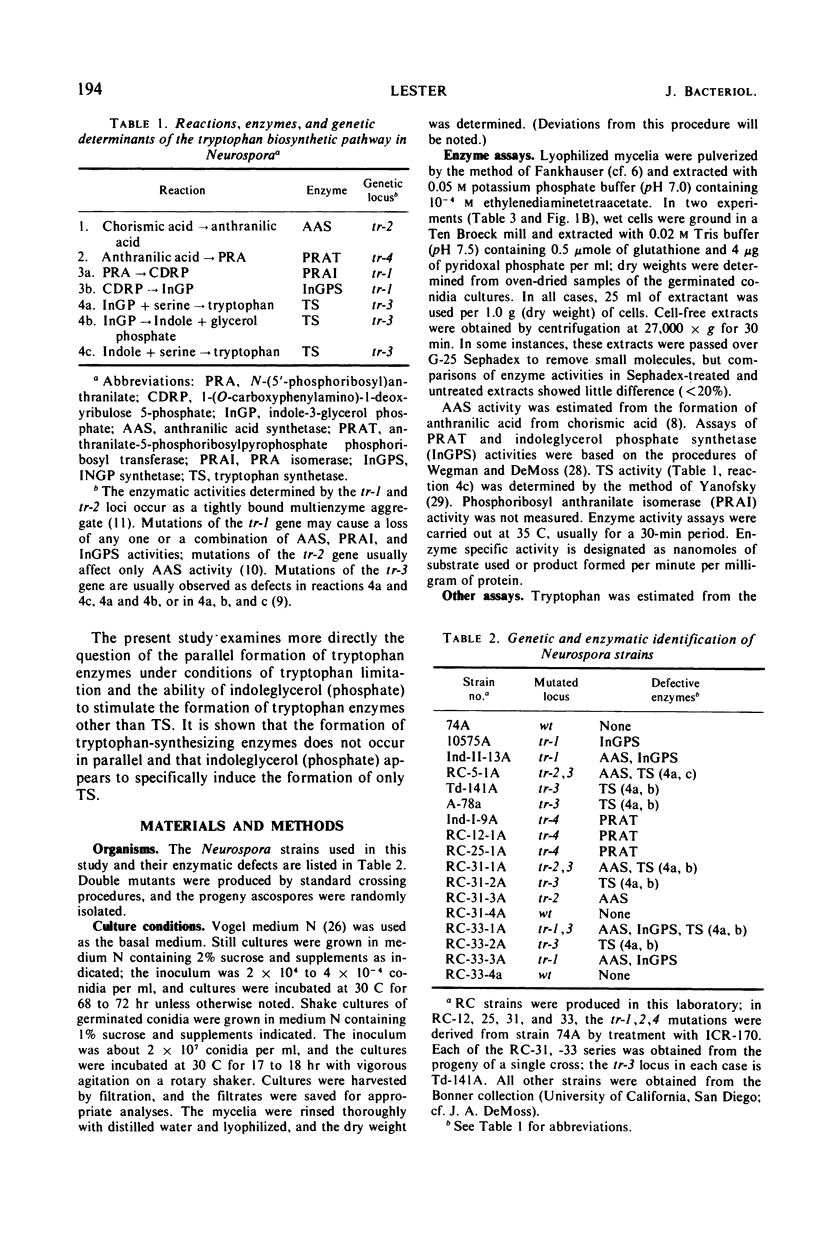
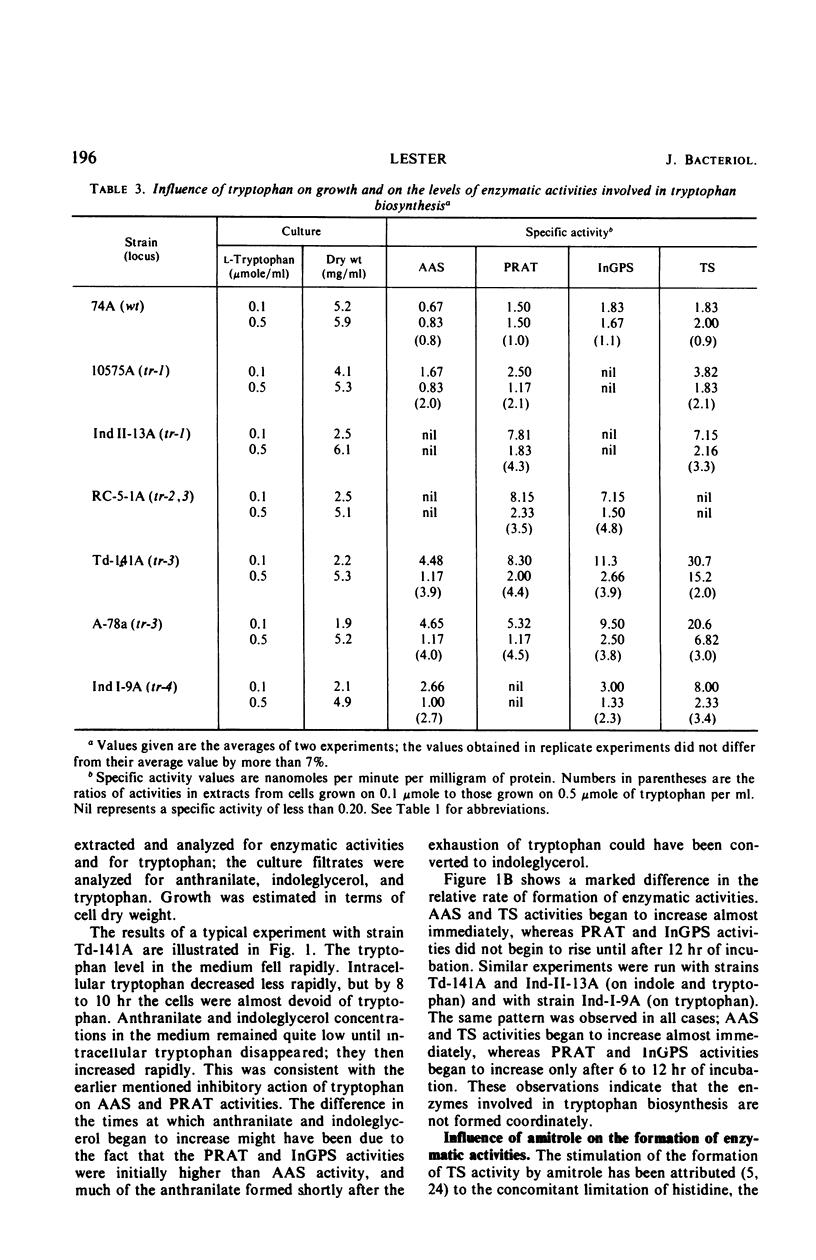
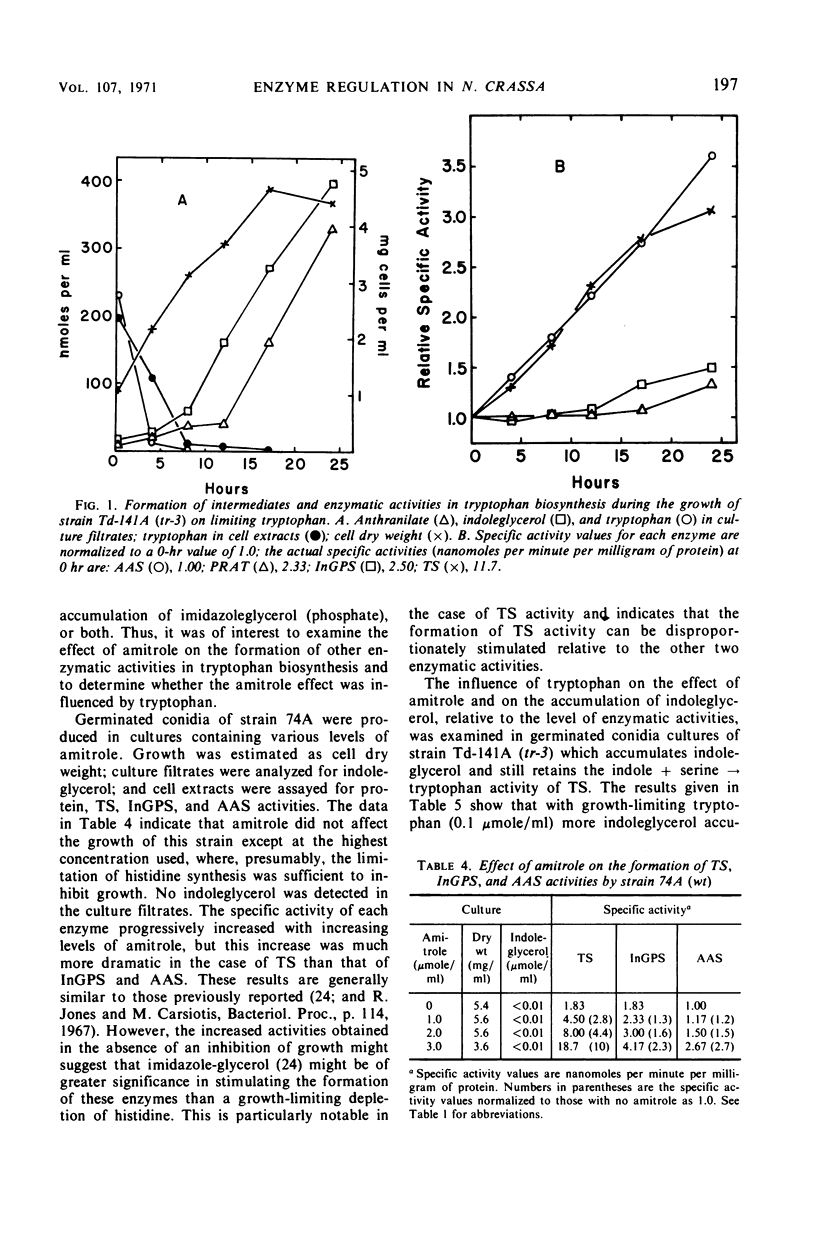
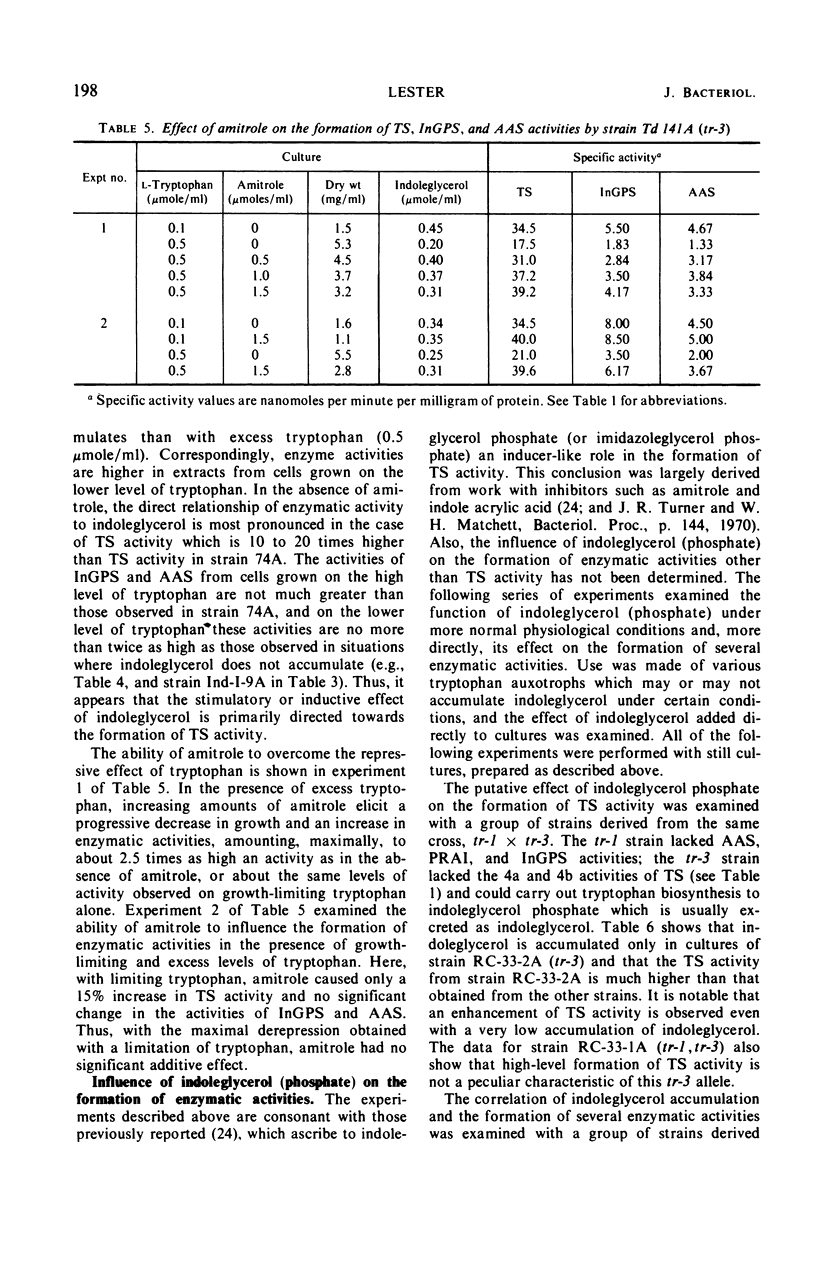
Abstract
The formation of enzymatic activities involved in the biosynthesis of tryptophan in Neurospora crassa was examined under various conditions in several strains. With growth-limiting tryptophan, the formation of four enzymatic activities, anthranilic acid synthetase (AAS), anthranilate-5-phosphoribosylpyrophosphate phosphoribosyl transferase (PRAT), indoleglycerol phosphate synthetase (InGPS), and tryptophan synthetase (TS) did not occur coordinately. AAS and TS activities began to increase immediately, whereas PRAT and InGPS activities began to increase only after 6 to 12 hr of incubation. In the presence of amitrole (3-amino-1,2,4-triazole), the formation of TS activity in a wild-type strain was more greatly enhanced than were AAS and InGPS activities. With a tr-3 mutant, which ordinarily exhibits an elevated TS activity, amitrole did not produce an increase in TS activity greater than that observed on limiting tryptophan. With tr-3 mutants, the increased levels of TS activity could be correlated with the accumulation of indoleglycerol in the medium; prior genetic blocks which prevented or reduced the synthesis of indoleglycerol also reduced the formation of TS activity. The addition of indoleglycerol to cultures of a double mutant (tr-1, tr-3) which