktail of biotinylated anti-rabbit and anti-mouse, or biotinylated anti-rat immunoglobulins, followed by a 30-min streptavidin-conjugated horseradish peroxidase incubation. Staining was completed after incubation with substrate—chromogen solution DAB (DakoCytomation, USA) for 5 min or NovaRED (Vector Laboratories, USA) for 15 min at room temperature, which give a brown or red-brown end-product, respectively. Sections were counter-stained in Mayer's haematoxylin, washed, dehydrated, cleared in xylene and mounted.
In order to quantify the effects of the different HA treatments, sections were assessed by two observers (blinded to treatment group) for intensity of immunostaining (0–3; 0 = no staining, 1 = mild, 2 = moderate and 3 = marked). Average scores for two fields per section were calculated.
Isolation of synovial fibroblasts
Non-fixed specimens of synovium were diced finely, washed twice with phosphate-buffered saline, pH 7.2 (PBS) then resuspended in 12 ml sterile 0.2% (w/v) Gibco BRL porcine trypsin/0.1% (w/v) EDTA in PBS (T&E). After incubation at 37°C for 1 h, 1 ml fetal bovine serum (FBS) was added to inactivate the trypsin. The digested tissue was then resuspended in 0.05% (w/v) clostridial collagenase (Sigma Aldrich) and 0.01% (w/v) DNase I (Sigma Aldrich) in DMEM/10% FBS and incubated at 37°C for 16 h. After washing twice with DMEM/10% FBS, the pellet of ovine synovial fibroblasts (OSFs) was resuspended in 6 ml DMEM/10% FBS. Three 2 ml aliquots of the OSF suspension per joint were placed into 3 × 10 cm2 wells of 6-well plates and incubated at 37°C. Media was changed every 2 or 3 days. When the cells were ∼70–80% confluent, they were used to determine de novo HA synthesis as described subsequently.
HA synthesis by synovial fibroblasts
3H-glucosamine (specific activity = 8.80 Ci/mmol; GE Healthcare) was added to DMEM/10% FBS at 1 µCi/ml. Two millilitres were added to each well (2 µCi per well) and exactly 24 h later, media was removed from cells, the cells rinsed with PBS and harvested for DNA determination using a modification of the Hoescht 33 258 dye-binding method described by Kim et al. [36] and Cake et al. [37].
After adjusting the pH to 6.0 with 10 µl 1 M acetic acid, 50 µl 20 mM sodium acetate/0.15 M NaCl pH 6.0 with and without 5 TRU Streptomyces hyaluronidase (SHase) was added to 500 µl aliquots of each media sample. All samples were incubated at 60°C for 3 h and then at 94°C for 5 min.
Changes in the amount of 3H-glucosamine incorporated into endogenous HA were measured as radioactivity peaks separated by Superose 6 (GE Healthcare) size exclusion chromatography. Digested media samples were centrifuged at 18 000 g for 10 min (room temperature) immediately prior to loading via a 200 µl sample loop and the pre-filtered degassed elution solvent was PBS, pH 7.2 run at 12 ml/h. The column eluent was collected for determination of radioactivity by scintillation spectometry in Ecolite® (ICN Biochemicals). CPM in hyaluronidase-digested samples was subtracted from CPM in undigested samples to obtain CPM specifically due to 3H-hyaluronan. The CPM value thus obtained for each well was corrected for number of cells present, as determine by the cellular DNA content. Total HA synthesised by the cells was determined from all Superose 6 fractions before the total column volume in SHase-digested fractions whereas HMW-HA was determined from CPM that voided the column.
Statistical methods
Non-parametric data (histological and immunohistological scoring) was analysed using the Kruskal–Wallis test for multiple groups and, if significance was found, Mann–Whitney U-tests for between-group comparisons (n = 12 joints per treatment) were used.
The α-level was set at 0.05, reducing to 0.03 following Benjamini–Hochberg post-hoc procedure for multiple comparisons [38] to correct for Type 1 errors. For parametric data (intimal fibrosis and HA synthesis), unpaired Student's t-tests were performed, with P < 0.05 considered to be statistically significant.
Results
Effect of meniscectomy—histological scoring
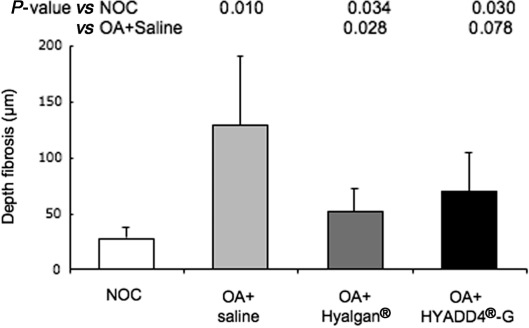
Marked differences were observed between knee joint synovia from NOC animals and saline-treated meniscectomized animals. Subintimal fibrosis (P = 0.0035), vascularity (P = 0.0035) and aggregate score of the non-parametric criteria (P = 0.0036), were all significantly higher in OA joint synovia (Table 2). The increase in intimal hyperplasia was not statistically significant (P = 0.044) while the increase in cellular infiltrate was highly variable (P = 0.13). Depth of intimal fibrosis (P = 0.010) was significantly increased in synovium from meniscectomized saline-injected joints compared with NOC (Fig. 1).
