Abstract
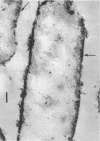
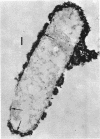
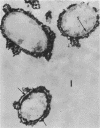
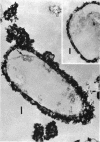
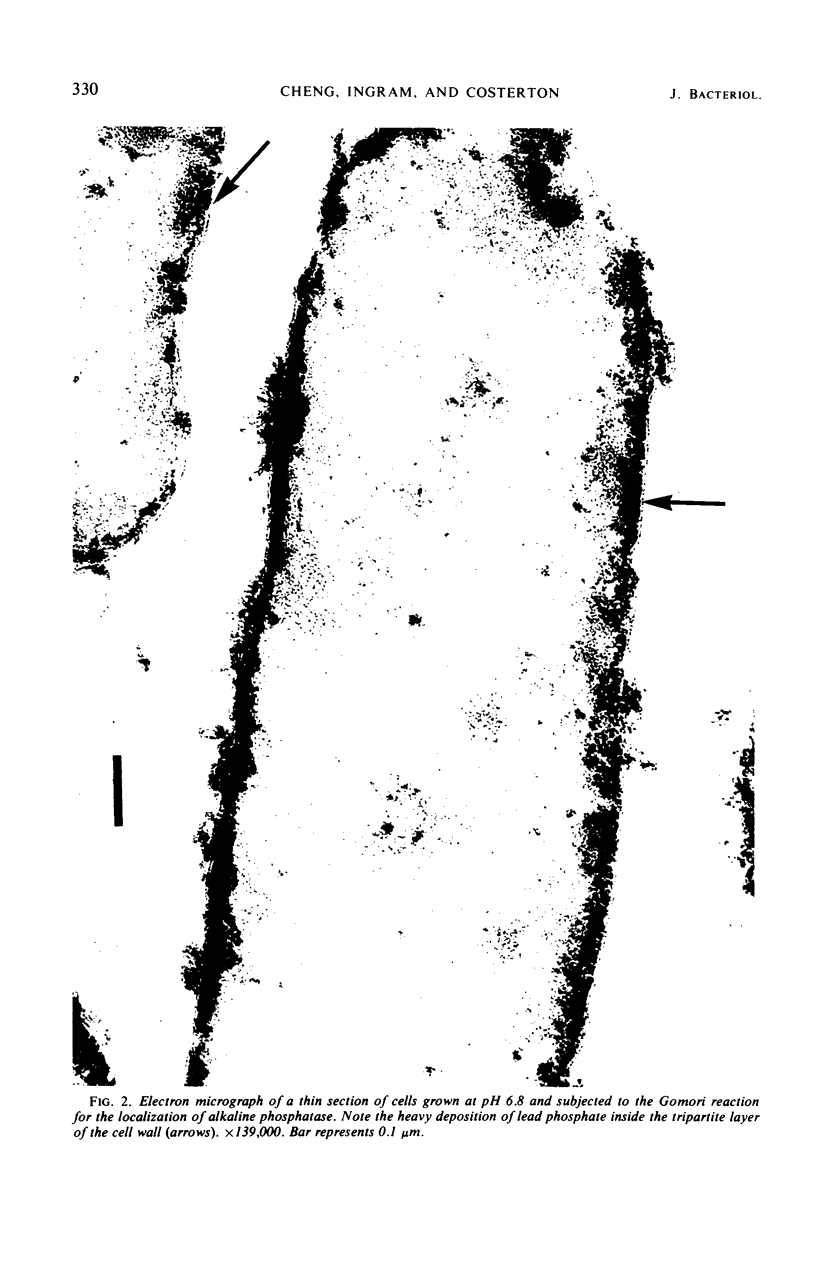
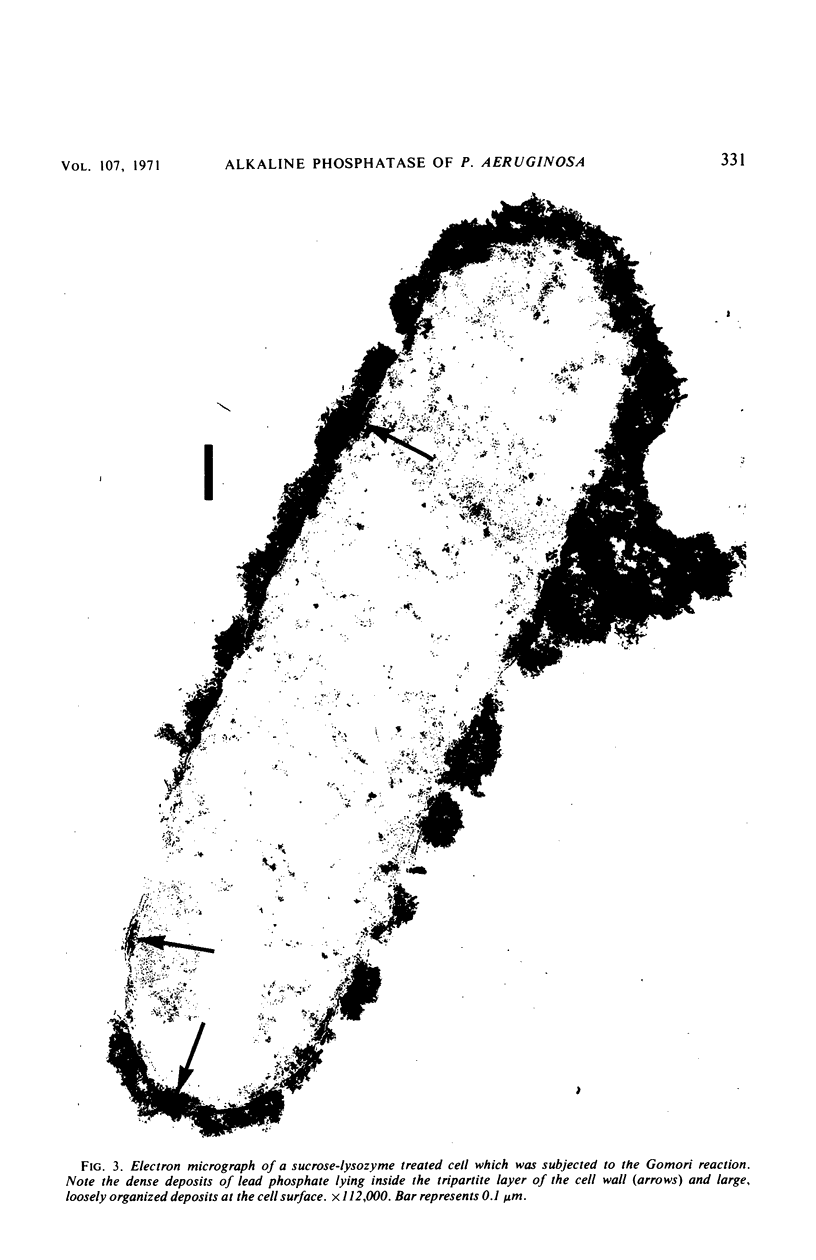
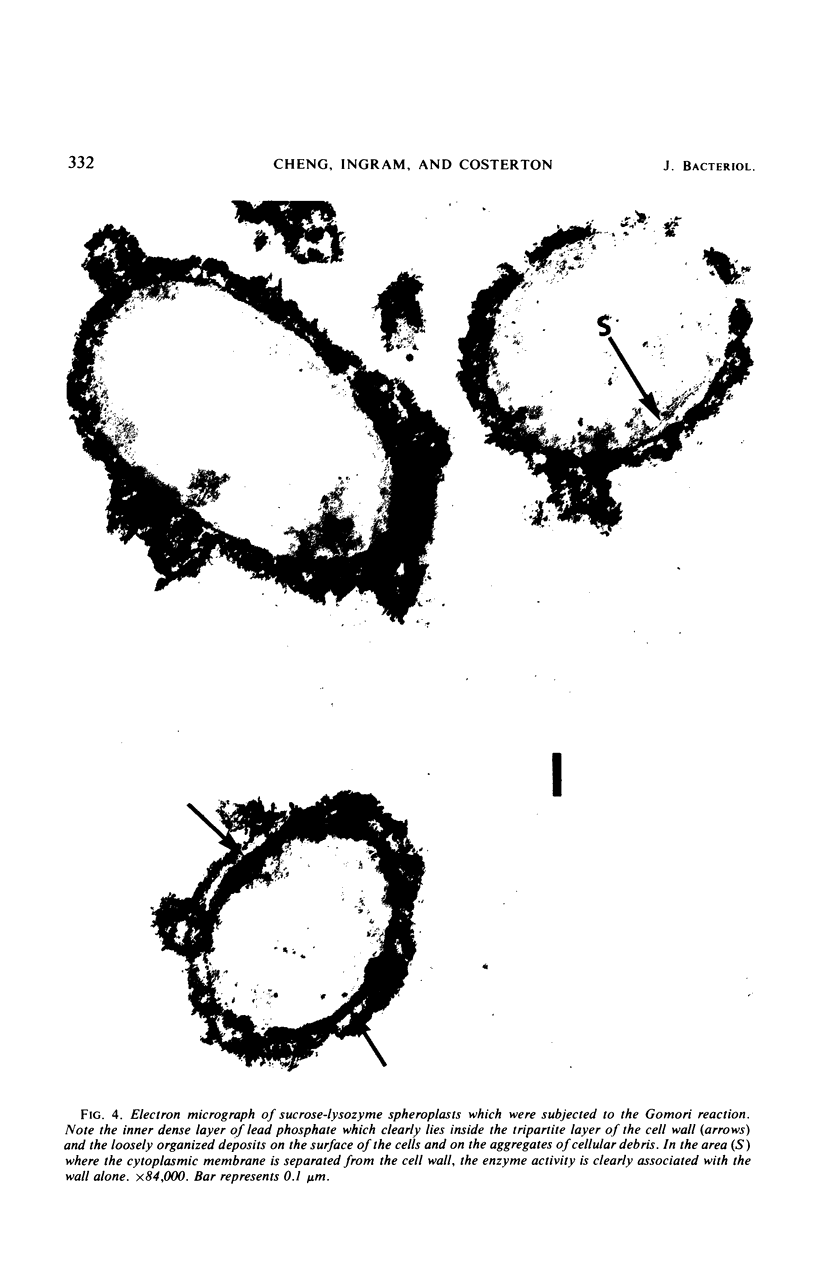
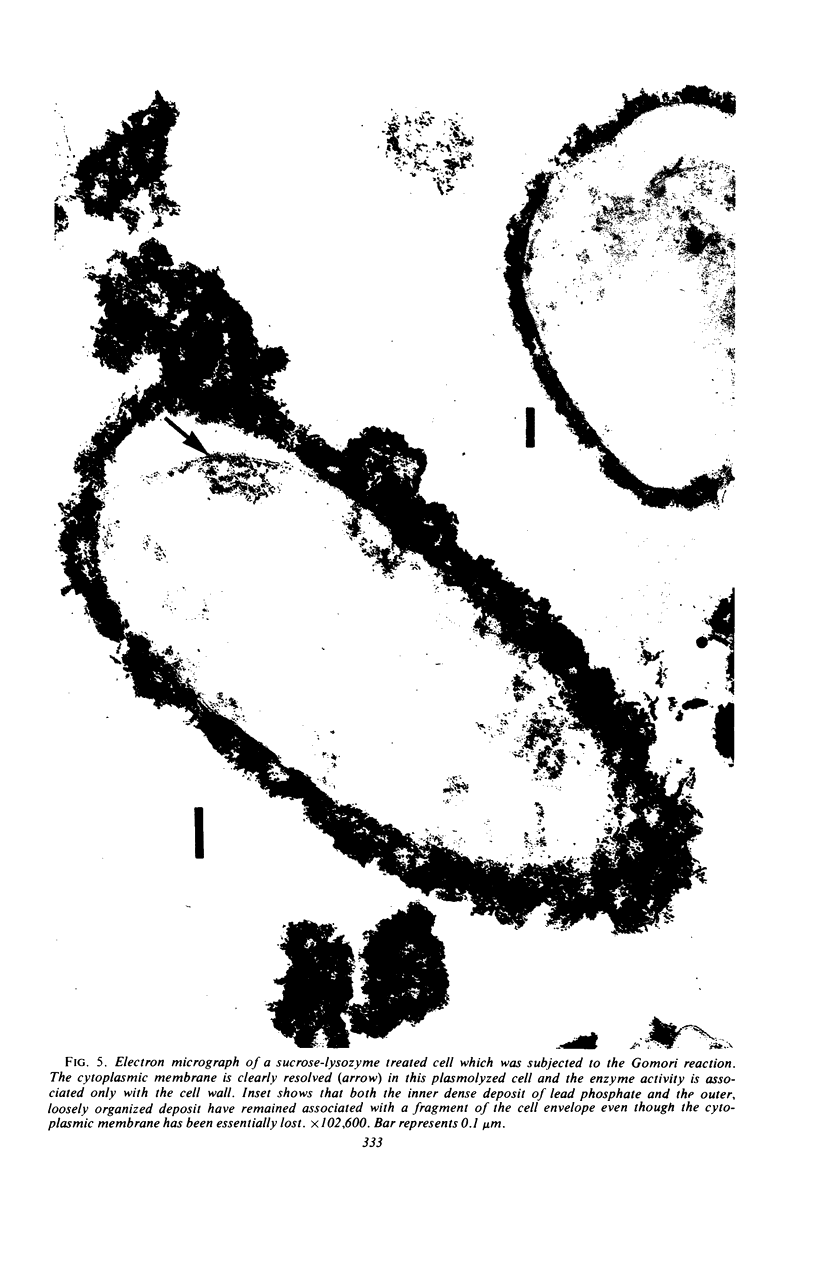
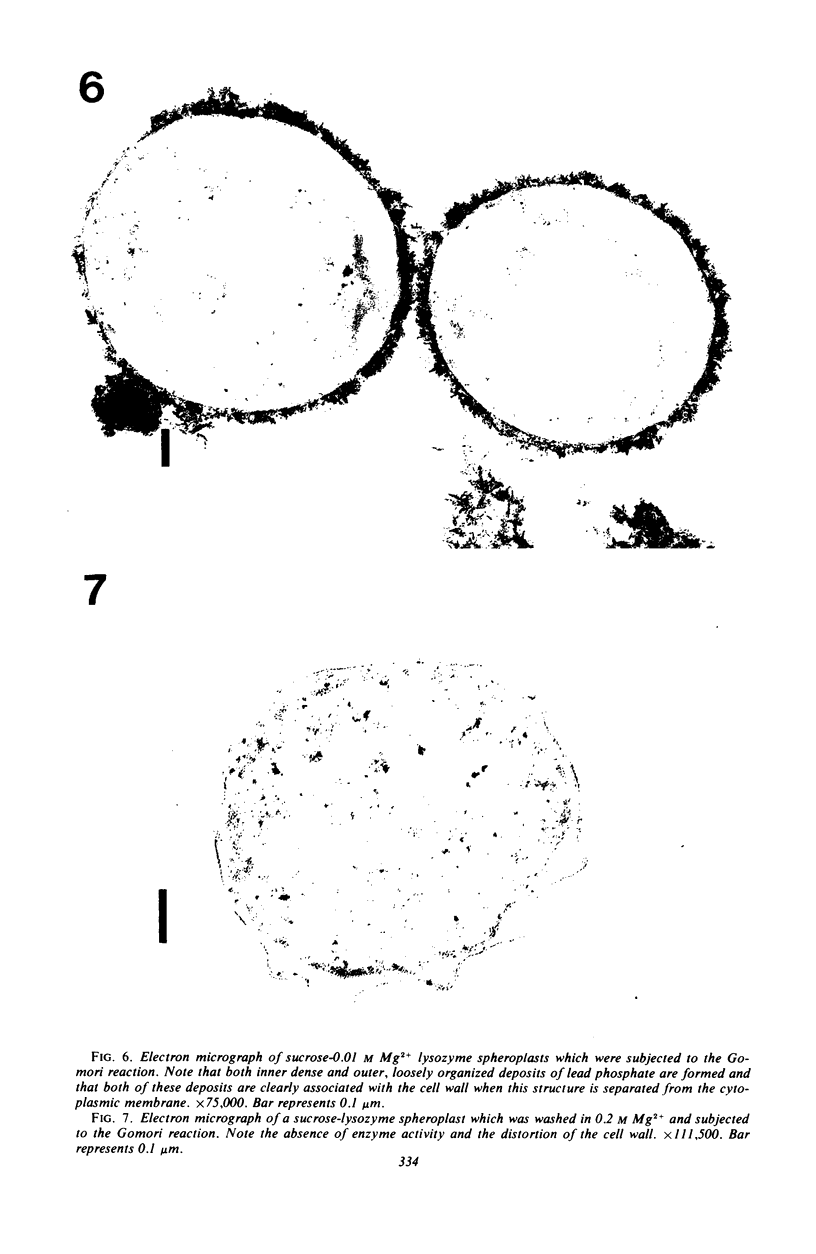
Spheroplasts prepared by lysozyme treatment of cells of Pseudomonas aeruginosa, suspended in 20% sucrose or 0.2 m MgCl2, were examined in detail. Preparation of spheroplasts in the presence of 0.2 m Mg2+ released periplasmic alkaline phosphatase, whereas preparation in the presence of 20% sucrose did not, even though untreated cells released phosphatase when