Abstract
Purpose
Our recent studies show specific localization of long-circulating liposomes (LCL) within the endosomal/lysosomal compartment of tumor-associated macrophages (TAM). Based on this finding, the present study aims to investigate whether clinically applied LCL formulations such as Doxil (LCL-encapsulated doxorubicin), have alternative mechanisms of action additionally to direct drug-mediated cytotoxicity towards tumor cells.
Methods
The antitumor activity of Doxil was evaluated in B16.F10 melanoma-bearing mice, in the presence and in the absence of TAM. To suppress TAM functions, liposomal clodronate (Lip-CLOD) was injected 24 h before the actual treatment. The effect of Doxil on the levels of angiogenic factors was determined using an angiogenic protein array. As positive control, the same experiments were conducted with LCL-encapsulated prednisolone phosphate (LCL-PLP), a tumor-targeted formulation with known strong anti-angiogenic/anti-inflammatory effects on TAM.
Results
Our results show that the antitumor efficacy of Doxil was only partially attributed to the inhibition of TAM-mediated angiogenesis whereas LCL-PLP inhibited tumor growth through strong suppressive effects on pro-angiogenic functions of TAM. As described previously, the main mechanism of Doxil might be a cytotoxic effect on tumor cells.
Conclusions
Our findings suggest that the antitumor activity of Doxil does not depend mainly on the presence of functional TAM in tumors.
KEY WORDS: angiogenic proteins, doxil, tumor-associated macrophages, tumor cells
INTRODUCTION
Long-circulating liposomes (LCL) such as poly(ethylene glycol) (PEG)-coated liposomes, possess a passive tumor-targeting property (1,2). The long-circulation property of intravenously (i.v.) administered LCL enables to exploit the enhanced permeability of the tumor microcirculation for extravasation into the tumor. LCL appear to accumulate in the interstitium area surrounding capillaries and tend to be taken up by tumor-associated macrophages (TAM; 3). TAM play an essential role in processes that drive tumor angiogenesis and inflammation (4–6). As a result of the natural tropism of LCL for TAM, angiogenic and tumor-associated inflammatory processes are possibly significantly affected by properly designed LCL-encapsulated drugs. Our previous studies showed that prednisolone phosphate (PLP) encapsulated in LCL (LCL-PLP) exerts strong inhibitory effects on tumor growth via inhibition of tumor angiogenesis in subcutaneous (s.c.) B16.F10 melanoma and C26 colon carcinoma murine tumor models (3,7). Specific localization of LCL within the endosomal/lysosomal compartment of TAM has been observed (3). Recent results strongly suggest that LCL-PLP act via their uptake by TAM leading to suppression of TAM-mediated production of pro-angiogenic factors (7). This also raises the question whether clinically applied LCL formulations, such as Doxil™ (Caelyx™ in Europe) (PEG-liposomes with encapsulated doxorubicin), may have alternative mechanisms of action additionally to their direct drug-mediated cytotoxicity towards tumor cells (8). Therefore, this study aims to investigate whether the mechanism of antitumor action of Doxil involves an anti-angiogenic/anti-inflammatory activity resulting from modulatory effects on TAM functions in B16.F10 melanoma-bearing mice. It is even not excluded that intracellularly accumulating Doxil particles kill TAM, as it has been reported that doxorubicin-containing liposomes could efficiently deplete part of the liver macrophage population after i.v. administration to rats (9,10). To evaluate whether TAM play an important role in the antitumor action of Doxil, clodronate-containing LCL (mean size about 100 nm) were used as a tool to deplete macrophages (11,12). Clodronate-containing liposomes as macrophage-suppressive agents have already been used in inflammatory and auto-immune diseases, where macrophages have been suggested to play a critical role in pathological processes (13). To study the antitumor activity of Doxil towards tumors with suppressed TAM function, tumor-bearing animals were pretreated with clodronate-liposomes before the actual treatment with Doxil. In addition, the effect of Doxil treatment on the tumor levels of pro-angiogenic and anti-angiogenic factors was determined in B16.F10 melanoma-bearing mice with and without pretreatment with liposomal clodronate (Lip-CLOD). As positive control, the same experiments were conducted with LCL-PLP, a tumor-targeted formulation with known strong anti-angiogenic/anti-inflammatory effects on TAM (14).
MATERIALS AND METHODS
Preparation of LCL-PLP
LCL were prepared as described previously (3). In brief, appropriate amounts of dipalmitoylphosphatidylcholine (Lipoid GmbH, Ludwigshafen, Germany), cholesterol (Sigma, St. Louis, MO, USA), and poly(ethylene glycol) PEG2000-distearoylphosphatidylethanolamine (Lipoid GmbH) in a molar ratio of 1.85:1.0:0.15, respectively, were dissolved in ethanol in a round-bottom flask. After lipid film formation, the film was hydrated with a solution of 100 mg/ml prednisolone disodium phosphate (PLP), (obtained from Bufa, Uitgeest, The Netherlands). Liposome size was reduced by multiple extrusion steps through polycarbonate membranes (Nuclepore, Pleasanton, CA, USA) with a final pore size of 50 nm. Mean particle size of the LCL was determined by dynamic light scattering and found to be 0.1 μm with a polydispersity value lower than 0.1. The polydispersity values obtained indicate limited variation in particle size. Phospholipid content was determined with a phosphate assay, performed on the organic phase after extraction of liposomal preparations with chloroform, according to Rouser (15). Unencapsulated drug was removed by dialysis in a Slide-A-Lyzer cassette with a molecular weight cut-off of 10kDa at 4°C with repeated changes of buffer. After extraction, the aqueous phase was used for determining the glucocorticoid phosphate content by high performance liquid chromatography as described previously (16). The type of column was RP18 (5 μm; Merck) and the mobile phase consisted of acetonitril and water (1:3 v/v), pH 2. The eluent was monitored with an ultraviolet detector set at 254 nm. The detection limit for the high performance liquid chromatography setup was 20 ng/ml. The liposomal preparation contained about 5 mg PLP/ml and ∼60 μmol phospholipid/ml. The encapsulation efficiency of PLP in LCL was about 5% (80 μg PLP/μmol phospholipid). The drug release from LCL was about 15%, at 37°C in the presence of phosphate buffered saline (PBS) for 3 weeks.