could not synthesize indoleglycerol markedly stimulated the production of TS activity but not PRAT activity; the production of TS activity reached the same level with limiting or with excess tryptophan. A model explaining these and other related observations on enzyme formation in N. crassa is proposed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Garry B. COORDINATE REPRESSION OF THE SYNTHESIS OF FOUR HISTIDINE BIOSYNTHETIC ENZYMES BY HISTIDINE. Proc Natl Acad Sci U S A. 1959 Oct;45(10):1453–1461. doi: 10.1073/pnas.45.10.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo-Begovich A., DeMoss J. A. In vitro formation of an active multienzyme complex in the tryptophan pathway of Neurospora crassa. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1072–1078. doi: 10.1073/pnas.64.3.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARRATT R. W., NEWMEYER D., PERKINS D. D., GARNJOBST L. Map construction in Neurospora crassa. Adv Genet. 1954;6:1–93. doi: 10.1016/s0065-2660(08)60127-3. [DOI] [PubMed] [Google Scholar]
- Bonner D. M., Yanofsky C., Partridge C. W. Incomplete Genetic Blocks in Biochemical Mutants of Neurospora. Proc Natl Acad Sci U S A. 1952 Jan;38(1):25–34. doi: 10.1073/pnas.38.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARSIOTIS M., LACY A. M. INCREASED ACTIVITY OF TRYPTOPHAN BIOSYNTHETIC ENZYMES IN HISTIDINE MUTANTS OF NEUROSPORA CRASSA. J Bacteriol. 1965 Jun;89:1472–1477. doi: 10.1128/jb.89.6.1472-1477.