Abstract
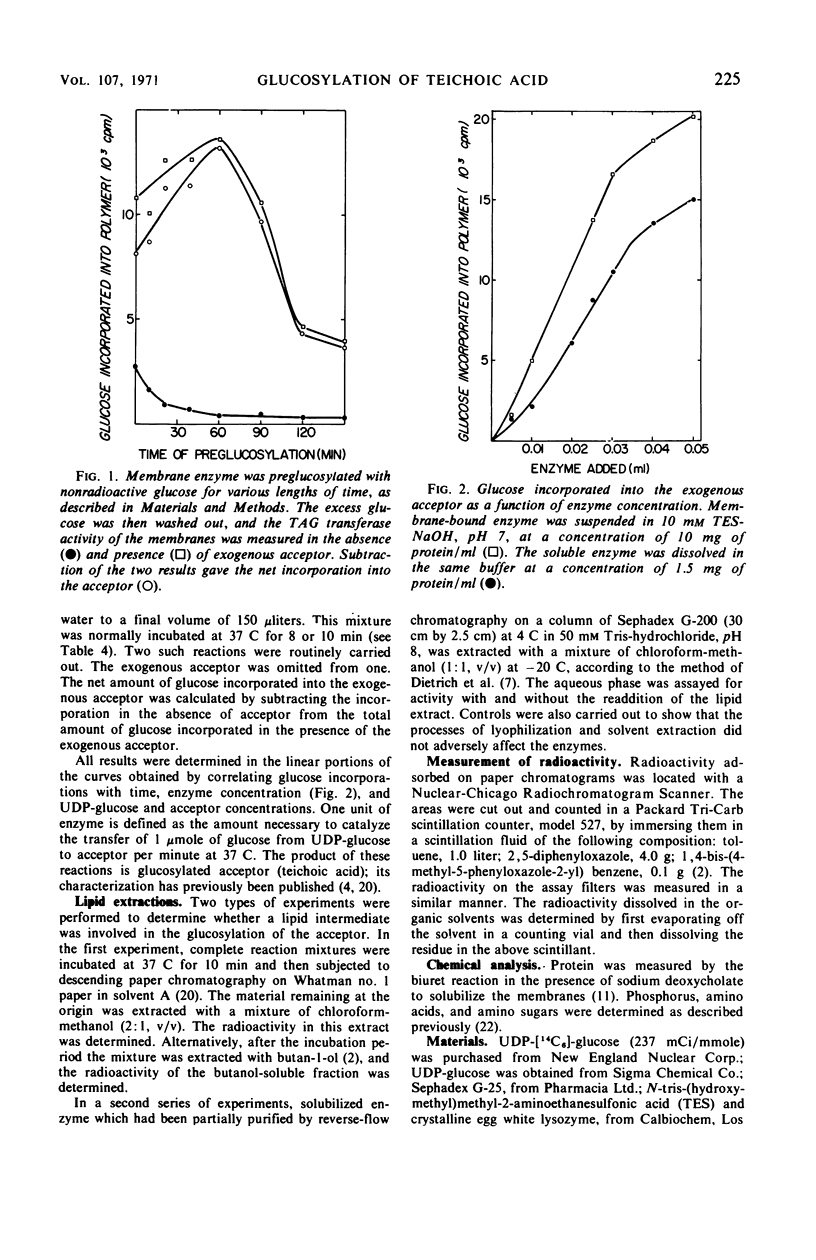
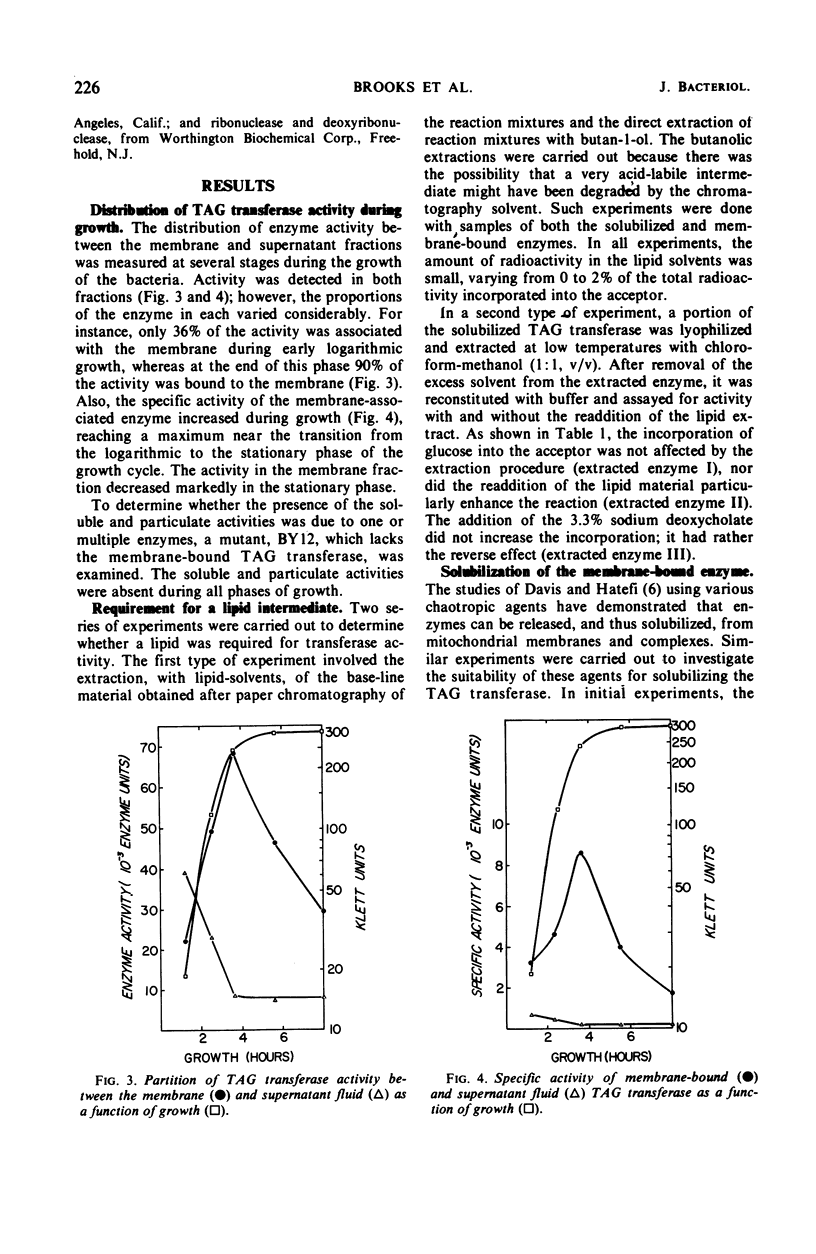
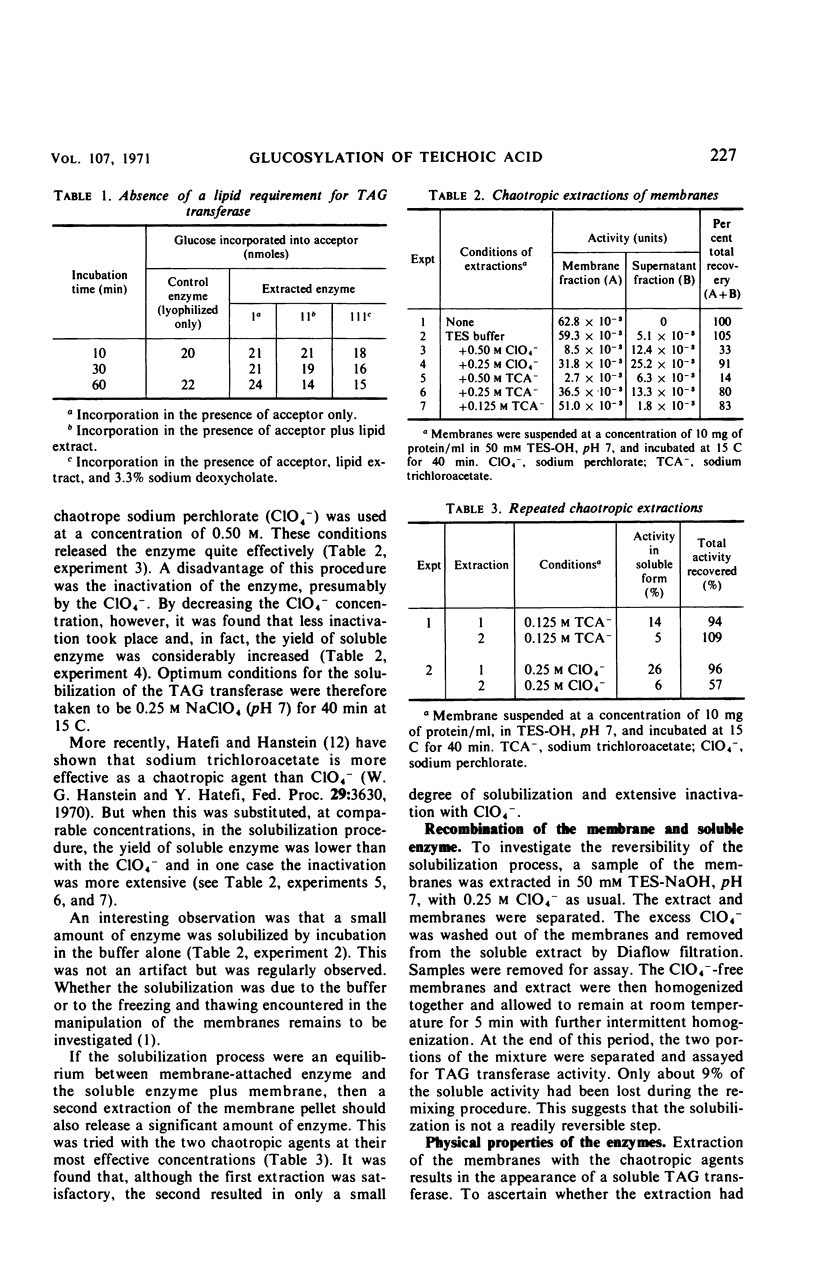
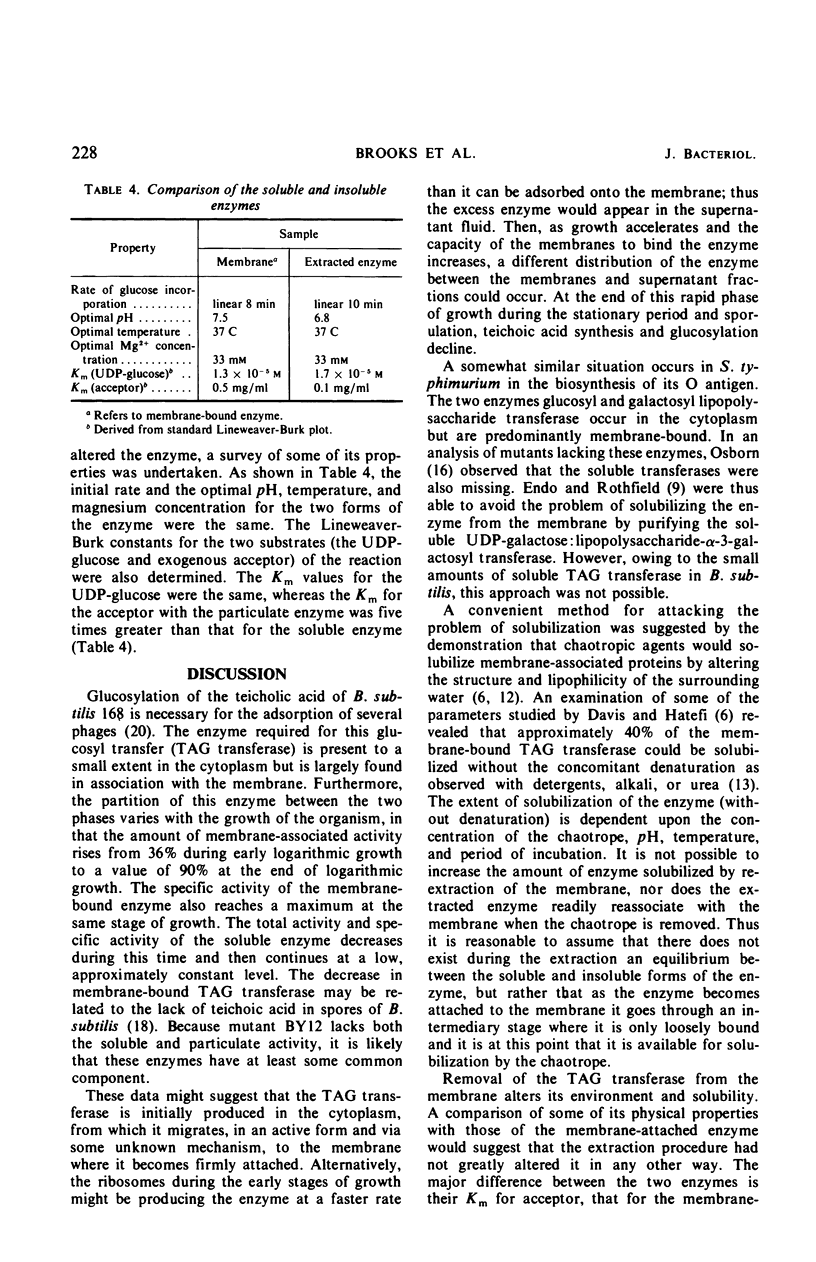
Polyglycerolteichoic acid:glucosyl transferase (TAG transferase), one of the three enzymes involved in the pathway leading to the glucosylation of teichoic acid in Bacillus subtilis 168, was investigated. During the early stages of the growth of B. subtilis, TAG transferase is predominantly a soluble enzyme found in the cytoplasm. As growth proceeds, the amount of soluble enzyme decreases and the proportion of insoluble, membrane-bound TAG transferase increases, reaching a maximal value at the close of the logarithmic phase. Data are presented which suggest that these are two forms of the same enzyme, or have some common component. The effects of chaotropic agents, such as sodium trichloroacetate and sodium perchlorate, on the cytoplasmic membrane were also studied. These data show that such compounds can effectively remove the TAG transferase from the membrane in a water-soluble form. A study of some of the physical properties of this solubilized enzyme suggests that there is little difference between the two forms of the enzyme. Experiments are described which indicate that the glucosyl transfer by both the membrane-bound and soluble enzymes is not mediated by lipids.