Abstract
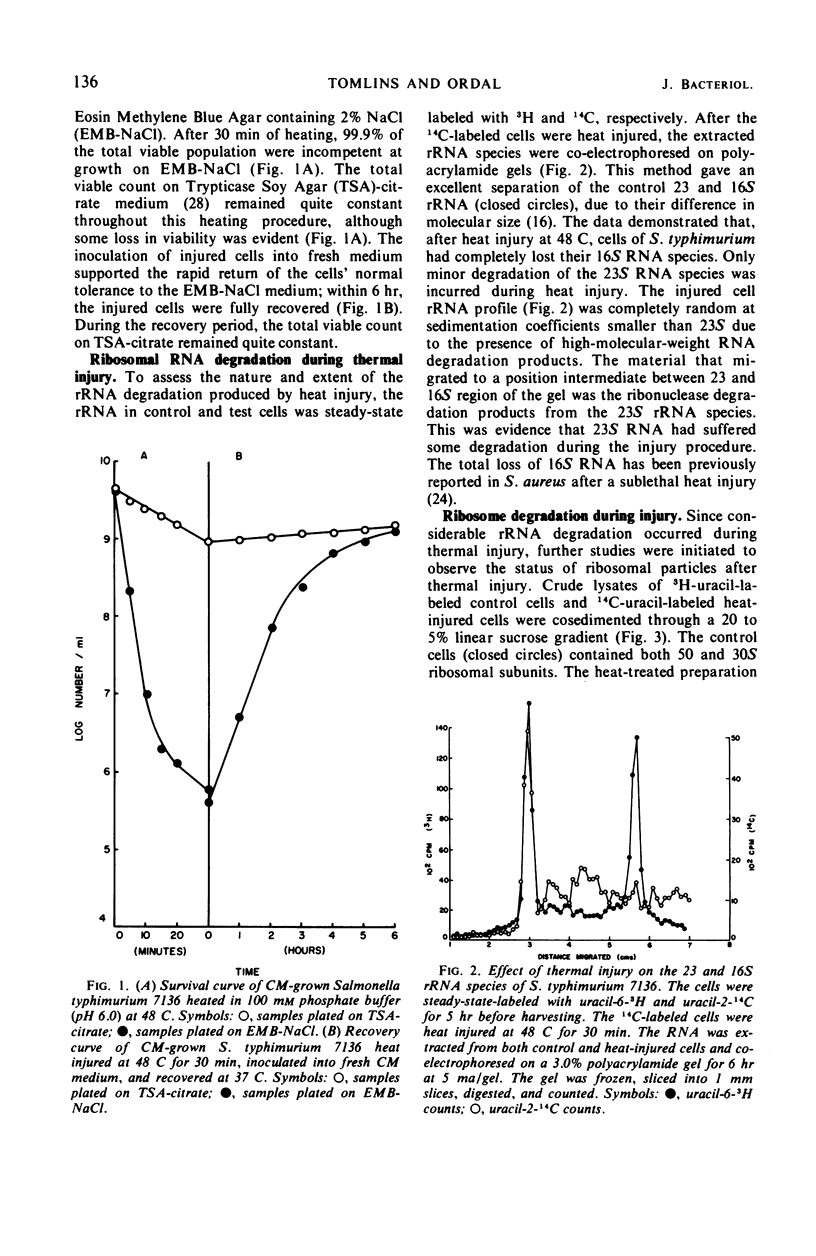
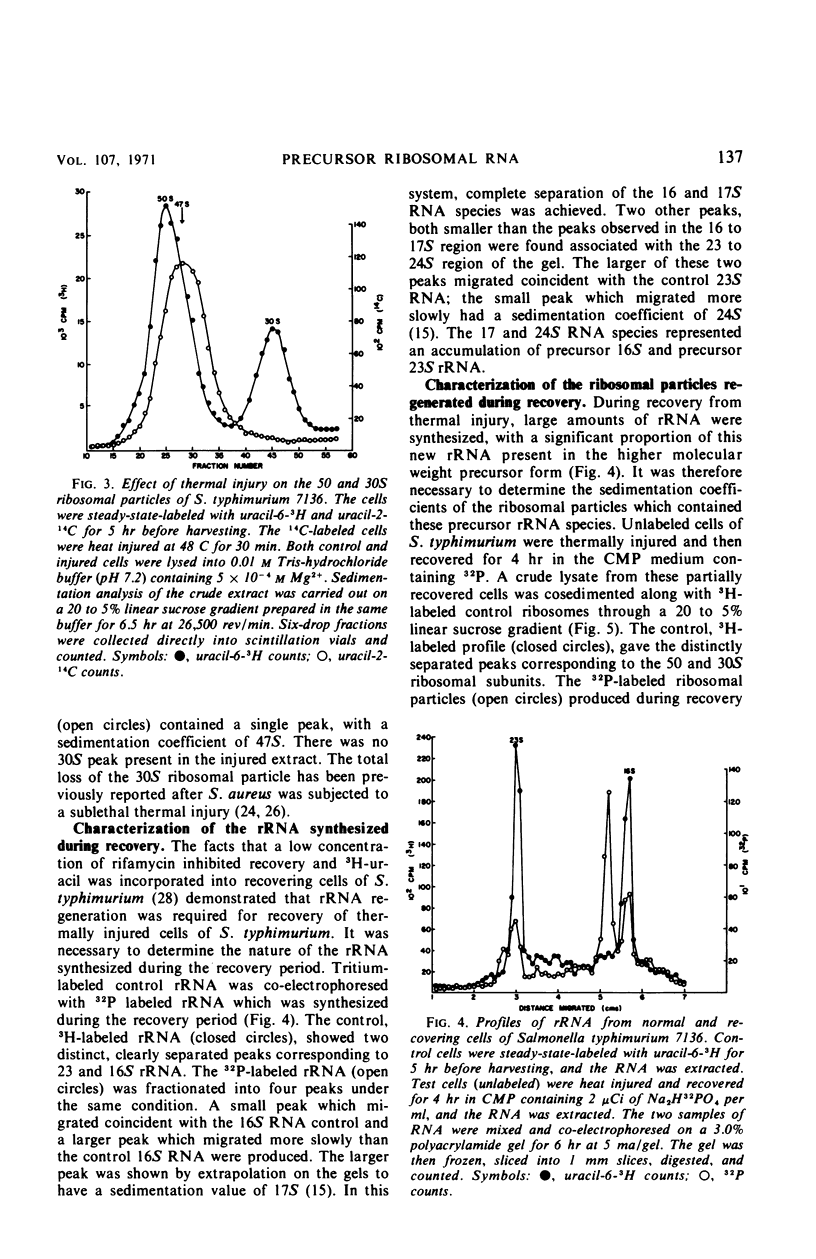
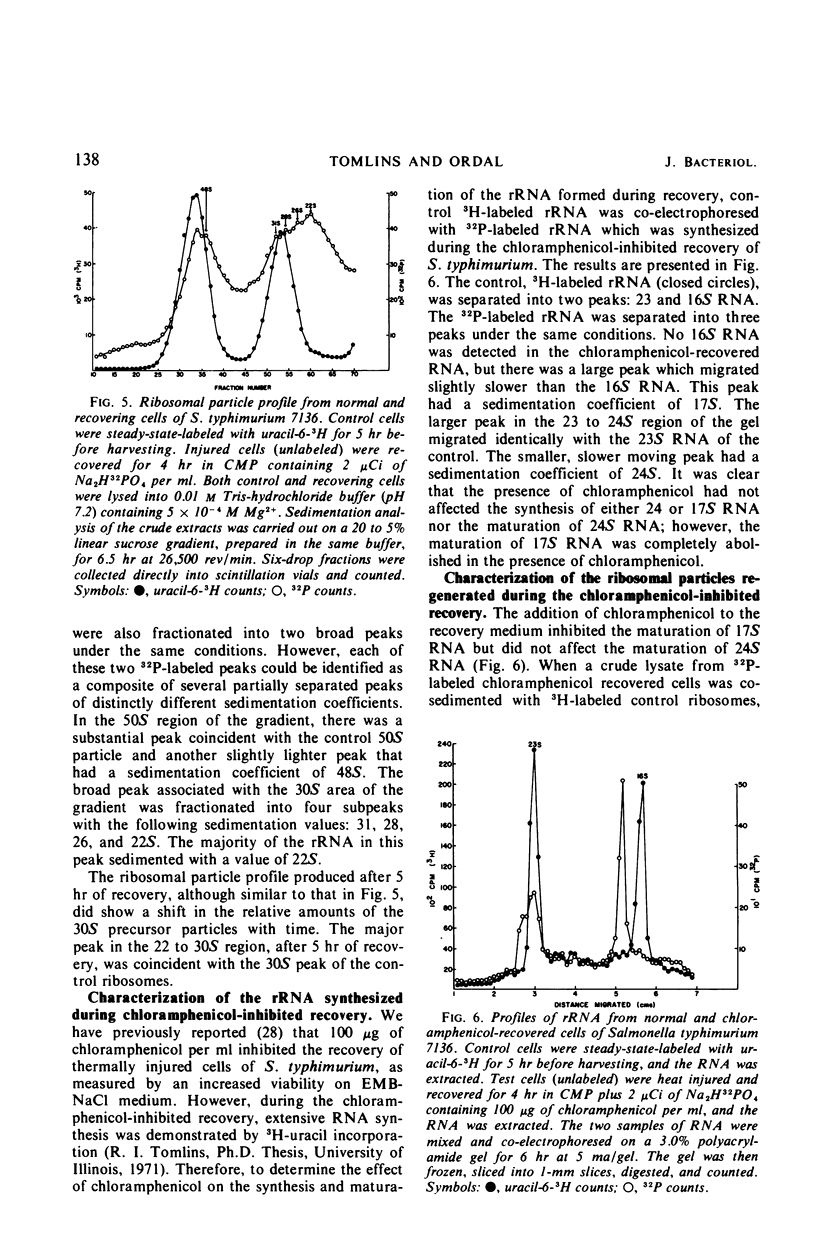
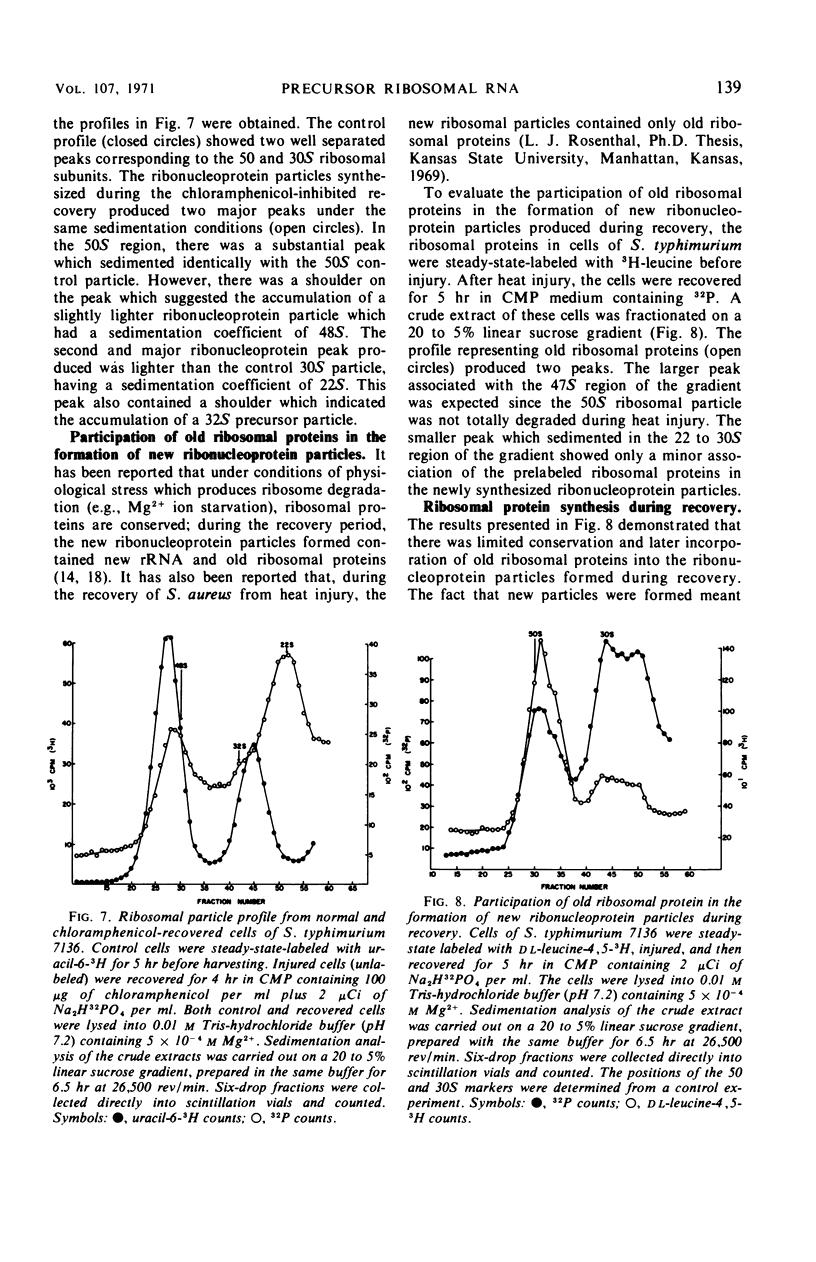
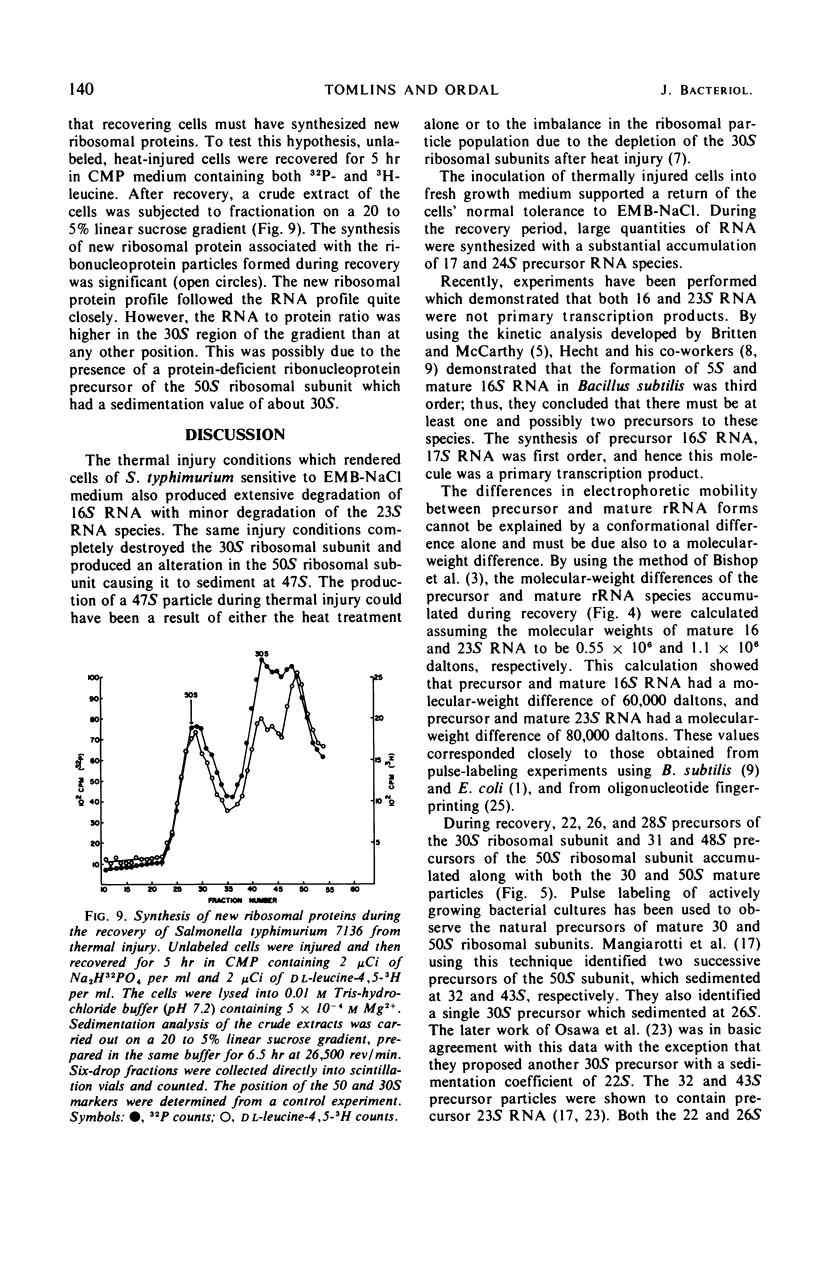
When cells of S. typhimurium were heated at 48 C for 30 min in phosphate buffer (pH 6.0), they became sensitive to Levine Eosin Methylene Blue Agar containing 2% NaCl (EMB-NaCl). The inoculation of injured cells into fresh growth medium supported the return of their normal tolerance to EMB-NaCl within 6 hr. The fractionation of ribosomal ribonucleic acid (rRNA) from unheated and heat-injured cells by polyacrylamide gel electrophoresis demonstrated that after injury the 16S RNA species was totally degraded and the 23S RNA was partially degraded. Sucrose gradient analysis demonstrated that after injury the 30S ribosomal subunit was totally destroyed and the sedimentation coefficient of the 50S particle was decreased to 47S. During the recovery of cells from thermal injury, four species of rRNA accumulated which were demonstrated to have the following sedimentation coefficients: 16, 17, 23, and 24S. Under identical recovery conditions, 22, 26, and 28S precursors of the 30S ribosomal subunit and 31 and 48S precursors of the 50S ribosomal subunit accumulated along with both the 30 and 50S mature particles. The addition of chloramphenicol to the