Preparation of Clodronate-Containing Liposomes (Lip-CLOD)
To deplete TAM, clodronate-containing LCL (3,12; mean size about 0.1 μm and polydispersity value lower than 0.1) were prepared as described previously for LCL-PLP. After lipid film formation, the film was hydrated with a solution of dichloromethylene bisphosphonate, disodium clodronate (Bonefos™ infusion (conc. 60 mg/ml); obtained from Schering, Weesp, The Netherlands). To reduce recruitment of new monocytes in tumors, large negatively charged liposomes (mean size about 1 μm and polydispersity value lower than 0.35) were used (17). Appropriate amounts of egg phosphatidylcholine and egg phosphatidylglycerol (both obtained from Lipoid GmbH, Ludwigshafen, Germany) cholesterol (Sigma, St. Louis, MO, USA) in a molar ratio of 1.85:0.3:1 were dissolved in ethanol. The hydration of lipid film was performed with 10 ml of clodronate or Bonefos infusion (60 mg/ml). Liposomes were extruded twice through a filter with a pore size of 8 μm. Phospholipid content was determined with a phosphate assay, performed on the organic phase after extraction of liposomal preparations with chloroform, according to Rouser (15). Unencapsulated drug was removed by dialysis in a Slide-A-Lyzer cassette with a molecular weight cut-off of 10kDa at 4°C with repeated changes of buffer. After extraction, the aqueous phase was used for determining the clodronate content by ultraviolet spectrophotometry at 238 nm after formation of clodronate complex with CuSO4 solution (18). Both types of liposomes contained about 5 mg clodronate/ml and ∼70 μmol phospholipid/ml with an encapsulation efficiency of clodronate about 8% (70 μg clodronate/μmol phospholipid).
Cells
B16.F10 murine melanoma cells were cultured as monolayers at 37°C in a 5% CO2-containing humidified atmosphere in DMEM medium (Gibco, Breda, The Netherlands) supplemented with 10% (v/v) heat-inactivated fetal calf serum (Gibco), 100IU/ml penicillin, 100 μg/ml streptomycin and 0.25 μg/ml amphotericin B (Gibco).
Murine Tumor Model
Male C57Bl/6 mice (6–8 weeks of age) were obtained from Charles River (The Netherlands) and kept in standard housing with standard rodent chow and water available ad libitum, and a 12 h light/dark cycle. Experiments were performed according to the national regulations and were approved by the local animal experiments ethical committee. For tumor induction, 1 × 106 B16.F10 melanoma cells were inoculated s.c. in the right flank of syngeneic C57Bl/6 mice. B16.F10 tumors became palpable at day 7 after tumor cell inoculation.
Effect of Liposomal Clodronate Pretreatment on the Antitumor Activity of Doxil
At 7 days after tumor cell inoculation, tumor size was measured and tumor volume calculated according to the formula V = 0.52 × a 2 × b, in which a is the smallest and b, the largest superficial diameter (in millimeter). The effect of Doxil and free doxorubicin in presence or absence of liposomal clodronate pretreatment on the growth of B16.F10 melanoma in mice were studied. As positive control, the same experiments were conducted with LCL-PLP, a tumor-targeted formulation with known strong anti-angiogenic/anti-inflammatory effects on TAM (7,14,19). In addition, as shown previously (7), LCL-PLP exerts only minor cytotoxic effects on B16.F10 murine melanoma cells in vitro.
To suppress macrophages a mixture of both clodronate liposomes (ratio 1:1 (w/w); Lip-CLOD) at a dose of 25 mg/kg (20) was injected i.v. at day 7 (when tumors became palpable; 24 h before the actual treatments). Doxil and free doxorubicin were administered i.v. at a dose of 2 mg/kg at days 8 and 11 after tumor cell inoculation. LCL-PLP and free PLP at a dose of 20 mg/kg were injected i.v. using the same dosing schedule as for Doxil. This dosing schedule was selected based on our previous studies on the effects of LCL-PLP and Lip-CLOD on TAM (14). As controls, tumor-bearing mice treated with PBS which did not receive Lip-CLOD treatment were used. Four to five animals were used per experimental group. On day 12, mice were sacrificed and tumor volumes were measured.
Effect of Liposomal Clodronate Pretreatment on the Anti-Angiogenic Actions of Doxil
To assess the effect of Doxil on TAM-mediated production of angiogenic factors, the same experimental setup as described above for testing antitumor activity of Doxil was used. At day 12 after tumor cell inoculation, mice were sacrificed and tumors were isolated. A screening of angiogenic proteins present in tumor tissues was performed using an angiogenic protein array (RayBio® Mouse Angiogenic protein Antibody Array membranes 1.1 (RayBiotech Inc. Norcross, GA)), according to manufacturers instructions (21). Each membrane contains 24 types of primary antibodies against certain angiogenic proteins. The tumor tissues for each group were lysed in 30 min with cell lysis buffer (RayBiotech), containing protease inhibitor cocktail (Sigma). After obtaining the pooled tumor tissue lysates, the protein content of the lysates was determined according to Peterson (22). Subsequently, the array membrane was subjected to different incubation steps, each for 2 h at room temperature followed by five washing-steps. First, the array membrane was incubated with 250 μg of protein from tissue lysates. Each membrane was incubated with a mixture of secondary biotin-conjugated antibodies, after which membranes were incubated with HRP-conjugated streptavidin. Thereafter, membranes were incubated with a mixture of two detection buffers (RayBiotech) for 1 min. X-ray film was exposed to the membranes for 4 min and then the film was developed. The experiment was perfomed in duplicate. Protein levels were quantified measuring the color intensity of each spot using GelPro Analyzer software, version 3.1, in comparison to positive control spots already bound to the membrane. Angiogenic protein levels in tumors were expressed as percentage of the levels of the same proteins in tumors from mice treated with PBS. Four to five animals were used per experimental group. The final results represent mean ± SD of two measurements.