Table 2.
Histological scores for H&E sections of stifle joint synovium sampled from NOC sheep and OA sheep subjected to various IA treatments (scored as described in Table 1)
| Treatment | Intimal hyperplasia | Cellular infiltrate | Subintimal fibrosis | Vascularity | Aggregate scores |
|---|---|---|---|---|---|
| NOC | 2.83 ± 0.31 | 0.83 ± 0.40 | 0.33 ± 0.33 | 3.50 ± 0.43 | 7.50 ± 0.71 |
| OA + saline | 4.00 ± 0.37 | 3.33 ± 1.09 | 3.67 ± 0.49 | 5.83 ± 0.17 | 16.8 ± 1.3 |
| P (vs NOC) | 0.044 | 0.13 | 0.0035 | 0.0035 | 0.0036 |
| OA + Hyalgan | 3.00 ± 0.52 | 1.67 ± 0.61 | 3.00 ± 0.45 | 4.50 ± 0.43 | 12.2 ± 0.7 |
| P (vs NOC) | 1.00 | 0.27 | 0.0049 | 0.14 | 0.0046 |
| P (vs OA + saline) | 0.14 | 0.19 | 0.32 | 0.020 | 0.015 |
| OA + HYADD4-G | 2.33 ± 0.56 | 1.83 ± 0.56 | 3.33 ± 0.80 | 4.50 ± 0.50 | 12.0 ± 1.5 |
| P (vs NOC) | 0.55 | 0.098 | 0.013 | 0.16 | 0.053 |
| P (vs OA + saline) | 0.032 | 0.25 | 0.87 | 0.023 | 0.076 |
Values are mean ± s.e.m, n = 12 joints, P-values by Mann–Whitney U-ranked tests, with P < 0.03 statistically significant after Benjamini–Hochberg post-hoc correction. There were no significant differences between Hyalgan®- and HYADD®4-G-treated groups (P > 0.5).
Fig. 1.
Depth of fibrosis of the synovial intima (mean ± s.d., n = 12 joints per group). There were no significant differences between IA treatments (P > 0.28) (unpaired Student's t-test).
Effect of meniscectomy—immunohistology
In the synovium of NOC joints, intimal cells and occasional fibroblastic subsynovial cells were positive for HSP-47. In contrast, TGF-β and TNF-α were localized to blood vessels as well as occasional intimal cells. Immunostaining for HSP-47 was unaltered in any meniscectomized joints, and although a slight increase in intimal cell staining for TGF-β and TNF-α was observed in some OA joints, this was highly variable and was not significant when sections were blindly scored (data not shown).
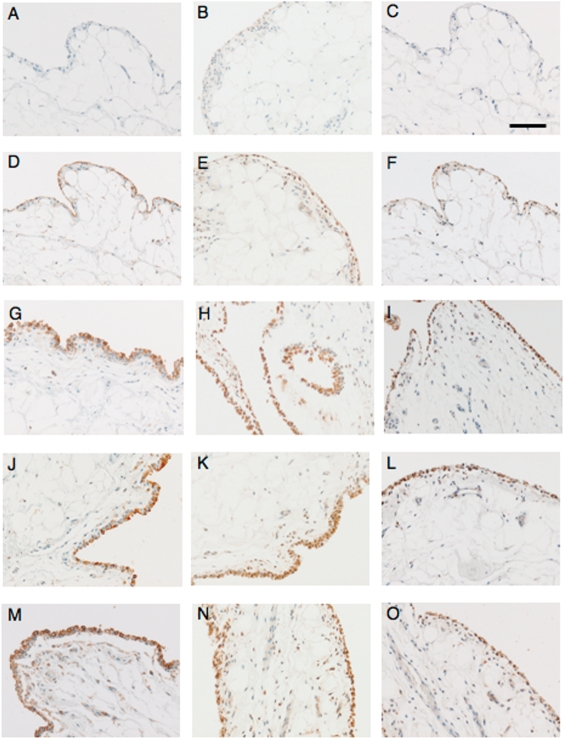
In NOC sheep, the antibody for CD44 stained the superficial synovial intima only, with staining deeper into the tissue rarely seen (Fig. 2D). Occasional lining cells had cell membrane staining but, as most lining cells are very elongated and fibroblastic, this was not always clear. In OA joints, the immunostaining was cell membrane-associated in all cases and appeared to be strongest on the cell membranes adjacent to the synovial space, with little staining in the subsynovial tissue. Synovial lining cells and some cells in the subsynovium were positive for CTGF in NOC joints (Fig. 2E). Staining appeared to be mainly cytoplasmic and varied between cells from no staining to very heavy staining within the same section of tissue. In OA joints, the tissue distribution of CTGF was similar to NOC but more intense staining was observed, and in addition some matrix staining was evident that was not present in the negative control sections. In synovia from NOC sheep, the antibody for iNOS stained only a very few lining cells in some specimens (not all) with no ECM or subsynovium staining in any specimen (Fig. 2F). After meniscectomy, many lining cells exhibit heavy staining, with some positive cells also appearing in the subsynovial tissue. There was more intense antibody staining for CD44, CTGF and iNOS on the synovial lining of meniscectomized joints when compared with NOC (all P = 0.004).