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baginsky M. L., Hatefi Y. Reconstitution of succinate-coenzyme Q reductase (complex II) and succinate oxidase activities by a highly purified, reactivated succinate dehydrogenase. J Biol Chem. 1969 Oct 10;244(19):5313–5319. [PubMed] [Google Scholar]
- Brooks D., Baddiley J. A lipid intermediate in the synthesis of a poly-(N-acetylglucosamine 1-phosphate) from the wall of Staphylococcus lactis N.C.T.C. 2102. Biochem J. 1969 Nov;115(2):307–314. doi: 10.1042/bj1150307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. C., Fraser D. K., Young F. E. Problems in purification of a Bacillus subtilis autolytic enzyme caused by association with teichoic acid. Biochim Biophys Acta. 1970 Feb 11;198(2):308–315. doi: 10.1016/0005-2744(70)90063-x. [DOI] [PubMed] [Google Scholar]
- Chin T., Burger M. M., Glaser L. Synthesis of teichoic acids. VI. The formation of multiple wall polymers in Bacillus subtilis W-23. Arch Biochem Biophys. 1966 Sep 26;116(1):358–367. doi: 10.1016/0003-9861(66)90042-7. [DOI] [PubMed] [Google Scholar]
- Davis K. A., Hatefi Y. Kinetics of the resolution of complex I (reduced diphosphopyridine nucleotide-coenzyme Q reductase) of the mitochondrial electron transport system by chaotropic agents. Biochemistry. 1969 Aug;8(8):3355–3361. doi: 10.1021/bi00836a033. [DOI] [PubMed] [Google Scholar]
- Dietrich C. P., Colucci A. V., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. V. Separation of protein and lipid components of the particulate enzyme from Micrococcus lysodeikticus and purification of the endogenous lipid acceptors. J Biol Chem. 1967 Jul 10;242(13):3218–3225. [PubMed] [Google Scholar]
- Douglas L. J., Baddiley J. A lipid intermediate in the biosynthesis of a teichoic acid. FEBS Lett. 1968 Aug;1(2):114–116. doi: 10.1016/0014-5793(68)80034-1. [DOI] [PubMed] [Google Scholar]
- Endo A., Rothfield L. Studies of a phospholipid-requiring bacterial enzyme. I. Purification and properties of uridine diphosphate galactose: lipopolysaccharide alpha-3-galactosyl transferase. Biochemistry. 1969 Sep;8(9):3500–3507. doi: 10.1021/bi00837a003. [DOI] [PubMed] [Google Scholar]
- GLASER L., BURGER M. M. THE SYNTHESIS OF TEICHOIC ACIDS. 3. GLUCOSYLATION OF POLYGLYCEROPHOSPHATE. J Biol Chem. 1964 Oct;239:3187–3191. [PubMed] [Google Scholar]