Statistical Analysis
Data from different experiments were reported as mean ± SD. For statistical analysis, Student’s t-test for independent means was used. A value of P < 0.05 was considered significant. The differences between the overall effects of different treatments on tumor growth were analyzed by one-way ANOVA with Dunnett’s Multiple Comparison Test. The differences between the effects of different treatments on angiogenic factor levels were analyzed by two-way ANOVA with Bonferroni correction for multiple comparisons using GraphPad Prism version 4.02 for Windows, GraphPad Software (San Diego, CA, USA).
RESULTS
Effect of Lip-CLOD Pretreatment on Antitumor Activity of Doxil
To investigate whether the antitumor activity of Doxil on B16.F10 melanoma model is dependent on the presence of TAM in tumor tissue, B16.F10 melanoma-bearing mice were pretreated with Lip-CLOD before i.v. administration of Doxil. The purpose of Lip-CLOD pretreatment is to create suppressed TAM functioning in the tumor before treatment with Doxil. To this end, we prepared a mixture of two types of clodronate liposomes (Lip-CLOD) in a ratio 1:1 (w/w). To deplete TAM, clodronate was encapsulated in LCL (mean size about 100 nm; 3,12). In addition, to reduce chemoattraction of new monocytes in tumors, clodronate-containing large negatively charged liposomes (mean size about 1 μm) were co-injected (17). Doxil treatment started 24 h after Lip-CLOD pretreatment and involved an i.v. dose of 2 mg/kg at days 8 and 11 after tumor cell inoculation. A separate positive control animal group was treated with LCL-PLP, as it has been shown recently that the antitumor activity of LCL-PLP is largely based on inhibition of TAM-associated angiogenesis and inflammation (14). LCL-PLP treatment (i.v. dose of 20 mg/kg at days 8 and 11 after tumor cell inoculation) started also 24 h after Lip-CLOD pretreatment. The antitumor activity of Doxil was compared to that of LCL-PLP, in the presence and in the absence of Lip-CLOD pretreatment (Figs. 1 and 2).
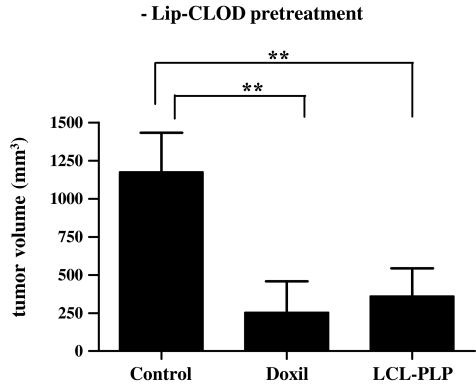
Fig. 1.
Antitumor activity of Doxil and LCL-PLP in B16.F10 murine melanoma model when animals were not pretreated with Lip-CLOD. Tumor volumes at day 12 (day of sacrifice) were compared to volumes of tumors from mice treated only with PBS. One-way ANOVA with Dunnett’s Multiple Comparison Test was used; ** P < 0.01. The results represent mean ± SD of 4–5 mice. −Lip-CLOD = no pretreatment with Lip-CLOD, Control = treatment with PBS, Doxil = treatment with Doxil, LCL-PLP = treatment with LCL-PLP.
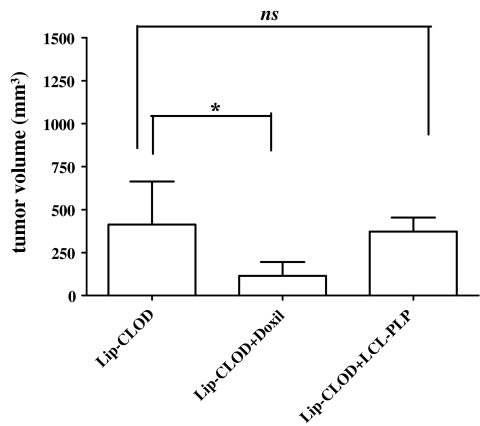
Fig. 2.
Effect of Lip-CLOD pretreatment on antitumor activity of Doxil and LCL-PLP in murine B16.F10 melanoma model. All groups were pretreated with Lip-CLOD 24 h before the actual treatment. Tumor volumes at day 12 (day of sacrifice) were compared to tumor volumes in mice treated with PBS. One-way ANOVA with Dunnett’s Multiple Comparison Test was used; ns, not significant (P > 0.05); * P < 0.05. The results represent mean ± SD of 4–5 mice. Lip-CLOD = treatment only with Lip-CLOD, Lip-CLOD + Doxil = pretreatment with Lip-CLOD followed by Doxil treatment, Lip-CLOD + LCL-PLP = pretreatment with Lip-CLOD followed by LCL-PLP treatment.
When Lip-CLOD pretreatment was not given, Doxil treatment alone inhibited tumor growth by 80% (P < 0.01) compared to the growth of control tumors (tumors in mice receiving only PBS). Similar inhibition of tumor growth (by 70%, P < 0.01) was induced by LCL-PLP treatment (Fig. 1).
Lip-CLOD alone (i.e. not followed by Doxil or LCL-PLP treatment) inhibited tumor growth by 65% (P < 0.05) compared to control tumors. When Lip-CLOD administration is followed by LCL-PLP treatment, no additional growth inhibitory effect was seen (Fig. 2). When Lip-CLOD pretreatment is followed by Doxil treatment, clearly Doxil had a strong additional antitumor effect (by 73% tumor growth inhibition P = 0.0436) when compared to that induced by Lip-CLOD-treated animals (Fig. 2). The combination treatment with Doxil and Lip-CLOD is 3.5-fold more effective than Lip-CLOD treatment alone.
