Abstract
The occurrence of succinic dehydrogenase [succinic:(acceptor) oxidoreductase, EC 1.3.99.1] in membrane fractions of Micrococcus lysodeikticus was investigated. The enzyme could be purified 10-fold, by deoxycholate treatment. Butanol extraction of membranes yielded an active fraction, nonsedimentable at 130,000 × g for 2 hr and altered in its phospholipid content relative to membranes. The activity of the enzyme in particulate preparations was decreased in the presence of competitive inhibitors and by compounds known to react with iron, sulfhydryl groups, and flavine. In this respect, the bacterial succinic dehydrogenase is similar to the enzyme derived from yeast and mammalian sources. In certain membrane fractions, Ca2+ and Mg2+ exhibited inhibitory effects whereas Triton X-100 caused activation. The enzyme could also be activated by substrate. In the phenazine reductase assay, incomplete reduction of electron acceptor was observed upon addition of divalent cations and iron binding agents.
Full text
PDF

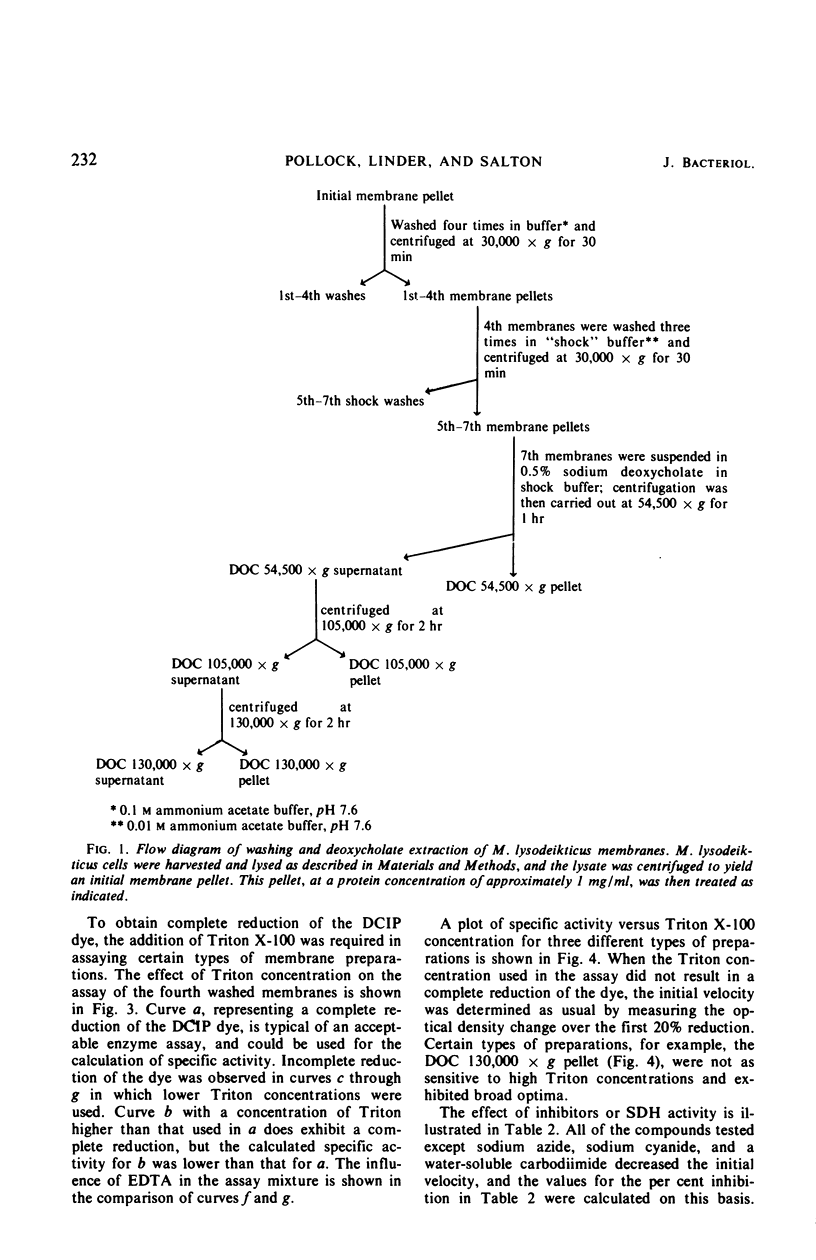
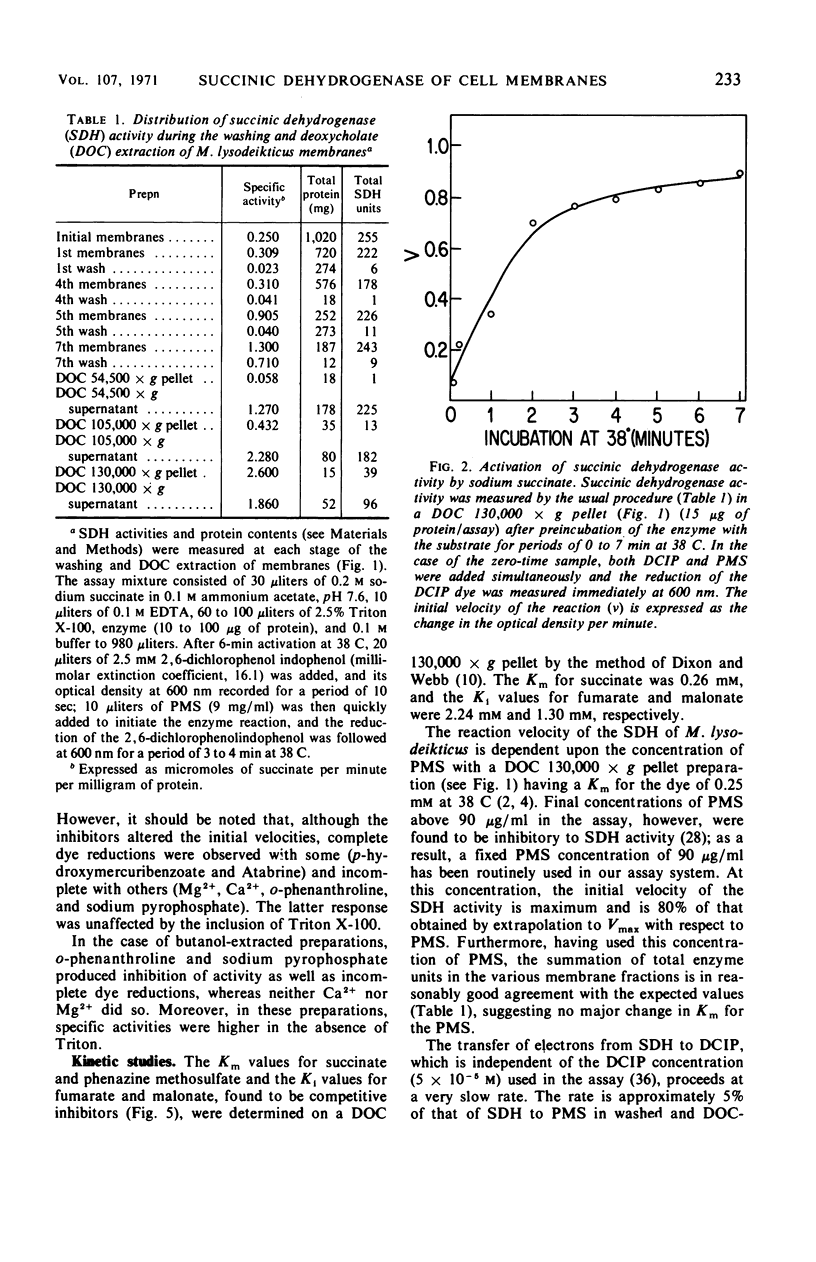
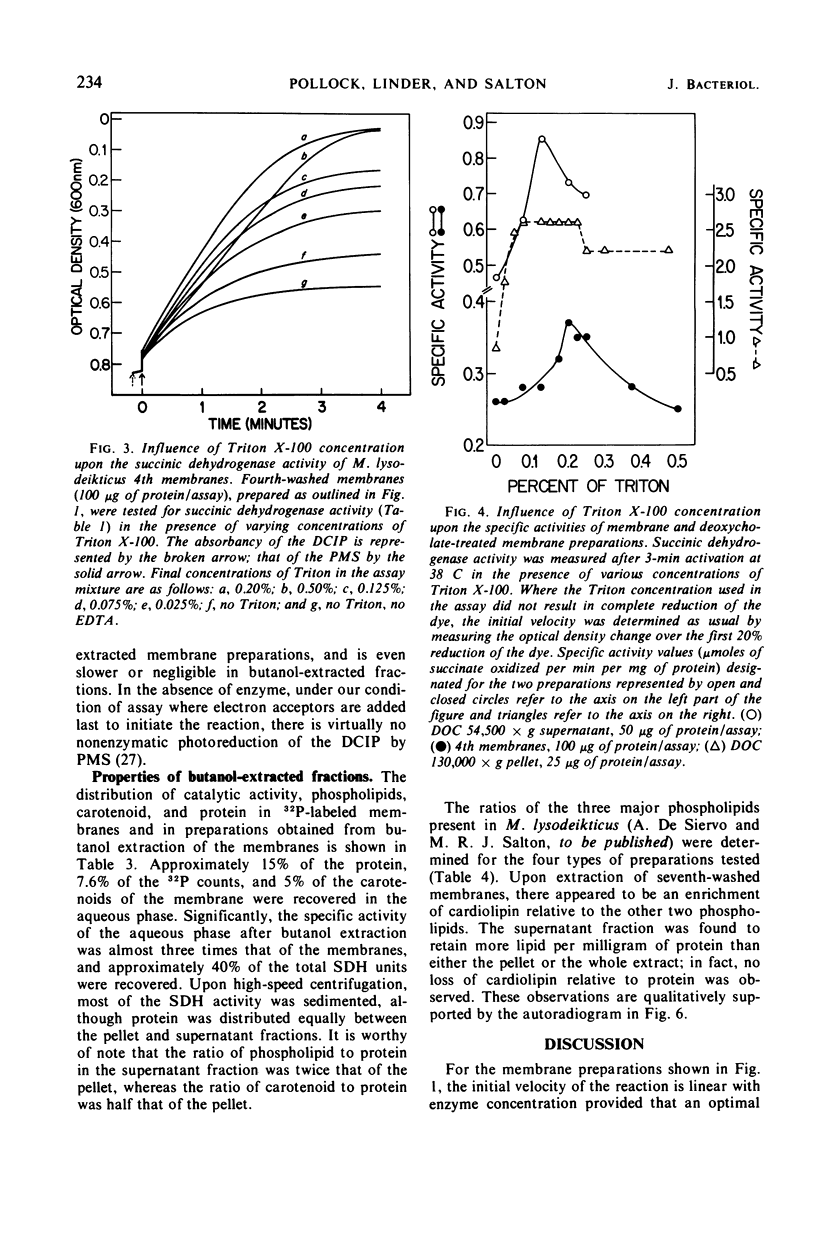

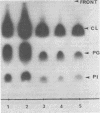
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAMSON M. B., KATZMAN R., WILSON C. E., GREGOR H. P. IONIC PROPERTIES OF AQUEOUS DISPERSIONS OF PHOSPHATIDIC ACID. J Biol Chem. 1964 Dec;239:4066–4072. [PubMed] [Google Scholar]
- ARRIGONI O., SINGER T. P. Limitations of the phenazine methosulphate assay for succinic and related dehydrogenases. Nature. 1962 Mar 31;193:1256–1258. doi: 10.1038/1931256a0. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Baginsky M. L., Hatefi Y. Reconstitution of succinate-coenzyme Q reductase (complex II) and succinate oxidase activities by a highly purified, reactivated succinate dehydrogenase. J Biol Chem. 1969 Oct 10;244(19):5313–5319. [PubMed] [Google Scholar]