recovery medium inhibited both the maturation of 17S RNA and the production of mature 30S ribosomal subunits, but permitted the accumulation of a single 22S precursor particle. Chloramphenicol did not affect either the maturation of 24S RNA or the mechanism of formation of 50S ribosomal subunits during recovery. Very little old ribosomal protein was associated with the new rRNA synthesized during recovery. New ribosomal proteins were synthesized during recovery and they were found associated with the new rRNA in ribosomal particles. The rate-limiting step in the recovery of S. typhimurium from thermal injury was in the maturation of the newly synthesized rRNA.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adesnik M., Levinthal C. Synthesis and maturation of ribosomal RNA in Escherichia coli. J Mol Biol. 1969 Dec 14;46(2):281–303. doi: 10.1016/0022-2836(69)90422-7. [DOI] [PubMed] [Google Scholar]
- Allwood M. C., Russell A. D. Thermally induced ribonucleic acid degradation and leakage of substances from the metabolic pool in Staphylococcus aureus. J Bacteriol. 1968 Feb;95(2):345–349. doi: 10.1128/jb.95.2.345-349.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRITTEN R. J., McCARTHY B. J. The synthesis of ribosomes in E. coli. II. Analysis of the kinetics of tracer incorporation in growing cells. Biophys J. 1962 Jan;2:49–55. doi: 