Effect of Doxil on Angiogenic Protein Production; Influence of Lip-CLOD Pretreatment
To assess the effect of Doxil on angiogenic protein production in the B16.F10 melanoma model, with and without Lip-CLOD pretreatment, angiogenic protein levels in tumor tissue were studied. A screening of 24 angiogenic proteins involved in angiogenesis, inflammation and apoptosis present in tumor tissue was performed using an angiogenic protein array of RayBio® Mouse Angiogenic protein Antibody Array membranes 1.1 (RayBiotech Inc., Norcross, GA, USA; 21). The effect of TAM suppression on the production of angiogenic factors was verified in tumors from mice which were treated with Lip-CLOD alone (i.e. not followed by Doxil or LCL-PLP administration). Again LCL-PLP was used as positive control, as this tumor-targeted formulation has strong reducing effects on production of pro-angiogenic/pro-inflammatory factors in tumors (7). When Lip-CLOD pretreatment was not administered, Doxil reduced the level of the majority of intratumoral pro-angiogenic factors only slightly (Table I and Fig. 3). The strongest reduction after Doxil treatment was noted for eotaxin, Fas ligand (FasL), and vascular endothelial growth factor (VEGF; by 50–70%).
Table I.
Effects of i.v. Administered Doxil and LCL-PLP on Pro-Angiogenic Protein Levels in s.c. B16.F10 Melanoma when Lip-CLOD Pretreatment was not given
| Pro-angiogenic factors | Reduction induced by Doxil (% of reduction as mean ± SD) | Reduction induced by LCL-PLP (% of reduction as mean ± SD) | Statistical differences |
|---|---|---|---|
| Granulocyte-colony stimulating factor (G-CSF) | 42.6 ± 4.0 | 85.2 ± 1.1 | *** |
| Granulocyte-macrophage- colony stimulating factor (GM-CSF) | 7.3 ± 2.0 | 73.4 ± 3.0 | *** |
| Monocyte-colony stimulating factor (M-CSF) | 8.0 ± 1.0 | 50.0 ± 0.1 | *** |
| Insulin growth factor II (IGF-II) | 24.5 ± 5.3 | 70.5 ± 0.7 | *** |
| Interleukin 1α (IL-1α) | 34.3 ± 0.0 | 69.1 ± 0.6 | *** |
| Interleukin 1β (IL-1β) | 17.2 ± 6.4 | 76.2 ± 5.2 | *** |
| Interleukin 6 (IL-6) | 21.5 ± 9.2 | 69.9 ± 1.2 | *** |
| Interleukin 9 (IL-9) | 15.5 ± 2.4 | 47.4 ± 9.2 | *** |
| Interleukin 12p40 (IL-12 p40) | 8.9 ± 1.6 | 63.0 ± 1.3 | *** |
| Tumor necrosis factor α (TNF α) | 37.3 ± 2.3 | 22.0 ± 9.8 | ** |
| Monocyte chemoattractant protein-1 (MCP1) | 6.3 ± 1.2 | 47.6 ± 0.2 | *** |
| Eotaxin | 55.5 ± 2.6 | 96.4 ± 5.1 | *** |
| Fas ligand (FasL) | 66.1 ± 8.3 | 86.0 ± 8.9 | *** |
| Basic fibroblast growth factor (bFGF) | 39.7 ± 3.5 | 52.6 ± 1.8 | * |
| Vascular endothelial growth factor (VEGF) | 62.5 ± 0.5 | 7.0 ± 4.3 | *** |
| Leptin | 24.7 ± 0.7 | 14.9 ± 0.4 | ns |
| Thrombopoietin (TPO) | 38.0 ± 0.9 | 3.3 ± 2.0 | *** |
Pro-angiogenic factors are defined as proteins reported in literature to favor angiogenesis and tumor-associated inflammation. The protein levels are compared to protein levels in control tumors (tumors from mice treated with PBS when the Lip-CLOD pretreatment was not given). The results were analyzed for statistically significant differences between the effects of Doxil and LCL-PLP on the levels of pro-angiogenic factors. A two-way ANOVA with Bonferroni correction for multiple comparisons was used. The results represent mean ± SD of two measurements
ns Not significant (P > 0.05)
*P < 0.05, **P < 0.01, *** P < 0.001
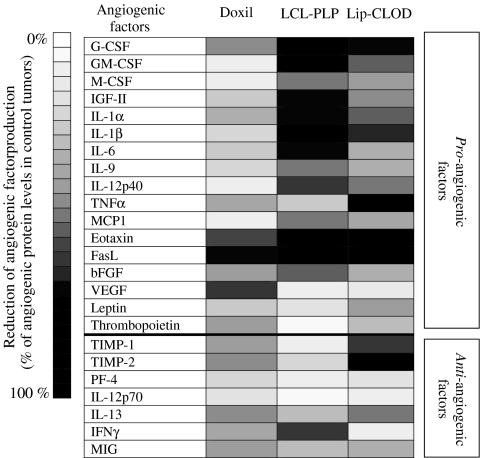
Fig. 3.
Comparison of the anti-angiogenic actions of Doxil, LCL-PLP, and Lip-CLOD in murine B16.F10 melanoma model. Results are presented as % reduction of the levels of tumor angiogenic factors ranging from 0% (white) to 100% (black) compared to the level of angiogenic factors in control tumors. Control tumors are defined as tumors from mice treated only with PBS. Doxil = treatment only with Doxil; LCL-PLP = treatment only with LCL-PLP; Lip-CLOD = treatment only with Lip-CLOD.
The production of most of the anti-angiogenic factors in tumors was also only slightly affected by the Doxil treatment (Table II and Fig. 3).
Table II.