Fig. 2.
Representative images of immunohistology of ovine synovium from NOC (A–F), saline-treated (G–I), Hyalgan®-treated (J–L) or HYADD®4-G treated (M–O) joints stained with negative control antibodies (A–C) or antibodies to CD44 (D, G, J, M); CTGF (E, H, K, N) or iNOS (F, I, L, O) as outlined in Methods section. Bar represents 100 µm for all images, which were captured at ×200 magnification.
Effect of IA HA treatment
IA treatment with either Hyalgan® (P = 0.015, significant) or HYADD®4-G (P = 0.076, not significant) decreased the aggregate scores to the same extent compared with saline treatment (Table 2). Compared with saline treatment, vascularity was significantly decreased by both Hyalgan® (P = 0.020) or HYADD®4-G (P = 0.023) while intimal hyperplasia was only reduced by HYADD®4-G (P = 0.032, not significant). Subintimal fibrosis score was not decreased by either HA preparation (Table 2). In contrast, intimal fibrosis depth was decreased significantly by Hyalgan® (P = 0.028) and not by HYADD®4-G (P = 0.078) compared with saline (Fig. 1). There were no differences between IA treatments with either Hyalgan® or HYADD®4-G in any of the synovial pathological changes (P > 0.5 for all variables measured). Neither of the HA treatments modulated intensity or distribution of immunostaining for any of the molecules examined (Fig. 2J–O).
HA synthesis by synovial fibroblasts
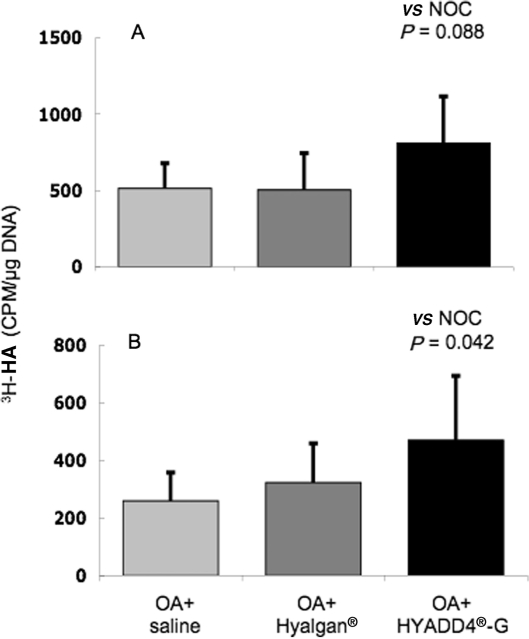
Preliminary experiments using OSFs established that cells from this species, unlike the corresponding human and lapine synovial cells, do not retain their morphology and phenotypic expression for many passages in culture (data not shown). For this reason, the HA synthesis by the isolated synovial fibroblasts from each sheep joint was determined using primary (previously uncultured) cells. There were no differences in total or HMW-HA synthesis by the synovial cells from Hyalgan®-treated joints compared with saline treatment (Fig. 3). In contrast, IA treatment with HYADD®4-G showed a trend towards increased quantity of total HA (P = 0.088, not significant) and a significantly increased HMW-HA (P = 0.042) synthesis by the ovine synovial cells when compared with cells from the joints of saline-treated sheep.
Fig. 3.
(A) Total and (B) HMW 3H-labelled HA synthesized and released by synovial fibroblasts isolated from knee joints of OA sheep subjected to different IA treatments. Values are mean ± s.d. (n = 6 sheep, two joints per sheep, three replicate cultures per joint) of CPM per microgram of cellular DNA.
Discussion
We have shown that there are significant pathological changes in the synovium of sheep 6 months following meniscectomy. The histological scoring system used was an extension of that previously described [33, 39] and specifically designed to detect differences in this ovine model. The changes induced by meniscectomy include increased intimal cells, intimal and subintimal fibrosis and vascularity when compared with synovium from unoperated sheep joints, which mimics the synovial pathological changes reported in human OA synovium [11]. In contrast, there was no consistent increase in plasma/inflammatory cell infiltration, which, together with the lack of increased staining for TNF-α, indicates minimal synovial inflammation at this 6-month time point in this model. It is plausible that meniscectomy may induce an early synovial inflammation that is mostly resolved by 6 months, leaving thickened intima, subsynovial fibrosis and increased vascularity as the predominant pathological changes. This agrees with the minimal synovial changes seen in OA compared with RA in humans [40]. In order to investigate potential mechanisms for the pathological changes observed we undertook immunolocalization for a variety of factors known to be involved in these processes.
Immunohistology revealed increases in CTGF, CD44 and iNOS 6 months after meniscectomy. CTGF has been implicated as a major downstream regulator in TGF-β-dependent fibrosis, particularly of liver, lungs, heart and skin (scarring) [41] and other fibroproliferative diseases. When transfected into the synovial lining of mouse knee joints, CTGF induced a transient synovial fibrosis with ECM accumulation [42], and TGF-β has been found at sites of fibrosis in patients with OA [43]. TGF-β may be expressed early to initiate matrix accumulation and induce CTGF, which is then persistently expressed as suggested in a mouse model of fibrosis [44], which may explain the lack of change in TGF-β detected at 6 months in our model. HSP-47 is a collagen chaperone that increases during fibrosis in other tissues [45], but its lack of regulation in the current study suggests that the export of collagen from the cell is not a rate-limiting step in synovial fibrosis.