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carsiotis M., Jones R. F., Lacy A. M., Cleary T. J., Fankhauser D. B. Histidine-mediated control of tryptophan biosynthetic enzymes in Neurospora crassa. J Bacteriol. 1970 Oct;104(1):98–106. doi: 10.1128/jb.104.1.98-106.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford I. P., Gunsalus I. C. Inducibility of tryptophan synthetase in Pseudomonas putida. Proc Natl Acad Sci U S A. 1966 Aug;56(2):717–724. doi: 10.1073/pnas.56.2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEMOSS J. A. THE CONVERSION OF SHIKIMIC ACID TO ANTHRANILIC ACID BY EXTRACTS OF NEUROSPORA CRASSA. J Biol Chem. 1965 Mar;240:1231–1235. [PubMed] [Google Scholar]
- DeMoss J. A., Jackson R. W., Chalmers J. H., Jr Genetic control of the structure and activity of an enzyme aggregate in the tryptophan pathway of Neurospora crassa. Genetics. 1967 Jul;56(3):413–424. doi: 10.1093/genetics/56.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMoss J. A., Wegman J. An enzyme aggregate in the tryptophan pathway of Neurospora crassa. Proc Natl Acad Sci U S A. 1965 Jul;54(1):241–247. doi: 10.1073/pnas.54.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demoss J. A., Bonner D. M. STUDIES ON NORMAL AND GENETICALLY ALTERED TRYPTOPHAN SYNTHETASE FROM NEUROSPORA CRASSA. Proc Natl Acad Sci U S A. 1959 Sep;45(9):1405–1412. doi: 10.1073/pnas.45.9.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaertner F. H., DeMoss J. A. Purification and characterization of a multienzyme complex in the tryptophan pathway of Neurospora crassa. J Biol Chem. 1969 May 25;244(10):2716–2725. [PubMed] [Google Scholar]