10.1016/s0006-3495(62)86840-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H., Claybrook J. R., Spiegelman S. Electrophoretic separation of viral nucleic acids on polyacrylamide gels. J Mol Biol. 1967 Jun 28;26(3):373–387. doi: 10.1016/0022-2836(67)90310-5. [DOI] [PubMed] [Google Scholar]
- Bluhm L., Ordal Z. J. Effect of sublethal heat on the metabolic activity of Staphylococcus aureus. J Bacteriol. 1969 Jan;97(1):140–150. doi: 10.1128/jb.97.1.140-150.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C. W., Ordal Z. J. Thermal injury and recovery of Salmonella typhimurium and its effect on enumeration procedures. Appl Microbiol. 1969 Sep;18(3):332–336. doi: 10.1128/am.18.3.332-336.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie C., Nashimoto H., Nomura M. Structure and function of E. coli ribosomes. 8. Cold-sensitive mutants defective in ribosome assembly. Proc Natl Acad Sci U S A. 1969 Jun;63(2):384–391. doi: 10.1073/pnas.63.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht N. B., Bleyman M., Woese C. R. The formation of 5S ribosomal ribonucleic acid in Bacillus subtilis by posttranscriptional modification. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1278–1283. doi: 10.1073/pnas.59.4.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht N. B., Woese C. R. Separation of bacterial ribosomal ribonucleic acid from its macromolecular precursors by polyacrylamide gel electrophoresis. J Bacteriol. 