CD44, a multivariate transmembrane glycoprotein known to be the principle cell surface receptor for HA [46], is present in the synovium [47] and SF [40] of OA patients and levels correlate with the degree of inflammation but not Kellgren grade [40]. Limited evidence of inflammation was noted in our sheep model despite synovial intima staining more strongly for CD44 than normal joints. The orientation of the CD44 staining to the synovial space we report in this study has previously been seen in rat temporomandibular joint synovium, where it co-localized with fibroblastic synoviocytes (Type B) and not macrophage-like (Type A) cells [48].
The increase in iNOS may be implicated in OA pathology by increasing NO, that induces inflammation in synovium [49], apoptosis of synoviocytes [50] and degradation of AC [51]. iNOS is expressed by many mammalian cells in response to inflammatory stimuli or mediators, such as IL-1 or TNF [52]. Increased TNF-α from synovium and SFs from OA patients [53] may induce increased vascularity through its effects on VEGF [54]. However, despite a mild increase in immunostaining in some joints, there was no significant difference in staining score for TNF-α in the ovine synovium (data not shown).
HA treatment is beneficial in OA patients with decreased pain and increased range of motion [18]. There are a number of potential mechanisms whereby HA may have these clinical benefits. There is evidence that HA functions as a DMOAD, potentially slowing cartilage degradation [20]. Despite previous studies where HA treatment reduced tibial cartilage lesion size [39] and histopathology scores [28, 55], there was no evidence of modulation of AC pathology after either HA treatment in the present study (data not shown). The lack of chondroprotection in the present study may be associated with the advanced age of the sheep used (7–8 yrs compared with 2–4 yrs in previous studies) and the timing of the HA administration (16 weeks after induction of OA). Decreased total and HMW-HA in SF is a consistent finding in OA [11]. IA HA therapy both directly supplements the endogenous HA concentration and may stimulate the fibroblasts of the synovium to produce more HA of higher quality [20, 23]. In the present study, HYADD4-G stimulated the synthesis of HMW-HA ex vivo and only this HA preparation decreased intimal hyperplasia, consistent with the inhibitory effect of HMW-HA on synoviocyte proliferation in vitro [56]. Previously, we showed that acute application of non-derivatized HA in vitro increased endogenous HA synthesis by synovial fibroblasts, which was dependent on the MW of the HA preparation. It is interesting that this effect was not observed in the present study 5 weeks after HA injection of non-derivatized HA, possibly due to the presence of HA turnover/clearance mechanisms that would not be present in vitro. The enhanced ability of HYADD4-G to remain in the joint cavity and hence in contact with the synovium could account for its more potent effects on the MW of endogenous HA production by synovial fibroblasts isolated 5 weeks after the last injection.
HA preparations that are of a sufficient HMW have analgesic properties when injected into both animal and human joints (reviewed in [57]). This has been confirmed both clinically and in laboratory experiments measuring neural discharges in nerves of cat [58] and rat [59] joints. The present results of reduced vascularization of OA synovium by both HA preparations are consistent with the well-recognised anti-angiogenic properties of HMW-HA [60] and could contribute to disease modification and analgesia.
Increased synovial fibrosis was experimentally measured in the synovium of patients who reported pain after cruciate ligament reconstructive surgery [61] and was uniquely correlated with knee pain in those OA patients with knee symptoms [4]. Neither of the HA preparation decreased the subintimal fibrosis score that assessed overall fibrosis in the section. However, Hyalgan® decreased intimal fibrosis depth, and this could contribute to the reduction in OA pain after HA IA treatment in patients. The reduction in fibrosis seen with the IA HA injections was not reflected in reduced CTGF levels, suggesting alternate mechanisms for the anti-fibrotic effect. CTGF has been shown to up-regulate tissue inhibitor of metalloproteinase-1 (TIMP-1) in mouse synovium [42], potentially preventing collagen catabolism by MMPs and facilitating matrix accumulation [62]. HMW-HA decreased TIMP-1 expression in isolated human synovial fibroblasts [63], and hence may allow increased MMP activity, normalizing collagen turnover and thus preventing the fibrosis. Whether the injected HA in the present joints may act to reduce TIMP-1 levels, allowing uninhibited MMP activity to counteract the effects of CTGF and thus decrease fibrosis requires further investigation.
Animal studies using radioactively labelled HA have shown that it is rapidly cleared from the synovial cavity, largely via the lymphatics [64]. In an attempt to prolong the half-life of HA in the joint, cross-linked or otherwise modified HA preparations with markedly increased MW and resistance to degradation have been examined. This research has produced alternative treatments for OA, which stay longer in the joint, and HA preparations with potentially different pharmacological properties [65]. HYADD®4-G is a novel amide derivative of 500–730 kDa HA (Hyalgan®) where aliphatic amine (hexadecylamine) is bound to HA at the carboxylic group of the glucuronic acid (2% substitution). HYADD®4-G has been observed to have superior rheological properties to Hyalgan® and to human SF [26]. Overall, both HA preparations in the current study have a beneficial effect on synovial pathological changes; however, the potential longer retention of HYADD®4-G, which also induced increased HMW-HA and decreased intimal hyperplasia with fewer injections, may suggest potential advantages of this preparation.