- Gibson F. Chorismic acid: purification and some chemical and physical studies. Biochem J. 1964 Feb;90(2):256–261. doi: 10.1042/bj0900256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S. A., Weber R. P. COLORIMETRIC ESTIMATION OF INDOLEACETIC ACID. Plant Physiol. 1951 Jan;26(1):192–195. doi: 10.1104/pp.26.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LESTER G. Regulation of early reactions in the biosynthesis of tryptophan in Neurospora crassa. J Bacteriol. 1963 Feb;85:468–475. doi: 10.1128/jb.85.2.468-475.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LESTER G. Some aspects of tryptophan synthetase formation in Neurospora crassa. J Bacteriol. 1961 Jun;81:964–973. doi: 10.1128/jb.81.6.964-973.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lester G. In vivo regulation of intermediate reactions in the pathway of tryptophan biosynthesis in Neurospora crassa. J Bacteriol. 1968 Nov;96(5):1768–1773. doi: 10.1128/jb.96.5.1768-1773.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATCHETT W. H., DEMOSS J. A. Direct evidence for a trytophan-anthranilic acid cycle in Neurospora. Biochim Biophys Acta. 1963 Jun 4;71:632–642. doi: 10.1016/0006-3002(63)91136-3. [DOI] [PubMed] [Google Scholar]