- Cerletti P., Strom R., Giordano M. G., Balestrero F., Giovenco M. A. Peptide bound flavins and succinic dehydrogenase activity in the brain. Biochem Biophys Res Commun. 1964;14:408–412. doi: 10.1016/0006-291x(64)90077-4. [DOI] [PubMed] [Google Scholar]
- DERVARTANIAN D. V., VEEGER C. STUDIES ON SUCCINATE DEHYDROGENASE. I. SPECTRAL PROPERTIES OF THE PURIFIED ENZYME AND FORMATION OF ENZYME-COMPETITIVE INHIBITOR COMPLEXES. Biochim Biophys Acta. 1964 Nov 22;92:233–247. [PubMed] [Google Scholar]
- HAUGE J. G. Purification and properties of glucose dehydrogenase and cytochrome b from Bacterium anitratum. Biochim Biophys Acta. 1960 Dec 4;45:250–262. doi: 10.1016/0006-3002(60)91449-9. [DOI] [PubMed] [Google Scholar]
- JACKSON F. L., LAWTON V. D. A cytochrome of the b group from Micrococcus lysodeikticus. Biochim Biophys Acta. 1959 Sep;35:76–84. doi: 10.1016/0006-3002(59)90336-1. [DOI] [PubMed] [Google Scholar]
- KEARNEY E. B. Studies on succinic dehydrogenase. IV. Activation of the beef heart enzyme. J Biol Chem. 1957 Nov;229(1):363–375. [PubMed] [Google Scholar]
- Kimura T., Hauber J., Singer T. P. Studies on succinate dehydrogenase. 13. Reversible activation of the mammalian enzyme. J Biol Chem. 1967 Nov 10;242(21):4987–4993. [PubMed] [Google Scholar]
- King T. E. Reconstitution of the respiratory chain. Adv Enzymol Relat Areas Mol Biol. 1966;28:155–236. doi: 10.1002/9780470122730.ch3. [DOI] [PubMed] [Google Scholar]