suspended in sucrose in the absence of lysozyme. Biochemical characterizations of the sucrose-lysozyme preparations indicated that lysozyme mediated a reassociation of the released phosphatase with the spheroplasts. In addition, the enzyme released from whole cells suspended in 20% sucrose (which represents 20 to 40% of the cell-bound phosphatase) reassociates with the cells in the presence of lysozyme. Electron microscopic examinations of various preparations revealed that phosphatase released in sucrose reassociated with the external cell wall layers in the presence of lysozyme, that sucrose-lysozyme prepared spheroplasts did not dissociate phosphatase which remained in the periplasm of sucrose-washed cells, and that phosphatase was never observed to be associated with the cytoplasmic membrane. A model to account for the binding of P. aeruginosa alkaline phosphatase to the internal portion of the tripartite layer of the cell wall rather than to the cytoplasmic membrane or peptidoglycan layer is presented.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheng K. J., Ingram J. M., Costerton J. W. Alkaline phosphatase localization and spheroplast formation of Pseudomonas aeruginosa. Can J Microbiol. 1970 Dec;16(12):1319–1324. doi: 10.1139/m70-218. [DOI] [PubMed] [Google Scholar]
- Cheng K. J., Ingram J. M., Costerton J. W. Release of alkaline phosphatase from cells of Pseudomonas aeruginosa by manipulation of cation concentration and of pH. J Bacteriol. 