- Hatefi Y., Hanstein W. G. Solubilization of particulate proteins and nonelectrolytes by chaotropic agents. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1129–1136. doi: 10.1073/pnas.62.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydanek M. G., Jr, Neuhaus F. C. The initial stage in peptidoglycan synthesis. IV. Solubilization of phospho-N-acetylmuramyl-pentapeptide translocase. Biochemistry. 1969 Apr;8(4):1474–1481. doi: 10.1021/bi00832a024. [DOI] [PubMed] [Google Scholar]
- Higashi Y., Strominger J. L., Sweeley C. C. Structure of a lipid intermediate in cell wall peptidoglycan synthesis: a derivative of a C55 isoprenoid alcohol. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1878–1884. doi: 10.1073/pnas.57.6.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn E. D. Cell membranes: structure and synthesis. Annu Rev Biochem. 1969;38:263–288. doi: 10.1146/annurev.bi.38.070169.001403. [DOI] [PubMed] [Google Scholar]
- Osborn M. J. Biochemical characterization of mutants of Salmonella typhimurium lacking glucosyl or galactosyl lipopolysaccharide transferases. Nature. 1968 Mar 9;217(5132):957–960. doi: 10.1038/217957a0. [DOI] [PubMed] [Google Scholar]
- Osborn M. J. Structure and biosynthesis of the bacterial cell wall. Annu Rev Biochem. 1969;38:501–538. doi: 10.1146/annurev.bi.38.070169.002441. [DOI] [PubMed] [Google Scholar]