Effects of i.v. Administered Doxil and LCL-PLP on Anti-Angiogenic Protein Levels in s.c. B16.F10 Melanoma when Lip-CLOD Pretreatment was not given
| Anti-angiogenic factors | Reduction induced by Doxil (% of reduction as mean ± SD) | Reduction induced by LCL-PLP (% of reduction as mean ± SD) | Statistical differences |
|---|---|---|---|
| Tissue inhibitor of metalloproteinase 1 (TIMP-1) | 38.1 ± 7.4 | 8.4 ± 1.5 | *** |
| Tissue inhibitor of metalloproteinase 2 (TIMP-2) | 40.5 ± 2.5 | 17.6 ± 1.2 | *** |
| Platelet factor 4 (PF4) | 19.4 ± 0.8 | 6.7 ± 2.5 | ns |
| Interleukin 12 p70 (IL-12 p70) | 14.2 ± 1.0 | 4.3 ± 0.1 | ns |
| Interleukin 13 (IL-13) | 40.5 ± 2.5 | 27.0 ± 4.3 | * |
| Interferon γ (IFN-γ) | 36.0 ± 1.0 | 60.2 ± 3.6 | *** |
| Monokine induced by IFN-γ (MIG) | 38.7 ± 0.6 | 25.6 ± 4.4 | * |
The anti-angiogenic factors are defined as proteins reported in literature to impede angiogenesis and tumor-associated inflammation. The protein levels are compared to protein levels in control tumors (tumors from mice treated with PBS when the Lip-CLOD pretreatment was not given). The results were analyzed for statistically significant differences between the effects of Doxil and LCL-PLP on the levels of anti-angiogenic factors. A two-way ANOVA with Bonferroni correction for multiple comparisons was used. The results represent mean ± SD of two measurements
ns Not significant (P > 0.05)
*P < 0.05, *** P < 0.001
The LCL-PLP formulation, however, exerted strong reducing effects on the intratumoral pro-angiogenic protein production, in the absence of Lip-CLOD pretreatment (Fig. 3). More specifically, LCL-PLP treatment reduced expression of the pro-angiogenic factors GM-CSF, M-CSF, IGF-II, IL-1α, IL-6, IL-12p40, bFGF (by 50–75%), and G-CSF, IL-1β, eotaxin, FasL (by 75–100%; Table I). The reduction exerted by LCL-PLP on most of the pro-angiogenic proteins was much stronger (P = 0.0086) when compared to the result obtained with Doxil (Fig. 3). The level of the majority of anti-angiogenic proteins was slightly suppressed after LCL-PLP (Table II and Fig. 3). Only the intratumoral production of IFNγ was strongly reduced (by 60%) by LCL-PLP treatment.
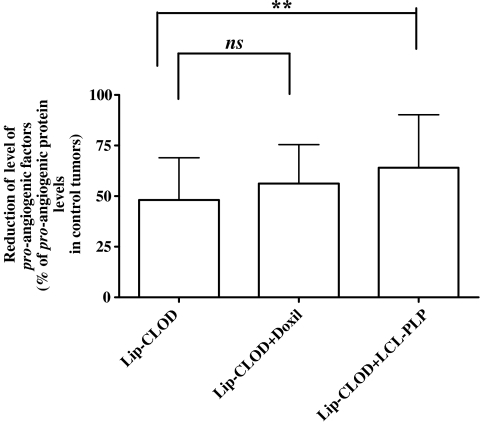
Lip-CLOD treatment alone strongly reduced the level of most of the pro-angiogenic tumor proteins (on average by 50%, P < 0.0001 compared to control tumors; Figs. 3 and 4). Interestingly, the level of two anti-angiogenic factors (TIMP-1 and TIMP-2) was also strongly reduced (about 60–100%, P < 0.0001; Fig. 3). When Lip-CLOD pretreatment was given, Doxil treatment had no additional reducing effects on pro-angiogenic (Fig. 4) and anti-angiogenic factor levels (data not shown). However, when Lip-CLOD pretreatment was followed by treatment with the positive control, LCL-PLP formulation, the reduction of pro-angiogenic protein production was somewhat enhanced (by 16%, P = 0.0001; Fig. 4). No additional effect of LCL-PLP on the level of anti-angiogenic tumor proteins was seen (data not shown).
Fig. 4.
The influence of Lip-CLOD pretreatment on the effect of Doxil and LCL-PLP on production of pro-angiogenic factors in tumors. Results are presented as % average reduction of the level of tumor pro-angiogenic factors compared to the level of pro-angiogenic factors in control tumors. Control tumors are defined as tumors from mice not treated with Lip-CLOD but treated with PBS. Mean ± SD; n = 17 pro-angiogenic factor levels determined in duplicate per experimental group, Lip-CLOD = treatment only with Lip-CLOD, Lip-CLOD + Doxil = pretreatment with Lip-CLOD followed by Doxil treatment, Lip-CLOD + LCL-PLP = pretreatment with Lip-CLOD followed by LCL-PLP treatment. One-way ANOVA with Dunnett’s Multiple Comparison Test was used; ns not significant (P > 0.05), and** P < 0.01.
DISCUSSION
Doxil is a commercially available LCL formulation containing the well-known antitumor agent, doxorubicin. It has been shown to significantly enhance the doxorubicin levels in the tumor and thereby antitumor activity, in various mouse and human xenograft tumor models (10,23–26). This study aimed to investigate whether the mechanism of the antitumor activity of Doxil involves inhibition of tumor angiogenesis through suppressive and possibly even lethal effects on TAM, as i.v. administered LCL extravasating in solid tumors have been shown to substantially localize in TAM (3).