There have been a number of reviews of the benefits of IA HA treatment in OA. One of the most recent systematic reviews of five HA trial meta-analyses (covering 11–37 studies each) concludes that this therapy results in modest improvement in validated outcomes [17], with four of the five meta-analyses rating HA as beneficial and safe. Contention arises, however, when attempts are made to explain how HA modulates the disease process, or which if any, of the various HA preparations are superior. It is clear from the present study that the actions of IA HA in OA synovium are multi-factorial. In the present animal model, IA HA (both Hyalgan® or HYADD®4-G) reduced the overall pathology of synovia from meniscectomized joints. Neither of the HA preparation modified immunostaining of the six potential effector molecules examined. Further studies are necessary to elucidate the processes by which the different HA treatments are generating a positive effect in OA synovium.

Acknowledgements
The authors would like to thank Dianne Pethick for animal care and maintenance, Dan Burkhardt for help with animal tissue processing, Sue Smith for histology and immunohistology technical assistance and Fidia Farmaceutici S.p.A for funding.
Funding: This study was funded by Fidia S.p.A., Abano Terme, Italy.
Disclosure statement: M.M.S. was the recipient of research funds and a travel grant from Fidia Farmaceutici. M.A.C. received a travel grant from Fidia. P.G. received a travel grant from Fidia. A.S. is employed by Fidia S.p.A. C.B.L. was the recipient of a research grant from Fidia Farmaceutici. R.A.R. has declared no conflicts of interest.
References
- 1.Pelletier JP, Martel-Pelletier J, Abramson SB. Osteoarthritis, an inflammatory disease. Potential implication for the selection of new therapeutic targets. Arthritis Rheum. 2001;44:1237–47. doi: 10.1002/1529-0131(200106)44:6<1237::AID-ART214>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 2.Yuan GH, Masuko-Hongo K, Kato T, Nishioka K. Immunologic intervention in the pathogenesis of osteoarthritis. Arthritis Rheum. 2003;48:602–11. doi: 10.1002/art.10768. [DOI] [PubMed] [Google Scholar]
- 3.Ghosh P, Smith M. The role of cartilage-derived antigens, pro-coagulant activity and fibrinolysis in the pathogenesis of osteoarthritis. Med Hypotheses. 1993;41:190–4. doi: 10.1016/0306-9877(93)90068-2. [DOI] [PubMed] [Google Scholar]
- 4.Hill CL, Gale DG, Chaisson CE, et al. Knee effusions, popliteal cysts, and synovial thickening: association with knee pain in osteoarthritis. J Rheumatol. 2001;28:1330–7. [PubMed] [Google Scholar]
- 5.Hill CL, Hunter DJ, Niu J, et al. Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann Rheum Dis. 2007;66:1599–603. doi: 10.1136/ard.2006.067470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steenvoorden MM, Tolboom TC, van der Pluijm G, et al. Transition of healthy to diseased synovial tissue in rheumatoid arthritis is associated with gain of mesenchymal/fibrotic characteristics. Arthritis Res Ther. 2006;8:R165–74. doi: 10.1186/ar2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J. 2004;18:816–27. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 8.Hatamochi A, Mori K, Ueki H. Role of cytokines in controlling connective tissue gene expression. Arch Dermatol Res. 1994;287:115–21. doi: 10.1007/BF00370729. [DOI] [PubMed] [Google Scholar]
- 9.Jay GD, Torres JR, Warman ML, Laderer MC, Breuer KS. The role of lubricin in the mechanical behavior of synovial fluid. Proc Natl Acad Sci USA. 2007;104:6194–9. doi: 10.1073/pnas.0608558104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blewis ME, Schumacher BL, Klein TJ, Schmidt TA, Voegtline MS, Sah RL. Microenvironment regulation of PRG4 phenotype of chondrocytes. J Orthop Res. 2007;25:685–95. doi: 10.1002/jor.20307. [DOI] [PubMed] [Google Scholar]
- 11.Dahl IMS, Husby G. Hyaluronic acid production in vitro by synovial lining cells from normal and rheumatoid joints. Ann Rheum Dis. 1985;44:647–57. doi: 10.1136/ard.44.10.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balazs EA. Viscosupplementation for treatment of osteoarthritis: from initial discovery to current status and results. Surg Technol Int. 2004;12:278–89. [PubMed] [Google Scholar]
- 13.Van Den Bekerom MP, Mylle G, Rys B, Mulier M. Viscosupplementation in symptomatic severe hip osteoarthritis: a review of the literature and report on 60 patients. Acta Orthop Belg. 2006;72:560–8. [PubMed] [Google Scholar]
