Abstract
Neuronal Ca2+ channels are inhibited by a variety of transmitter receptors coupled to Go-type GTP-binding proteins. Go has been postulated to work via a direct interaction between an activated G protein subunit and the Ca2+ channel complex. Here we show that the inhibition of sensory neuron N-type Ca2+ channels produced by γ-aminobutyric acid involves a novel, rapidly activating tyrosine kinase signaling pathway that is mediated by Gαo and a src-like kinase. In contrast to other recently described G protein-coupled tyrosine kinase pathways, the Gαo-mediated modulation requires neither protein kinase C nor intracellular Ca2+. The results suggest that this pathway mediates rapid receptor-G protein signaling in the nervous system and support the existence of a previously unrecognized form of crosstalk between G protein and tyrosine kinase pathways.
Receptor-mediated modulation of ion channels is an important means of regulating intercellular communication in the nervous system. Voltage-dependent Ca2+ channels are effective targets for such modulation by virtue of their involvement in a host of cellular functions. Of the many calcium channel types now recognized, it has been the N-type (or class B) channels that have received the most attention in studies of receptor-mediated regulation. A large number of receptors and a variety of signaling pathways have been identified to target N channels (1, 2).
High voltage-activated Ca2+ currents evoked from cultured embryonic chicken sensory neurons are predominantly N-type (as they are blocked ≈90% by ω-conotoxin GVIA) and are inhibited by several neurotransmitters, including γ-aminobutyric acid (GABA) and norepinephrine (NE). The inhibition is characterized by a slowing in the kinetics of N current activation (kinetic slowing) and an attenuation of the overall current amplitude without changes in current waveform (steady-state inhibition). Previous work has shown that these two components can be separated on the basis of a differential sensitivity to voltage (3).
All inhibition by GABA is rapid and mediated through GABAB receptors (4) coupled to G proteins of the Go class (5). The speed of inhibition, coupled with the fact that it is independent of protein kinases A and C, cyclic nucleotides, intracellular Ca2+, and phosphatases 1 and 2A (6, 7), has led to the suggestion that GABA evokes inhibition through a direct effect of G protein subunits on the Ca2+ channel (1, 2). Results reported below, however, suggest that steady-state inhibition is mediated indirectly through a novel tyrosine kinase pathway that is activated by Gαo.
MATERIALS AND METHODS
Embryonic chicken sensory neurons were grown in culture, and tight-seal whole-cell recording was performed after 1–3 days as in ref. 5. For intracellular application of kinase inhibitors, agents were diluted into intracellular recording solution and delivered to the cell via passive diffusion from the patch pipette. For extracellular application, agents were diluted into standard extracellular saline and applied via a wide-bore (140 μm i.d.) “sewer pipe,” which exchanges solutions with sub-second kinetics.
Phosphotyrosine Determination.
Neurons were plated at a density of 200,000 cells per 35-mm dish. After 24 hr, cultures were washed three times with phosphate-free DMEM (GIBCO), incubated for 2 hr at 37°C in medium containing 32Pi (2 mCi/ml), followed by washing three times with phosphate-free medium. Cells then were exposed for 20, 40, or 60 s with buffer ± 100 μM GABA (both containing 100 μM bicuculline to block GABAA receptors) and lysed with ice-cold buffer [PBS, pH 7.4, containing 250 μM sodium pervanadate, 1% (vol/vol) Nonidet P-40, 1 mM Pefabloc, 1 mM EDTA, 1 mM ethylene glycol bis(β-aminoethyl ether-N,N,N′,N′-tetraacetic acid, 10 μg/ml pepstatin, 10 μg/ml leupeptin, and 100 μg/ml soybean trypsin inhibitor]. Lysates containing 2.0 mg of total protein were subjected to the following immunoprecipitation methods.
Immunoprecipitation.
Lysates were centrifuged at 15,000 × g for 30 min, and supernatant removed and exposed to untreated strepavidin-Sepharose beads. The cleared preparation was incubated in 6 μg/ml mouse mAb against phosphotyrosine, 4G10 (8) for 16 hr at 4°C followed by incubation (4 hr, 4°C) in biotinylated antibody-strepavidin-Sepharose bead complex (Pierce). Precipitates were collected by centrifugation, washed three times in ice-cold PBS containing 250 μM sodium pervanadate, 1% BSA, 1 mM Pefabloc, 10 μg/ml pepstatin, 10 μg/ml leupeptin, and 100 μg/ml soybean trypsin inhibitor, and resuspended in nonreducing sample buffer. Samples (10 μl) were electrophoresed on a sodium dodecylsulfate 4–15% acrylamide gel and visualized on x-ray film (Kodak).