The anti-angiogenic actions of Doxil were compared with those induced by LCL-PLP, a tumor-targeted formulation with known strong anti-angiogenic/anti-inflammatory activity in tumors (7,19). Recent studies reported that i.v. administration of LCL-PLP results in strong inhibition of tumor growth (3). The mechanism of antitumor action of LCL-PLP appeared to be primarily based on a reduction of intratumoral level of pro-angiogenic factors (7). The anti-angiogenic effects exerted by LCL-PLP are enabled by the tumor-targeting capability of LCL, which is a combined result of the long circulation time of the liposomal formulation and the enhanced permeability of tumor vasculature as compared to healthy endothelium (7,27). LCL can extravasate through the permeable pathological vasculature and thereby accumulate into the malignant tissue (referred to as the “enhanced permeability and retention (EPR) effect”; 19). Once extravasated into the tumor, LCL were observed to localize in the immediate vicinity of tumor blood vessels and in the endosomal/lysosomal compartment of TAM (3). It is known that TAM have a main role in tumor growth progression, being an important source of inflammatory and angiogenic factors involved in all steps of tumor angiogenesis (11,27–30).
To investigate whether Doxil in addition to direct cytotoxic effects on tumor cells, also exerts antitumor activity via suppression of TAM, we investigated the effect of pretreatment with Lip-CLOD (12,31,32) on the antitumor activity of Doxil. Previous studies already showed the feasibility of clodronate encapsulated in liposomes for suppression of TAM activity from s.c. tumor tissue (11). Lip-CLOD treatment alone strongly inhibited tumor growth. Furthermore, Lip-CLOD induced strong reduction of the production of most of the pro-angiogenic factors as well as of certain anti-angiogenic factors (Fig. 3). These results clearly suggest that TAM play a vital role in tumor growth by producing angiogenic factors critical for tumor growth progression.
In the absence of Lip-CLOD pretreatment, both Doxil and LCL-PLP exerted strong tumor growth inhibitory effects. Tumor growth was inhibited by 70–80% compared to the growth of control tumors (Fig. 1).
In the presence of Lip-CLOD pretreatment, a significantly stronger antitumor effect was observed after Doxil administration. An additional antitumor effect was not observed in case of LCL-PLP treatment (Fig. 2). With Lip-CLOD already establishing antitumor activity via TAM suppression, the lack of any additional effect confered by the subsequent LCL-PLP treatment suggests that the LCL-PLP localizing in the tumor area is not able to further downregulate the functioning of the already suppressed TAM, illustrating the effectiveness of the Lip-CLOD treatment. The observation though that Doxil is able to induce additional tumor growth inhibition, would indicate that its antitumor activity does not depend on the presence of functional TAM in tumor tissue, and that Doxil is killing tumor cells via direct cytotoxic effects of doxorubicin on the tumor cells (33–35).
This suggestion based on the tumor growth inhibition results is confirmed by the results obtained at the level of the intratumoral production of angiogenic proteins. Lip-CLOD treatment alone appeared to strongly reduce the production of particularly the pro-angiogenic factors, which is in good agreement with its potent antitumor activity (Fig. 3). As it was shown previously (7,14) LCL-PLP treatment alone induces a similar strong degree of suppression, albeit the intensity of the suppressive effect of both formulations varies with the type of angiogenic factor. If the LCL-PLP is administered after the anti-angiogenic Lip-CLOD treatment, only a slight additive effect was seen, which is in line with the similar anti-angiogenic mode of action of LCL-PLP via suppressive effects on TAM (Fig. 4). Doxil, however, was much less effective in reducing the angiogenic protein levels as compared to LCL-PLP and Lip-CLOD, suggesting that the strong antitumor activity of Doxil is not mediated by TAM-related effects, although the mild degree of suppression of angiogenic factor production observed might have been caused by Doxil particles localizing in TAM and inhibiting their function. That Doxil mainly acts via direct cytotoxic effects on tumor cells, is indicated by the observation that only Doxil treatment was able to strongly reduce the intratumoral level of VEGF, a key angiogenic protein produced in high amounts by melanoma cells (36). Both the anti-angiogenic LCL-PLP and Lip-CLOD formulations did not show this reducing effect on VEGF, supporting that both formulations lack direct cytotoxic effects on melanoma cells.
Although localization of extravasated Doxil particles in TAM is a realistic possibility, the antitumor activity is likely for a large part based on other mechanisms. Doxorubicin may be released from extracellularly localized Doxil particles and subsequently entering tumor cells (37,38). In addition, Doxil particles are likely being taken up by TAM (3). Intracellular processing of Doxil particles within TAM involves degradation of the LCL bilayers within the endosomal/lysosomal compartment. This degradation process likely leads to liberation of doxorubicin molecules within TAM (33). As doxorubicin molecules have been reported to be chemically stable in the harsh environment encountered, they may pass cellular membranes, and act intracellularly by inhibiting the functionality and even viability of TAM explaining the observed mild suppressive effects on the production of angiogenic proteins. Alternatively, the liberated doxorubicin molecules may be released in the extracellular tumor interstitium, followed by passive diffusion into tumor cells, and in this way contributing to the cytotoxicity of Doxil towards tumor cells (33–35).
CONCLUSION
The present data suggest that the antitumor activity of Doxil in the B16.F10 melanoma tumor model is not dependent on the presence of functional TAM in tumor tissue.
Acknowledgments
The authors would like to thank Marcel Fens for his help with animal studies.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- ANOVA
analysis of variance
- bFGF
basic fibroblast growth factor
- EPR
enhanced permeability and retention
- FasL
Fas ligand
- GC
glucocorticoids
- G-CSF
granulocyte-colony stimulating factor
- GM-CSF
granulocyte-macrophage-colony stimulating factor
- IFN-γ
Interferon γ
- IGF-II
Insulin growth factor II
- IL-1α
Interleukin 1α
- IL-1β
Interleukin 1β
- IL-6
Interleukin 6
- IL-9
Interleukin 9
- IL-12 p40
Interleukin 12 p40
- IL-12 p70
Interleukin 12 p70
- IL-13
Interleukin 13
- i.v.
