Abstract
N-acetylaspartate/creatine (NAA/Cr) ratios, assessed with proton magnetic resonance spectroscopy, are increasingly used as a surrogate marker for axonal dysfunction and degeneration in multiple sclerosis (MS). The purpose of this study was to test short-time reproducibility of NAA/Cr ratios in patients with clinically stable MS. In 35 MS patients we analysed NAA/Cr ratios obtained with 1H-MR spectroscopic imaging at the centrum semiovale either with lateral ventricles partially included (group 1; n=15) or more cranially with no ventricles included (group 2; n=20). To test short-term reproducibility of the NAA/Cr measurements, patients were scanned twice 4 weeks apart. We determined mean NAA/Cr and Cho/Cr ratios of 12 grey matter and 24 white matter voxels. Mean NAA/Cr ratios of both the white and grey matter did not change after 4 weeks. Overall 4-week reproducibility of the NAA/Cr ratio, expressed as coefficient of variation, was 4.8% for grey matter and 3.5% for white matter. Reproducibility of cranial scanning of the ventricles was slightly better than with cerebrospinal fluid included. Our study shows good short-term reproducibility of NAA/Cr ratio measurements in the centrum semiovale, which supports the reliability of this technique for longitudinal studies.
Keywords: Multiple sclerosis, Magnetic resonance, Spectroscopy, Reproducibility
Introduction
Multiple sclerosis (MS) is a chronic disorder of the central nervous system with inflammatory demyelination and axonal degeneration [1]. The disease usually starts between the ages of 20 and 40 years, and by the age of 55 years, about half of the patients need unilateral assistance to walk [2]. The disease progresses slowly, and clinical scales currently used are not sensitive enough to quantify progression [3]. Using surrogate markers of progression can shorten the duration of clinical trials in progressive MS.
¹H-magnetic resonance spectroscopy (¹H-MRS) of the brain in MS patients can detect changes in cell metabolites in both focal lesions and normal-appearing white matter (NAWM) [4]. Compared to controls, N-acetylaspartate (NAA) concentrations in MS patients are lower both in the lesions and in NAWM, whereas creatine (Cr) concentrations are equal to slightly increased [5]. NAA is a marker of neuroaxonal metabolism and integrity, and reductions in the white matter are due to axonal dysfunction or loss. It has been suggested that whole-brain NAA decline rates in patients with MS may predict future disease course [6]. Cr is present in neurons, astrocytes and oligodendrocytes, and elevations in white matter of MS patients can be due to axonal transection, astrocytic proliferation, and demyelination. Because absolute concentrations of metabolites with ¹H-MRS are difficult to interpret, the NAA/Cr ratio is often used as a valid surrogate marker of ‘cerebral tissue integrity’ [5]. Decreases in NAA/Cr, which in MS patients are found in lesions and NAWM, are indicative of neuroaxonal disturbance, oligodendroglial disturbance, or astrocytic proliferation. The third metabolite well observable in long TE ¹H-MRS is choline (Cho), which is regarded as a marker of glial proliferation [7].
Next to validity, good surrogate markers need to be reliable, especially when used for monitoring white matter integrity in longitudinal studies. In this study we investigated the reproducibility of NAA/Cr ratios obtained with 2D spectroscopic imaging at two different levels of the centrum semiovale within a time span of 4 weeks in patients with clinically stable MS.
Materials and methods
Patients
All patients co-operated in the study with informed consent and local medical ethical committee approval. Thirty-five patients participated in the study. Patients were randomly divided in two groups. All patients had clinically definitive MS with a relapsing remitting or secondary progressive disease course [8, 9]. None of the patients was using immunomodulatory medication. Patients were relapse free for at least 4 weeks and had not taken corticosteroids 4 weeks prior to the first MRI and during the study period. Disability was assessed with the Expanded Disability Status Scale (EDSS), which gives a score ranging from 0 to 10 with higher scores, indicating more disability [10].