1968 Mar;95(3):986–990. doi: 10.1128/jb.95.3.986-990.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iandolo J. J., Ordal Z. J. Repair of thermal injury of Staphylococcus aureus. J Bacteriol. 1966 Jan;91(1):134–142. doi: 10.1128/jb.91.1.134-142.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRBY K. S. A new method for the isolation of ribonucleic acids from mammalian tissues. Biochem J. 1956 Nov;64(3):405–408. doi: 10.1042/bj0640405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KONO M., OSAWA S. INTERMEDIARY STEPS OF RIBOSOME FORMATION IN ESCHERICHIA COLI. Biochim Biophys Acta. 1964 Jun 22;87:326–334. doi: 10.1016/0926-6550(64)90228-2. [DOI] [PubMed] [Google Scholar]
- KURLAND C. G., NOMURA M., WATSON J. D. The physical properties of the chloromycetin particles. J Mol Biol. 1962 May;4:388–394. doi: 10.1016/s0022-2836(62)80019-9. [DOI] [PubMed] [Google Scholar]
- Lefkovits I., Di Girolamo M. Reutilization of ribosomal proteins in vivo for the formation of new ribosomal particles in Escherichia coli B. Biochim Biophys Acta. 1969 Feb 18;174(2):566–573. doi: 10.1016/0005-2787(69)90286-x. [DOI] [PubMed] [Google Scholar]