intravenous administration
- LCL
long-circulating liposomes
- LCL-PLP
long-circulating liposome-encapsulated prednisolone disodium phosphate
- Lip-CLOD
a mixture of two types of clodronate liposomes: clodronate-containing LCL and clodronate-containing large negatively charged liposomes (ratio 1:1 (w/w))
- MCP1
monocyte chemoattractant protein-1
- M-CSF
monocyte-colony stimulating factor
- MIG
monokine induced by IFN-γ
- PBS
phosphate buffered saline
- PEG
Poly(ethylene glycol)
- PF4
platelet factor 4
- PLP
prednisolone disodium phosphate
- s.c.
subcutaneous administration
- SD
standard deviation
- TAM
tumor-associated macrophages
- TIMP-1
tissue inhibitor of metalloproteinase 1
- TIMP-2
tissue inhibitor of metalloproteinase 2
- TNF α
tumor necrosis factor α
- TPO
thrombopoietin
- VEGF
vascular endothelial growth factor
References
- 1.Storm G., Crommelin D. J. Colloidal systems for tumor targeting. Hybridoma. 1997;16:119–125. doi: 10.1089/hyb.1997.16.119. [DOI] [PubMed] [Google Scholar]
- 2.Gabizon A. A. Stealth liposomes and tumor targeting: one step further in the quest for the magic bullet. Clin Cancer Res. 2001;7:223–225. [PubMed] [Google Scholar]
- 3.Schiffelers R. M., Metselaar J. M., Fens M. H., Janssen A. P., Molema G., Storm G. Liposome-encapsulated prednisolone phosphate inhibits growth of established tumors in mice. Neoplasia. 2005;7:118–127. doi: 10.1593/neo.04340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salcedo R., Young H. A., Ponce M. L., Ward J. M., Kleinman H. K., Murphy W. J., Oppenheim J. J. Eotaxin (CCL11) induces in vivo angiogenic responses by human CCR3+ endothelial cells. J Immunol. 2001;166:7571–7578. doi: 10.4049/jimmunol.166.12.7571. [DOI] [PubMed] [Google Scholar]
- 5.Reale M., Intorno R., Tenaglia R., Feliciani C., Barbacane R. C., Santoni A., Conti P. Production of MCP-1 and RANTES in bladder cancer patients after bacillus Calmette-Guerin immunotherapy. Cancer Immunol Immunother. 2002;51:91–98. doi: 10.1007/s00262-001-0254-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biancone L., Martino A. D., Orlandi V., Conaldi P. G., Toniolo A., Camussi G. Development of inflammatory angiogenesis by local stimulation of Fas in vivo. J Exp Med. 1997;186:147–152. doi: 10.1084/jem.186.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banciu M., Schiffelers R. M., Fens M. H., Metselaar J. M., Storm G. Anti-angiogenic effects of liposomal prednisolone phosphate on B16 melanoma in mice. J Control Release. 2006;113:1–8. doi: 10.1016/j.jconrel.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 8.Tejada-Berges T., Granai C. O., Gordinier M., Gajewski W. Caelyx/Doxil for the treatment of metastatic ovarian and breast cancer. Expert Rev Anticancer Ther. 2002;2:143–150. doi: 10.1586/14737140.2.2.143. [DOI] [PubMed] [Google Scholar]
- 9.Daemen T., Hofstede G., Ten Kate M. T., Bakker-Woudenberg I. A., Scherphof G. L. Liposomal doxorubicin-induced toxicity: depletion and impairment of phagocytic activity of liver macrophages. Int J Cancer. 1995;61:716–721. doi: 10.1002/ijc.2910610520. [DOI] [PubMed] [Google Scholar]
- 10.Storm G., ten Kate M. T., Working P. K., Bakker-Woudenberg I. A. Doxorubicin entrapped in sterically stabilized liposomes: effects on bacterial blood clearance capacity of the mononuclear phagocyte system. Clin Cancer Res. 1998;4:111–115. [PubMed] [Google Scholar]
- 11.Zeisberger S. M., Odermatt B., Marty C., Zehnder-Fjallman A. H., Ballmer-Hofer K., Schwendener R. A. Clodronate-liposome-mediated depletion of tumour-associated macrophages: a new and highly effective antiangiogenic therapy approach. Br J Cancer. 2006;95:272–281. doi: 10.1038/sj.bjc.6603240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oussoren C., Storm G. Role of macrophages in the localisation of liposomes in lymph nodes after subcutaneous administration. Int J Pharm. 1999;183:37–41. doi: 10.1016/S0378-5173(99)00040-X. [DOI] [PubMed] [Google Scholar]
- 13.van Rooijen N., van Kesteren-Hendrikx E. Clodronate liposomes: perspectives in research and therapeutics. J Liposome Res. 2002;12:81–94. doi: 10.1081/LPR-120004780. [DOI] [PubMed] [Google Scholar]
- 14.Banciu M., Metselaar J. M., Schiffelers R. M., Storm G. Antitumor activity of liposomal prednisolone phosphate depends on the presence of functional tumor-associated macrophages in tumor tissue. Neoplasia. 2008;10:108–117. doi: 10.1593/neo.07913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rouser G., Fkeischer S., Yamamoto A. Two dimensional thin layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 1970;5:494–496. doi: 10.1007/BF02531316. [DOI] [PubMed] [Google Scholar]
- 16.Metselaar J. M., Wauben M. H., Wagenaar-Hilbers J. P., Boerman O. C., Storm G. Complete remission of experimental arthritis by joint targeting of glucocorticoids with long-circulating liposomes. Arthritis Rheum. 2003;48:2059–2066. doi: 10.1002/art.11140. [DOI] [PubMed] [Google Scholar]