- LARA F. J. The succinic dehydrogenase of Propionibacterium pentosaceum. Biochim Biophys Acta. 1959 Jun;33(2):565–567. doi: 10.1016/0006-3002(59)90153-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LUKOIANOVA M. A., GEL'MAN N. S., BIRIUZOVA V. I. [Structure of cytoplasmic membranes of Micrococcus lysodeikticus and succinic oxidase and succinic dehydrogenase activity]. Biokhimiia. 1961 Sep-Oct;26:916–925. [PubMed] [Google Scholar]
- MADSEN N. B. The oxidation of succinate in extracts of xanthomonas phaseoli. Can J Biochem Physiol. 1960 May;38:481–492. [PubMed] [Google Scholar]
- MARINETTI G. V. CHROMATOGRAPHY OF LIPIDS ON COMMERCIAL SILICA GEL LOADED FILTER PAPER. J Lipid Res. 1965 Apr;6:315–317. [PubMed] [Google Scholar]
- Maddy A. H. The properties of the protein of the plasma membrane of ox erythrocytes. Biochim Biophys Acta. 1966 Mar 28;117(1):193–200. doi: 10.1016/0304-4165(66)90166-8. [DOI] [PubMed] [Google Scholar]
- Muñoz E., Nachbar M. S., Schor M. T., Salton M. R. Adenosinetriphosphatase of Micrococcus lysodeikticus: selective release and relationship to membrane structure. Biochem Biophys Res Commun. 1968 Aug 13;32(3):539–546. doi: 10.1016/0006-291x(68)90696-7. [DOI] [PubMed] [Google Scholar]
- Muñoz E., Salton M. R., Ng M. H., Schor M. T. Membrane adenosine triphosphatase of Micrococcus lysodeikticus. Purification, properties of the "soluble" enzyme and properties of the membrane-bound enzyme. Eur J Biochem. 1969 Feb;7(4):490–501. [PubMed] [Google Scholar]