1H-MR spectroscopy
MRI scans of the brain were obtained at baseline and after 4 weeks at a 3.0-T unit (Philips, Best, The Netherlands). 1H-MR spectroscopic imaging was preceded by the acquisition of a transverse multiple-slice MRI series in order to position the volume of interest (VOI). The standard transmitter/receiver coil was used, and the volume localisation was done by a combination of slice selection and 2D phase encoding with outer volume suppression with 12 rest slabs. All scan parameters were the same: TE =144 ms, TR =2.5 s, FOV =230 mm, slice thickness =20 mm, matrix =24×24, and turbo factor =3. Total scan time was around 8 min.
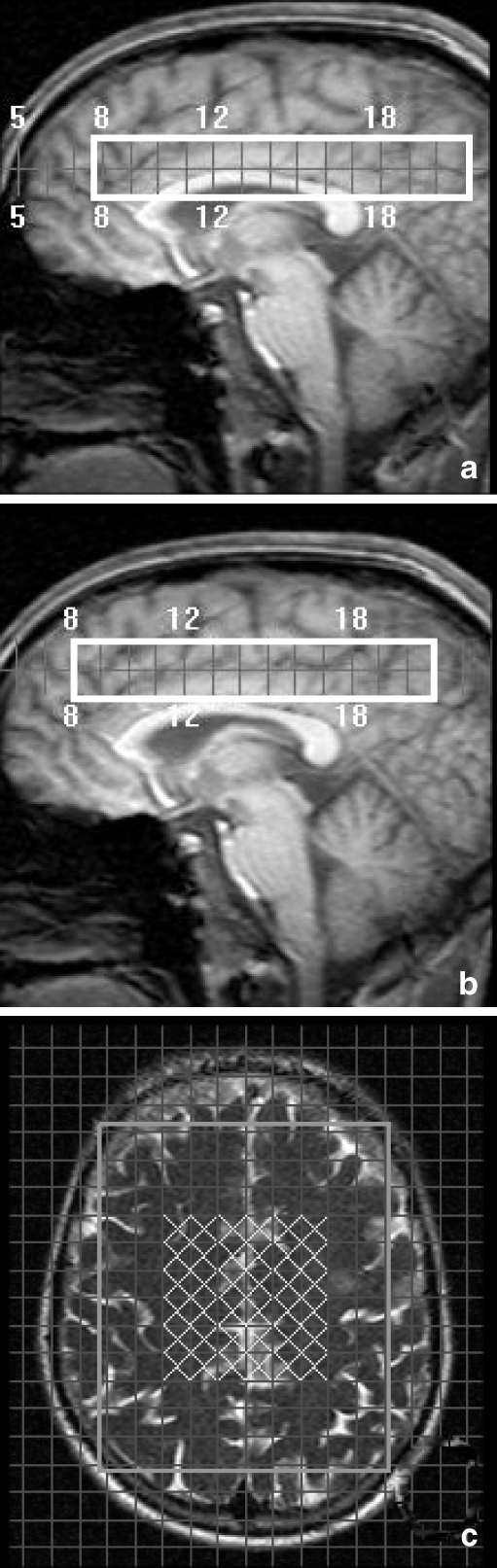
Group 1 was scanned around the lateral ventricles with some cerebrospinal fluid included in the VOI (Fig. 1a). Group 2 was scanned above the lateral ventricles with no cerebrospinal fluid included in the VOI (Fig. 1b). Individual anatomic landmarks were used for voxel repositioning.
Fig. 1.
Sagittal MRI images showing the volume of interest scanned in group 1 (a) and group 2 (b) with an axial image (c) displaying the grey (inner 12) and white matter (outer 12 in both hemispheres) voxels
To select voxels that mainly contained grey matter, 12 spectroscopic imaging (SI) voxels were selected in the midline grey matter in each subject (Fig. 1c). To select voxels that mainly contained white matter, 24 SI voxels were selected (12 in each hemisphere) in the deep white matter in each subject (Fig. 1c). Metabolite peak areas of NAA and Cho were determined with the manufacturer's software (Philips SPECTROVIEW) and are expressed as ratios to Cr. All voxels were visually checked for quality of the spectra and quality of the fitting of the peak areas and rejected if inaccurate according to the opinion of the two observers.
In every patient the NAA/Cr and Cho/Cr ratios per voxel were determined, and average ratios of the voxels in grey and white matter were calculated.