- Lewicki P. P., Sinskey A. J. Precision of RNA separation by polyacrylamide gel electrophoresis. Anal Biochem. 1970 Feb;33(2):273–278. doi: 10.1016/0003-2697(70)90297-6. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiarotti G., Apirion D., Schlessinger D., Silengo L. Biosynthetic precursors of 30S and 50S ribosomal particles in Escherichia coli. Biochemistry. 1968 Jan;7(1):456–472. doi: 10.1021/bi00841a058. [DOI] [PubMed] [Google Scholar]
- Nakada D., Marquisee M. J. Relaxed synthesis of ribosomal RNA by a stringent strain of Escherichia coli. J Mol Biol. 1965 Sep;13(2):351–361. doi: 10.1016/s0022-2836(65)80102-4. [DOI] [PubMed] [Google Scholar]
- Nomura M. Bacterial ribosome. Bacteriol Rev. 1970 Sep;34(3):228–277. doi: 10.1128/br.34.3.228-277.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozawa R., Horiuchi T., Mizuno D. Degradation of ribosomal RNA in a temperature-sensitive Escherichia coli. Arch Biochem Biophys. 1967 Feb;118(2):402–409. doi: 10.1016/0003-9861(67)90367-0. [DOI] [PubMed] [Google Scholar]
- Osawa S., Otaka E., Itoh T., Fukui T. Biosynthesis of 50 s ribosomal subunit in Escherichia coli. J Mol Biol. 1969 Mar 28;40(3):321–351. doi: 10.1016/0022-2836(69)90158-2. [DOI] [PubMed] [Google Scholar]
- Osawa S. Ribosome formation and structure. Annu Rev Biochem. 1968;37:109–130. doi: 10.1146/annurev.bi.37.070168.000545. [DOI] [PubMed] [Google Scholar]