- 17.Van Rooijen N., Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 18.Perugini P., Genta I., Conti B., Modena T., Pavanetto F. Long-term release of clodronate from biodegradable microspheres. AAPS PharmSciTech. 2001;2:E10. doi: 10.1208/pt020310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schiffelers R. M., Banciu M., Metselaar J. M., Storm G. Therapeutic application of long-circulating liposomal glucocorticoids in auto-immune diseases and cancer. J Liposome Res. 2006;16:185–194. doi: 10.1080/08982100600851029. [DOI] [PubMed] [Google Scholar]
- 20.Rozemuller H., Knaan-Shanzer S., Hagenbeek A., van Bloois L., Storm G., Martens A. C. Enhanced engraftment of human cells in RAG2/gammac double-knockout mice after treatment with CL2MDP liposomes. Exp Hematol. 2004;32:1118–1125. doi: 10.1016/j.exphem.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Huang R. P. Detection of multiple proteins in an antibody-based protein microarray system. J Immunol Methods. 2001;255:1–13. doi: 10.1016/S0022-1759(01)00394-5. [DOI] [PubMed] [Google Scholar]
- 22.Peterson G. L. Determination of total protein. Methods Enzymol. 1983;91:95–119. doi: 10.1016/S0076-6879(83)91014-5. [DOI] [PubMed] [Google Scholar]
- 23.Vaage J., Mayhew E., Lasic D., Martin F. Therapy of primary and metastatic mouse mammary carcinomas with doxorubicin encapsulated in long circulating liposomes. Int J Cancer. 1992;51:942–948. doi: 10.1002/ijc.2910510618. [DOI] [PubMed] [Google Scholar]
- 24.Huang S. K., Mayhew E., Gilani S., Lasic D. D., Martin F. J., Papahadjopoulos D. Pharmacokinetics and therapeutics of sterically stabilized liposomes in mice bearing C-26 colon carcinoma. Cancer Res. 1992;52:6774–6781. [PubMed] [Google Scholar]
- 25.Mayhew E. G., Lasic D., Babbar S., Martin F. J. Pharmacokinetics and antitumor activity of epirubicin encapsulated in long-circulating liposomes incorporating a polyethylene glycol-derivatized phospholipid. Int J Cancer. 1992;51:302–309. doi: 10.1002/ijc.2910510221. [DOI] [PubMed] [Google Scholar]
- 26.Vaage J., Barbera-Guillem E., Abra R., Huang A., Working P. Tissue distribution and therapeutic effect of intravenous free or encapsulated liposomal doxorubicin on human prostate carcinoma xenografts. Cancer. 1994;73:1478–1484. doi: 10.1002/1097-0142(19940301)73:5<1478::AID-CNCR2820730526>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 27.Crowther M., Brown N. J., Bishop E. T., Lewis C. E. Microenvironmental influence on macrophage regulation of angiogenesis in wounds and malignant tumors. J Leukoc Biol. 2001;70:478–490. [PubMed] [Google Scholar]
- 28.Bingle L., Brown N. J., Lewis C. E. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 29.Luo Y., Zhou H., Krueger J., Kaplan C., Lee S. H., Dolman C., Markowitz D., Wu W., Liu C., Reisfeld R. A., Xiang R. Targeting tumor-associated macrophages as a novel strategy against breast cancer. J Clin Invest. 2006;116:2132–2141. doi: 10.1172/JCI27648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin E. Y., Li J. F., Gnatovskiy L., Deng Y., Zhu L., Grzesik D. A., Qian H., Xue X. N., Pollard J. W. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 2006;66:11238–11246. doi: 10.1158/0008-5472.CAN-06-1278. [DOI] [PubMed] [Google Scholar]
- 31.Buiting A. M., Van Rooijen N. Liposome mediated depletion of macrophages: an approach for fundamental studies. J Drug Target. 1994;2:357–362. doi: 10.3109/10611869408996810. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt-Weber C. B., Rittig M., Buchner E., Hauser I., Schmidt I., Palombo-Kinne E., Emmrich F., Kinne R. W. Apoptotic cell death in activated monocytes following incorporation of clodronate-liposomes. J Leukoc Biol. 1996;60:230–244. doi: 10.1002/jlb.60.2.230. [DOI] [PubMed] [Google Scholar]
- 33.Storm G., Steerenberg P. A., Emmen F., van Borssum Waalkes M., Crommelin D. J. Release of doxorubicin from peritoneal macrophages exposed in vivo to doxorubicin-containing liposomes. Biochim Biophys Acta. 1988;965:136–145. doi: 10.1016/0304-4165(88)90049-9. [DOI] [PubMed] [Google Scholar]
- 34.Gabizon A., Shmeeda H., Barenholz Y. Pharmacokinetics of pegylated liposomal Doxorubicin: review of animal and human studies. Clin Pharmacokinet. 2003;42:419–436. doi: 10.2165/00003088-200342050-00002. [DOI] [PubMed] [Google Scholar]
- 35.Fornari F. A., Randolph J. K., Yalowich J. C., Ritke M. K., Gewirtz D. A. Interference by doxorubicin with DNA unwinding in MCF-7 breast tumor cells. Mol Pharmacol. 1994;45:649–656. [PubMed] [Google Scholar]
- 36.Torisu H., Ono M., Kiryu H., Furue M., Ohmoto Y., Nakayama J., Nishioka Y., Sone S., Kuwano M. Macrophage infiltration correlates with tumor stage and angiogenesis in human malignant melanoma: possible involvement of TNFalpha and IL-1alpha. Int J Cancer. 2000;85:182–188. [PubMed] [Google Scholar]
- 37.Woodle M. C., Storm G., Newman M. S., Jekot J. J., Collins L. R., Martin F. J., Szoka F. C., Jr. Prolonged systemic delivery of peptide drugs by long-circulating liposomes: illustration with vasopressin in the Brattleboro rat. Pharm Res. 1992;9:260–265. doi: 10.1023/A:1018953810705. [DOI] [PubMed] [Google Scholar]
- 38.Symon Z., Peyser A., Tzemach D., Lyass O., Sucher E., Shezen E., Gabizon A. Selective delivery of doxorubicin to patients with breast carcinoma metastases by stealth liposomes. Cancer. 1999;86:72–78. doi: 10.1002/(SICI)1097-0142(19990701)86:1<72::AID-CNCR12>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]