Statistical analysis
Reproducibility was tested by a coefficient of variation 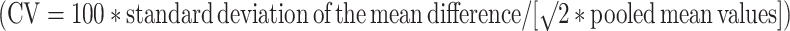 [11]. Changes in grey matter and white matter after 4 weeks and differences between baseline grey and white matter NAA/Cr ratios in patients were tested with paired t-tests. Differences between baseline grey matter and white matter NAA/Cr ratios of the two VOIs were tested with unpaired t-tests.
[11]. Changes in grey matter and white matter after 4 weeks and differences between baseline grey and white matter NAA/Cr ratios in patients were tested with paired t-tests. Differences between baseline grey matter and white matter NAA/Cr ratios of the two VOIs were tested with unpaired t-tests.
Results
Baseline characteristics of the patients are shown in Table 1. Patients were scanned after a mean ± standard deviation of 27.3±2.2 days. The mean number of excluded voxels was 1.51±2.93 for NAA/Cr and 4.54±5.40 for Cho/Cr (out of 36 voxels). White matter NAA/Cr and Cho/Cr ratios were higher than grey matter ratios (Table 2). The mean NAA/Cr did not change in either white matter or grey matter in both groups after 4 weeks (Table 2). The NAA/Cr ratios in grey and white matter were significantly higher in the group scanned with the ventricles partially included (p<0.001). Mean Cho/Cr in white and grey matter was 1.14 and 1.07 in group 1 and 0.98 and 0.92 in group 2 (p<0.001). Overall, the CV for NAA/Cr and Cho/Cr was 3.5% and 4.1% for white matter and 4.8% and 5.2% for grey matter. In group 1, the CVs of NAA/Cr in white matter and grey matter were 4.3% and 5.7%, and in group 2, 2.5% and 3.7%.
Table 1.
Characteristics of the patients
| All | Group 1 | Group 2 | |
|---|---|---|---|
| Number | 35 | 15 | 20 |
| Gender: male | 16 (46%) | 8 (53%) | 8 (40%) |
| Disease course: relapsing remitting/secondary progressive | 31/4 | 13/2 | 18/2 |
| Age: mean (sd) in years | 40 (9) | 43 (10) | 37 (8) |
| Disease duration: mean (SD) in years | 11 (7) | 12 (9) | 11 (6) |
| EDSS: median (IQR) | 3.0 (1.5–4.0) | 3.0 (1.5–4.5) | 3.0 (2.0–4.0) |
SD = standard deviation; IQR = interquartile range
Table 2.
The NAA/Cr ratios in the centrum semiovale at week 0 and week 4: mean (SD)
| Week 0 | Week 4 | p- value* | ||
|---|---|---|---|---|
| All (N = 35) | NAA/Cr white matter | 2.27 (0.26) | 2.26 (0.25) | 0.60 |
| NAA/Cr grey matter | 1.90 (0.23) # | 1.88 (0.21) | 0.42 | |
| Cho/Cr white matter | 1.05 (0.13) | 1.03 (0.15) | 0.11 | |
| Cho/Cr grey matter | 0.98 (0.12) # | 0.98 (0.13) | 0.60 | |
| Group 1 (N = 15) | NAA/Cr white matter | 2.45 (0.26) | 2.45 (0.25) | 0.82 |
| NAA/Cr grey matter | 2.08 (0.23) # | 2.03 (0.20) | 0.30 | |
| Group 2 (N = 20) | NAA/Cr white matter | 2.14 (0.15) | 2.13 (0.14) | 0.53 |
| NAA/Cr grey matter: | 1.76 (0.11) # | 1.77 (0.12) | 0.87 |
*p value: comparison week 0 with week 4
#p < 0.001: comparison of grey matter with white matter
SD = standard deviation
Discussion
Several small clinical trials in MS patients have used NAA/Cr ratios at the centrum semiovale as surrogate outcome measure [12–16]. Narayanan et al. reported an increase in NAA/Cr in ten relapsing MS patients after being treated with interferon β for 12 months [12]. Khan et al. reported an elevation of NAA/Cr in 18 patients in a large central brain volume after 2 years of treatment with glatiramer acetate [15]. An increase in NAA/Cr at the centrum semiovale in 11 MS patients after 2 weeks of treatment with fluoxetine was observed [16]. These studies suggest beneficial effects of therapy on axonal functioning or integrity. However, interpretation of the results is hampered by a lack of studies assessing reproducibility of NAA/Cr measurements in MS patients.