- 14.Kotz R, Kolarz G. Intra-articular hyaluronic acid: duration of effect and results of repeated treatment cycles. Am J Orthop. 1999;28(Suppl 11):5–7. [PubMed] [Google Scholar]
- 15.Maheu E, Ayral X, Dougados M. A hyaluronan preparation (500-730 kDa) in the treatment of osteoarthritis: a review of clinical trials with Hyalgan. Int J Clin Pract. 2002;56:804–13. [PubMed] [Google Scholar]
- 16.Hamburger MI, Lakhanpal S, Mooar PA, Oster D. Intra-articular hyaluronans: a review of product-specific safety profiles. Semin Arthritis Rheum. 2003;32:296–309. doi: 10.1053/sarh.2002.50008. [DOI] [PubMed] [Google Scholar]
- 17.Divine JG, Zazulak BT, Hewett TE. Viscosupplementation for knee osteoarthritis: a systematic review. Clin Orthop Relat Res. 2007;455:113–22. doi: 10.1097/BLO.0b013e31802f5421. [DOI] [PubMed] [Google Scholar]
- 18.Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006 doi: 10.1002/14651858.CD005321.pub2. CD005321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldberg VM, Buckwalter JA. Hyaluronans in the treatment of osteoarthritis of the knee: evidence for disease-modifying activity. Osteoarthr Cartilage. 2005;13:216–24. doi: 10.1016/j.joca.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 20.Ghosh P, Guidolin D. Potential mechanism of action of intra-articular hyaluronan therapy in osteoarthritis: are the effects molecular weight dependent? Semin Arthritis Rheum. 2002;32:10–37. doi: 10.1053/sarh.2002.33720. [DOI] [PubMed] [Google Scholar]
- 21.Frizziero L, Govoni E, Bacchini P. Intra-articular hyaluronic acid in the treatment of osteoarthritis of the knee: clinical and morphological study. Clin Exp Rheumatol. 1998;16:441–9. [PubMed] [Google Scholar]
- 22.Pasquali Ronchetti I, Guerra D, Taparelli F, et al. Morphological analysis of knee synovial membrane biopsies from a randomized controlled clinical study comparing the effects of sodium hyaluronate (Hyalgan®) and methylprednisolone acetate (Depomedrol®) in osteoarthritis. Rheumatology. 2001;40:158–69. doi: 10.1093/rheumatology/40.2.158. [DOI] [PubMed] [Google Scholar]
- 23.Smith MM, Ghosh P. The synthesis of hyaluronic acid by human synovial fibroblasts is influenced by the nature of the hyaluronate in the extracellular environment. Rheumatol Int. 1987;7:113–22. doi: 10.1007/BF00270463. [DOI] [PubMed] [Google Scholar]
- 24.Naor D, Nedvetzki S. CD44 in rheumatoid arthritis. Arthritis Res Ther. 2003;5:105–15. doi: 10.1186/ar746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fraser JRE, Kimpton WG, Pierscionek BK, Cahill RNP. The kinetics of hyaluronan in normal and acutely inflamed synovial joints: exploratory observations with experimental arthritis in sheep. Semin Arthritis Rheum. 1993;22(Suppl 1):9–17. doi: 10.1016/s0049-0172(10)80015-0. [DOI] [PubMed] [Google Scholar]
- 26.Borzacchiello A, Mayol L, Ambrosio I, Nicolais L, Schiavinato A. Evaluation of a novel hyaluronic acid derivative on synovial fluid viscoelastic properties. 19th European Conference on Biomaterials. European Society of Biomaterials, Italy, 2005:19. [Google Scholar]
- 27.Smith MM, Little CB. Experimental models of osteoarthritis. In: Moskowitz RW, Altman RD, Hochberg MC, editors. Osteoarthritis. Diagnosis and medical/surgical management. Philadelphia. London, New York, St. Louis, Sydney, Toronto: WB Saunders Company; 2007. pp. 107–25. [Google Scholar]
- 28.Ghosh P, Holbert C, Read R, Armstrong S. Hyaluronic acid (hyaluronan) in experimental osteoarthritis. J Rheumatol. 1995;22(Suppl 43):155–7. [PubMed] [Google Scholar]
- 29.Ghosh P, Numata Y, Smith S, Read R, Armstrong S, Johnson K. The metabolic response of articular cartilage to abnormal mechanical loading induced by medial or lateral meniscectomy. Agents Actions Suppl. 1993;39:89–93. doi: 10.1007/978-3-0348-7442-7_9. [DOI] [PubMed] [Google Scholar]
- 30.Ghosh P, Read R, Armstrong S, Wilson D, Marshall R, McNair P. The effects of intra-articular administration of hyaluronan in a model of early osteoarthritis in sheep. I. Gait analysis, radiological and morphological studies. Semin Arthritis Rheum. 1993;22(Suppl 1):18–30. doi: 10.1016/s0049-0172(10)80016-2. [DOI] [PubMed] [Google Scholar]