- Rosenthal L. J., Iandolo J. J. Thermally induced intracellular alteration of ribosomal ribonucleic acid. J Bacteriol. 1970 Sep;103(3):833–835. doi: 10.1128/jb.103.3.833-835.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogin S. J., Ordal Z. J. Regeneration of ribosomes and ribosomal ribonucleic acid during repair of thermal injury to Staphylococcus. J Bacteriol. 1967 Oct;94(4):1082–1087. doi: 10.1128/jb.94.4.1082-1087.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai P. C., Kessler D. P., Ingraham J. Cold-sensitive mutations in Salmonella typhimurium which affect ribosome synthesis. J Bacteriol. 1969 Mar;97(3):1298–1304. doi: 10.1128/jb.97.3.1298-1304.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlins R. I., Ordal Z. J. Requirements of Salmonella typhimurium for recovery from thermal injury. J Bacteriol. 1971 Feb;105(2):512–518. doi: 10.1128/jb.105.2.512-518.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub P., Nomura M. Structure and function of Escherichia coli ribosomes. I. Partial fractionation of the functionally active ribosomal proteins and reconstitution of artificial subribosomal particles. J Mol Biol. 1968 Jun 28;34(3):575–593. doi: 10.1016/0022-2836(68)90182-4. [DOI] [PubMed] [Google Scholar]
- Traub P., Nomura M. Structure and function of Escherichia coli ribosomes. VI. Mechanism of assembly of 30 s ribosomes studied in vitro. J Mol Biol. 1969 Mar 28;40(3):391–413. doi: 10.1016/0022-2836(69)90161-2. [DOI] [PubMed] [Google Scholar]