One study in five healthy subjects, assessing intraday and interday reproducibility of NAA/Cr ratios in the centrum semiovale, found CVs ranging between 5.1% and 7.2% [17]. Another study found a mean CV of 3.7% in six healthy subjects scanned twice, within 15 days apart, at a large volume of supratentorial brain [18]. Our study for the first time shows good short-term (4-week apart) reproducibility of NAA/Cr ratio measurements in the centrum semiovale of patients with MS.
In agreement with previous studies, NAA/Cr ratios in grey matter were lower than in white matter [19, 20]. Patients scanned cranially of the ventricles had lower NAA/Cr and Cho/Cr ratios compared to patients scanned with the ventricles partially included. Since the corpus callosum has the highest white matter NAA/Cr and Cho/Cr ratios, the larger area of the corpus callosum included in the VOI when scanning more caudally might explain this difference [21]. This underlines the importance of correct positioning of the VOI and voxel selection in longitudinal MRS studies.
Our study indicates that NAA/Cr ratio measurements with 1H-MRS at the centrum semiovale can be used as a reliable surrogate marker of cerebral tissue integrity in longitudinal MS studies. Selecting the VOI above the ventricles is preferred above placing the VOI partially in the ventricles.
Acknowledgements
This study was sponsored by Multiple Sclerosis anders (Amsterdam, The Netherlands).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Compston A, Coles A. Multiple sclerosis. Lancet. 2002;359:1221–1231. doi: 10.1016/S0140-6736(02)08220-X. [DOI] [PubMed] [Google Scholar]
- 2.Confavreux C, Vukusic S. Age at disability milestones in multiple sclerosis. Brain. 2006;129:595–605. doi: 10.1093/brain/awh714. [DOI] [PubMed] [Google Scholar]
- 3.Liu C, Blumhardt LD. Disability outcome measures in therapeutic trials of relapsing-remitting multiple sclerosis: effects of heterogeneity of disease course in placebo cohorts. J Neurol Neurosurg Psychiatry. 2000;68:450–457. doi: 10.1136/jnnp.68.4.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Filippi M, Rocca MA, Comi G. The use of quantitative magnetic-resonance-based techniques to monitor the evolution of multiple sclerosis. Lancet Neurol. 2003;2:337–346. doi: 10.1016/S1474-4422(03)00408-3. [DOI] [PubMed] [Google Scholar]
- 5.Caramanos Z, Narayanan S, Arnold DL. 1H-MRS quantification of tNA and tCr in patients with multiple sclerosis: a meta-analytic review. Brain. 2005;128:2483–2506. doi: 10.1093/brain/awh640. [DOI] [PubMed] [Google Scholar]
- 6.Gonen O, Oberndorfer TA, Inglese M, Babb JS, Herbert J, Grossman RI. Reproducibility of three whole-brain N-acetylaspartate decline cohorts in relapsing-remitting multiple sclerosis. AJNR Am J Neuroradiol. 2007;28:267–271. [PMC free article] [PubMed] [Google Scholar]
- 7.Chard DT, Griffin CM, McLean MA, Kapeller P, Kapoor R, Thompson AJ, Miller DH. Brain metabolite changes in cortical grey and normal-appearing white matter in clinically early relapsing-remitting multiple sclerosis. Brain. 2002;125:2342–2352. doi: 10.1093/brain/awf240. [DOI] [PubMed] [Google Scholar]
- 8.McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, McFarland HF, Paty DW, Polman CH, Reingold SC, Sandberg-Wollheim M, Sibley W, Thompson A, van den NS, Weinshenker BY, Wolinsky JS. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121–127. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- 9.Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology. 1996;46:907–911. doi: 10.1212/wnl.46.4.907. [DOI] [PubMed] [Google Scholar]