- 31.Ghosh P, Armstrong S, Read R, et al. Animal models of early osteoarthritis: their use for the evaluation of potential chondroprotective agents. Agents Actions Suppl. 1993;39:195–206. doi: 10.1007/978-3-0348-7442-7_22. [DOI] [PubMed] [Google Scholar]
- 32.Smith GN, Jr, Myers SL, Brandt KD, Mickler EA. Effect of intraarticular hyaluronan injection in experimental canine osteoarthritis. Arthritis Rheum. 1998;41:976–85. doi: 10.1002/1529-0131(199806)41:6<976::AID-ART4>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 33.Cake MA, Smith MM, Young AA, Ghosh P, Read RA. Synovial pathology in an ovine model of osteoarthritis: effect of intraarticular hyaluronan (Hyalgan) Clin Exp Rheumatol. 2008 (in press) [PubMed] [Google Scholar]
- 34.Cake MA, Read RA, Guillou B, Ghosh P. Modification of articular cartilage and subchondral bone pathology in an ovine meniscectomy model of osteoarthritis by avocado and soya unsaponifiables (ASU) Osteoarthr Cartilage. 2000;8:404–11. doi: 10.1053/joca.1999.0315. [DOI] [PubMed] [Google Scholar]
- 35.Little CB, Ghosh P, Bellenger CR. Topographic variation in biglycan and decorin synthesis by articular cartilage in the early stages of osteoarthritis: an experimental study in sheep. J Orthop Res. 1996;14:433–44. doi: 10.1002/jor.1100140314. [DOI] [PubMed] [Google Scholar]
- 36.Kim YJ, Sah RLY, Doong JY, Grodzinsky AJ. Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Anal Biochem. 1988;174:168–76. doi: 10.1016/0003-2697(88)90532-5. [DOI] [PubMed] [Google Scholar]
- 37.Cake MA, Appleyard RC, Read RA, Smith MM, Murrell GA, Ghosh P. Ovariectomy alters the structural and biomechanical properties of ovine femoro-tibial articular cartilage and increases cartilage iNOS. Osteoarthr Cartilage. 2005;13:1066–75. doi: 10.1016/j.joca.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 38.Reiner A, Yekutieli D, Benjamini Y. Identifying differentially expressed genes using false discovery rate controlling procedures. Bioinformatics. 2003;19:368–75. doi: 10.1093/bioinformatics/btf877. [DOI] [PubMed] [Google Scholar]
- 39.Ghosh P, Smith MM, Burkhardt D, et al. Preclinical studies on the pharmacology of hyaluronan in relation to its application as an intra-articular therapy for the treatment of osteoarthritis. In: Balazs EA, Hascall VC, editors. Hyaluronan, structure, metabolism, biological activities, therapeutic applications. NJ, USA: Matrix Biology Institute; 2005. pp. 491–502. [Google Scholar]
- 40.Fuchs S, Rolauffs B, Arndt S, Tibesku CO, Prehm P. CD44H and the isoforms CD44v5 and CD44v6 in the synovial fluid of the osteoarthritic human knee joint. Osteoarthr Cartilage. 2003;11:839–44. doi: 10.1016/j.joca.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 41.Ihn H. Pathogenesis of fibrosis: role of TGF-beta and CTGF. Curr Opin Rheumatol. 2002;14:681–5. doi: 10.1097/00002281-200211000-00009. [DOI] [PubMed] [Google Scholar]
- 42.Blaney Davidson EN, Vitters EL, Mooren FM, Oliver N, Berg WB, van der Kraan PM. Connective tissue growth factor/CCN2 overexpression in mouse synovial lining results in transient fibrosis and cartilage damage. Arthritis Rheum. 2006;54:1653–61. doi: 10.1002/art.21795. [DOI] [PubMed] [Google Scholar]
- 43.Hallbeck AL, Walz TM, Briheim K, Wasteson A. TGF-alpha and ErbB2 production in synovial joint tissue: increased expression in arthritic joints. Scand J Rheumatol. 2005;34:204–11. doi: 10.1080/03009740510017715. [DOI] [PubMed] [Google Scholar]
- 44.Chujo S, Shirasaki F, Kawara S, et al. Connective tissue growth factor causes persistent proalpha2(I) collagen gene expression induced by transforming growth factor-beta in a mouse fibrosis model. J Cell Physiol. 2005;203:447–56. doi: 10.1002/jcp.20251. [DOI] [PubMed] [Google Scholar]
- 45.Razzaque MS, Taguchi T. The possible role of colligin/HSP47, a collagen-binding protein, in the pathogenesis of human and experimental fibrotic diseases. Histol Histopathol. 1999;14:1199–212. doi: 10.14670/HH-14.1199. [DOI] [PubMed] [Google Scholar]
- 46.Aruffso A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61:1303–13. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- 47.Hale LP, Haynes BF, McCachren SS. Expression of CD44 variants in human inflammatory synovitis. J Clin Immunol. 1995;15:300–11. doi: 10.1007/BF01541320. [DOI] [PubMed] [Google Scholar]