1970 Nov;104(2):748–753. doi: 10.1128/jb.104.2.748-753.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGON R. G., CARSON K. J. LYSIS OF CELL WALLS AND INTACT CELLS OF PSEUDOMONAS AERUGINOSA BY ETHYLENEDIAMINE TETRAACETIC ACID AND BY LYSOZYME. Can J Microbiol. 1965 Apr;11:193–201. doi: 10.1139/m65-025. [DOI] [PubMed] [Google Scholar]
- MALAMY M. H., HORECKER B. L. RELEASE OF ALKALINE PHOSPHATASE FROM CELLS OF ESCHERICHIA COLI UPON LYSOZYME SPHEROPLAST FORMATION. Biochemistry. 1964 Dec;3:1889–1893. doi: 10.1021/bi00900a017. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Patterson D., Weinstein M., Nixon R., Gillespie D. Interaction of ribosomes and the cell envelope of Escherichia coli mediated by lysozyme. J Bacteriol. 1970 Feb;101(2):584–591. doi: 10.1128/jb.101.2.584-591.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiss J. W. The complexing of lysozyme with poly C and other homopolymers. Biophys J. 1968 Nov;8(11):1201–1210. doi: 10.1016/S0006-3495(68)86550-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessa G., Weissmann G. Incorporation of lysozyme into liposomes. A model for structure-linked latency. J Biol Chem. 1970 Jul 10;245(13):3295–3301. [PubMed] [Google Scholar]
- Wilson L. A., Spitznagel J. K. Molecular and structural damage to Escherichia coli produced by antibody, complement, and lysozyme systems. J Bacteriol. 1968 Oct;96(4):1339–1348. doi: 10.1128/jb.96.4.1339-1348.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]