- Owen P., Freer J. H. Factors influencing the activity of succinate dehydrogenase in membrane preparations from Micrococcus lysodeikticus. Biochem J. 1970 Nov;120(2):237–243. doi: 10.1042/bj1200237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINGER T. P., MASSEY V., KEARNEY E. B. Studies on succinic dehydrogenase. V. Isolation and properties of the dehydrogenase from baker's yeast. Arch Biochem Biophys. 1957 Jul;69:405–421. doi: 10.1016/0003-9861(57)90506-4. [DOI] [PubMed] [Google Scholar]
- Salton M. R., Freer J. H. Composition of the membranes isolated from several Gram-positive bacteria. Biochim Biophys Acta. 1965 Oct 18;107(3):531–538. doi: 10.1016/0304-4165(65)90197-2. [DOI] [PubMed] [Google Scholar]
- Salton M. R., Freer J. H., Ellar D. J. Electron transport components localized in a lipid-depleted sheet isolated from Micrococcus lysodeikticus membranes by deoxycholate extraction. Biochem Biophys Res Commun. 1968 Dec 30;33(6):909–915. doi: 10.1016/0006-291x(68)90398-7. [DOI] [PubMed] [Google Scholar]
- Salton M. R. Isolation and characterization of bacterial membranes. Trans N Y Acad Sci. 1967 Apr;29(6):764–781. doi: 10.1111/j.2164-0947.1967.tb02300.x. [DOI] [PubMed] [Google Scholar]
- Slater E. C. Effect of sulphydryl-combining compounds on the activity of the succinic oxidase system. Biochem J. 1949;45(2):130–142. doi: 10.1042/bj0450130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THORN M. B. Activation of succinate dehydrogenase in heart-muscle preparations. Biochem J. 1962 Oct;85:116–127. doi: 10.1042/bj0850116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeijlemaker W. P., Dervartanian D. V., Veeger C., Slater E. C. Studies on succinate dehydrogenase. IV. Kinetics of the overall reaction catalysed by preparations of the purified enzyme. Biochim Biophys Acta. 1969 Apr 22;178(2):213–224. doi: 10.1016/0005-2744(69)90391-x. [DOI] [PubMed] [Google Scholar]