- 48.Suzuki A, Nozawa-Inoue K, Amizuka N, Ono K, Maeda T. Localization of CD44 and hyaluronan in the synovial membrane of the rat temporomandibular joint. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:646–52. doi: 10.1002/ar.a.20331. [DOI] [PubMed] [Google Scholar]
- 49.Takahashi K, Hashimoto S, Kubo T, Hirasawa Y, Lotz M, Amiel D. Hyaluronan suppressed nitric oxide production in the meniscus and synovium of rabbit osteoarthritis model. J Orthop Res. 2001;19:500–3. doi: 10.1016/S0736-0266(00)90024-X. [DOI] [PubMed] [Google Scholar]
- 50.Jovanovic DV, Mineau F, Notoya K, Reboul P, Martel-Pelletier J, Pelletier JP. Nitric oxide induced cell death in human osteoarthritic synoviocytes is mediated by tyrosine kinase activation and hydrogen peroxide and/or superoxide formation. J Rheumatol. 2002;29:2165–75. [PubMed] [Google Scholar]
- 51.Amin AR, Abramson SB. The role of nitric oxide in articular cartilage breakdown in osteoarthritis. Curr Opin Rheumatol. 1998;10:263–8. doi: 10.1097/00002281-199805000-00018. [DOI] [PubMed] [Google Scholar]
- 52.Wahl SM, McCartney-Francis N, Chan J, Dionne R, Ta L, Orenstein JM. Nitric oxide in experimental joint inflammation. Benefit or detriment? Cells Tissues Organs. 2003;174:26–33. doi: 10.1159/000070572. [DOI] [PubMed] [Google Scholar]
- 53.Westacott CI, Sharif M. Cytokines in osteoarthritis: mediators or markers of joint destruction. Semin Arthritis Rheum. 1996;25:254–72. doi: 10.1016/s0049-0172(96)80036-9. [DOI] [PubMed] [Google Scholar]
- 54.Haywood L, McWilliams DF, Pearson CI, et al. Inflammation and angiogenesis in osteoarthritis. Arthritis Rheum. 2003;48:2173–7. doi: 10.1002/art.11094. [DOI] [PubMed] [Google Scholar]
- 55.Armstrong S, Read R, Ghosh P. The effects of intraarticular hyaluronan on cartilage and subchondral bone changes in an ovine model of early osteoarthritis. J Rheumatol. 1994;21:680–8. [PubMed] [Google Scholar]
- 56.Goldberg RL, Toole BP. Hyaluronate inhibition of cell proliferation. Arthritis Rheum. 1987;30:769–78. doi: 10.1002/art.1780300707. [DOI] [PubMed] [Google Scholar]
- 57.Balazs EA. Analgesic effect of elastoviscous hyaluronan solutions and the treatment of arthritic pain. Cells Tissues Organs. 2003;174:49–62. doi: 10.1159/000070574. [DOI] [PubMed] [Google Scholar]
- 58.Pozo MA, Balazs EA, Belmonte C. Reduction of sensory responses to passive movements of inflamed knee joints by hylan, a hyaluronan derivative. Exp Brain Res. 1997;116:3–9. doi: 10.1007/pl00005742. [DOI] [PubMed] [Google Scholar]
- 59.Pawlak M, Gomis A, Just S, Heppelmann B, Belmonte C, Schmidt RF. Mechanoprotective actions of elastoviscous hylans on articular pain receptors. In: Kennedy JF, Philips GO, editors. Hyaluronan 2000. Cambridge: Woodhead Publishing; 2002. pp. 341–51. [Google Scholar]
- 60.Slevin M, Krupinski J, Gaffney J, et al. Hyaluronan-mediated angiogenesis in vascular disease: uncovering RHAMM and CD44 receptor signaling pathways. Matrix Biol. 2007;26:58–68. doi: 10.1016/j.matbio.2006.08.261. [DOI] [PubMed] [Google Scholar]
- 61.Murakami S, Muneta T, Ezura Y, Furuya K, Yamamoto H. Quantitative analysis of synovial fibrosis in the infrapatellar fat pad before and after anterior cruciate ligament reconstruction. Am J Sports Med. 1997;25:29–34. doi: 10.1177/036354659702500106. [DOI] [PubMed] [Google Scholar]
- 62.Bonniaud P, Margetts PJ, Kolb M, et al. Adenoviral gene transfer of connective tissue growth factor in the lung induces transient fibrosis. Am J Respir Crit Care Med. 2003;168:770–8. doi: 10.1164/rccm.200210-1254OC. [DOI] [PubMed] [Google Scholar]
- 63.Wang CT, Lin YT, Chiang BL, Lin YH, Hou SM. High molecular weight hyaluronic acid down-regulates the gene expression of osteoarthritis-associated cytokines and enzymes in fibroblast-like synoviocytes from patients with early osteoarthritis. Osteoarthr Cartilage. 2006;14:1237–47. doi: 10.1016/j.joca.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 64.Sakamoto T, Mizuno S, Miyazaki K, Yamaguchi T, Toyoshima H, Namiki O. Biological fate of sodium hyaluronate (SPH) (1) studies on distribution, metabolism and excretion of 14C-SPH in rabbits after intra-articular administration. Pharmacometrics. 1984;28:375–87. [Google Scholar]
- 65.Marshall KW, Manolopoulos V, Mancer K, Staples J, Damyanovich A. Amelioration of disease severity by intraarticular hylan therapy in bilateral canine osteoarthritis. J Orthop Res. 2000;18:416–25. doi: 10.1002/jor.1100180313. [DOI] [PubMed] [Google Scholar]