- 10.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 11.van der Worp HB, Claus SP, Bar PR, Ramos LM, Algra A, van GJ, Kappelle LJ. Reproducibility of measurements of cerebral infarct volume on CT scans. Stroke. 2001;32:424–430. doi: 10.1161/01.str.32.2.424. [DOI] [PubMed] [Google Scholar]
- 12.Narayanan S, De Stefano N, Francis GS, Arnaoutelis R, Caramanos Z, Collins DL, Pelletier D, Arnason BGW, Antel JP, Arnold DL. Axonal metabolic recovery in multiple sclerosis patients treated with interferon beta-1b. J Neurol. 2001;248:979–986. doi: 10.1007/s004150170052. [DOI] [PubMed] [Google Scholar]
- 13.Parry A, Corkill R, Blamire AM, Palace J, Narayanan S, Arnold D, Styles P, Matthews PM. Beta-Interferon treatment does not always slow the progression of axonal injury in multiple sclerosis. J Neurol. 2003;250:171–178. doi: 10.1007/s00415-003-0965-8. [DOI] [PubMed] [Google Scholar]
- 14.Schubert F, Seifert F, Elster C, Link A, Walzel M, Mientus S, Haas J, Rinneberg H. Serial 1H-MRS in relapsing-remitting multiple sclerosis: effects of interferon-beta therapy on absolute metabolite concentrations. MAGMA. 2002;14:213–222. doi: 10.1007/BF02668215. [DOI] [PubMed] [Google Scholar]
- 15.Khan O, Shen Y, Caon C, Bao F, Ching W, Reznar M, Buccheister A, Hu J, Latif Z, Tselis A, Lisak R. Axonal metabolic recovery and potential neuroprotective effect of glatiramer acetate in relapsing-remitting multiple sclerosis. Mult Scler. 2005;11:646–651. doi: 10.1191/1352458505ms1234oa. [DOI] [PubMed] [Google Scholar]
- 16.Mostert JP, Sijens PE, Oudkerk M, De Keyser J. Fluoxetine increases cerebral white matter NAA/Cr ratio in patients with multiple sclerosis. Neurosci Lett. 2006;402:22–24. doi: 10.1016/j.neulet.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 17.Rotondo E, Bruschetta G, Sacca A, Bramanti P, Di Pasquale MR. Straightforward relative quantitation and age-related human standards of N-acetylaspartate at the centrum semiovale level by CSI (1)H-MRS. Magn Reson Imaging. 2003;21:1055–1060. doi: 10.1016/S0730-725X(03)00211-X. [DOI] [PubMed] [Google Scholar]
- 18.Pelletier D, Nelson SJ, Grenier D, Lu Y, Genain C, Goodkin DE. 3-D echo planar (1)HMRS imaging in MS: metabolite comparison from supratentorial vs. central brain. Magn Reson Imaging. 2002;20:599–606. doi: 10.1016/S0730-725X(02)00533-7. [DOI] [PubMed] [Google Scholar]
- 19.Safriel Y, Pol-Rodriguez M, Novotny EJ, Rothman DL, Fulbright RK. Reference values for long echo time MR spectroscopy in healthy adults. AJNR Am J Neuroradiol. 2005;26:1439–1445. [PMC free article] [PubMed] [Google Scholar]
- 20.Lundbom N, Barnett A, Bonavita S, Patronas N, Rajapakse J, Tedeschi G, Di Chiro G. MR image segmentation and tissue metabolite contrast in 1H spectroscopic imaging of normal and aging brain. Magn Reson Med. 1999;41:841–845. doi: 10.1002/(SICI)1522-2594(199904)41:4<841::AID-MRM25>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 21.Cecil KM, Hills EC, Sandel ME, Smith DH, McIntosh TK, Mannon LJ, Sinson GP, Bagley LJ, Grossman RI, Lenkinski RE. Proton magnetic resonance spectroscopy for detection of axonal injury in the splenium of the corpus callosum of brain-injured patients. J Neurosurg. 1998;88:795–801. doi: 10.3171/jns.1998.88.5.0795. [DOI] [PubMed] [Google Scholar]