- MATCHETT W. H., DEMOSS J. A. Factors affecting increased production of tryptophan synthetase by a TD mutant of Neurospora crassa. J Bacteriol. 1962 Jun;83:1294–1300. doi: 10.1128/jb.83.6.1294-1300.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH O. H., YANOFSKY C. 1-(o-Carboxyphenylamino)-1-deoxyribulose 5-phosphate, a new intermediate in the biosynthesis of tryptophan. J Biol Chem. 1960 Jul;235:2051–2057. [PubMed] [Google Scholar]
- Turner J. R., Matchett W. H. Alteration of tryptophan-mediated regulation in Neurospora crassa by indoleglycerol phosphate. J Bacteriol. 1968 May;95(5):1608–1614. doi: 10.1128/jb.95.5.1608-1614.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbarger H. E. Regulation of amino acid metabolism. Annu Rev Biochem. 1969;38:323–370. doi: 10.1146/annurev.bi.38.070169.001543. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Wegman J., DeMoss J. A. The enzymatic conversion of anthranilate to indolylglycerol phosphate in Neurospora crassa. J Biol Chem. 1965 Oct;240(10):3781–3788. [PubMed] [Google Scholar]
- YANOFSKY C., LENNOX E. S. Transduction and recombination study of linkage relationships among the genes controlling tryptophan synthesis in Escherichia coli. Virology. 1959 Aug;8:425–447. doi: 10.1016/0042-6822(59)90046-7. [DOI] [PubMed] [Google Scholar]
- YANOFSKY C. The enzymatic conversion of anthranilic acid to indole. J Biol Chem. 1956 Nov;223(1):171–184. [PubMed] [Google Scholar]