- Wright A., Dankert M., Fennessey P., Robbins P. W. Characterization of a polyisoprenoid compound functional in O-antigen biosynthesis. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1798–1803. doi: 10.1073/pnas.57.6.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUNG F. E., SPIZIZEN J., CRAWFORD I. P. BIOCHEMICAL ASPECTS OF COMPETENCE IN THE BACILLUS SUBTILIS TRANSFORMATION SYSTEM. I. CHEMICAL COMPOSITION OF CELL WALLS. J Biol Chem. 1963 Sep;238:3119–3125. [PubMed] [Google Scholar]
- YOUNG F. E., TIPPER D. J., STROMINGER J. L. AUTOLYSIS OF CELL WALLS OF BACILLUS SUBTILIS. MECHANISM AND POSSIBLE RELATIONSHIP TO COMPETENCE. J Biol Chem. 1964 Oct;239:PC3600–PC3602. [PubMed] [Google Scholar]
- Young F. E. Requirement of glucosylated teichoic acid for adsorption of phage in Bacillus subtilis 168. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2377–2384. doi: 10.1073/pnas.58.6.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young F. E., Smith C., Reilly B. E. Chromosomal location of genes regulating resistance to bacteriophage in Bacillus subtilis. J Bacteriol. 1969 Jun;98(3):1087–1097. doi: 10.1128/jb.98.3.1087-1097.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]