Recombinant Proteins.
Recombinant, myristoylated Gαo was expressed in and purified from Escherichia coli according to previously published methods (9). Recombinant, myristoylated Gαi2 was expressed in and purified from Sf9 cells according to previously published methods (10) and provided by M. Lindorfer and J. Garrison (University of Virginia). Before its use in electrophysiological assays, Gαi2 was tested for its ability to bind GTP and Gβγ and to couple to A1 adenosine receptors (see ref. 10 for methods). The Gα proteins (0.4 μM) were activated with guanosine 3′-[γ-thio]triphosphate (GTPγS) according to methods of Harden and coworkers (11), diluted into internal recording solution, and introduced intracellularly via the patch pipette at the specified concentrations.
RESULTS AND DISCUSSION
Two Types of GABA-Mediated Inhibition.
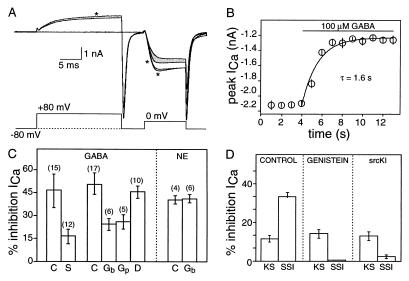
Somatic N-type Ca2+ currents were recorded from embryonic chicken sensory neurons from 1–3 days in vitro. Rapid application of 100 μM GABA through a wide-bore, gravity-fed pipette reduced charge entry through N channels by an average 45% (from 11 ± 3 pC in control to 6 ± 2 pC, n = 24). The inhibition was associated with a slowing of the activation kinetics whose voltage-dependence was demonstrated using the two-pulse paradigm in Fig. 1A. A depolarizing conditioning pulse reversed GABA-induced kinetic slowing without altering steady-state inhibition. An 80-mV, 15-ms conditioning pulse (with a 5-ms interval between conditioning and test pulses) reversed, on average, ≈85% of the voltage-dependent inhibition; this paradigm was used throughout the studies to distinguish the two modulatory components. Both types of inhibition were rapid (Fig. 1B), proceeding with a time constant of 1.6 s, and no differences in onset rate were observed for the two.
Figure 1.
GABA-mediated inhibition involves a tyrosine kinase. (A) Illustration of kinetic slowing and steady-state inhibition produced by GABA. Four superimposed currents (Upper) recorded in response to voltage protocol indicated; traces taken before (larger two currents) or during application of 100 μM GABA. Traces evoked by test pulses after a 15-ms conditioning depolarization to 80 mV are indicated by ∗. Shaded region highlights kinetic slowing. This voltage protocol reverses, on average, 87% of kinetic slowing, leaving steady-state inhibition unaffected (unpublished experiments); it is used throughout to quantitate the proportions of the two modulatory components. (B) Time course of N current inhibition. Currents evoked at 1 Hz using 2-ms test pulses to 0 mV; 100 μM GABA applied as indicated. Data are means ± SEM (n = 5). (C) Mean percentage inhibition of sensory neuron Ca2+ current produced by 100 μM GABA or NE ± tyrosine kinase inhibitors (n noted in parentheses). Control cells (C) or cells exposed to bath application of 100 μM genistein (Gb) or daidzein (D) or intracellular application of genistein (10 μM, Gp) or srcKI (70 μM, S). (D) Magnitude of kinetic slowing (KS) and steady-state inhibition (SSI) determined using prepulse paradigm in A for control cells or cells exposed to genistein or srcKI (as marked). Data are means ± SEM (n = 12).
GABA-Induced Steady-State Inhibition Involves a Tyrosine Kinase.
We tested the efficacy of two tyrosine kinase inhibitors against GABA-mediated modulation. Genistein (12), applied at 10 μM in the patch pipette, significantly attenuated GABA-induced inhibition without affecting control current (Fig. 1C). Using the two-pulse paradigm to separate kinetic slowing from steady-state inhibition, genistein was observed to target the latter, leaving kinetic slowing unaffected (Fig. 1D). This concentration of genistein is below the IC50 (25 μM) reported for inhibition of tyrosine kinase activity in vitro (12). Bath application of genistein (at 100 μM) was also effective against GABA-mediated inhibition, but produced no more inhibition than did 10 μM, demonstrating saturation of the response at the lower concentration. The involvement of tyrosine kinase is further supported by experiments with a peptide inhibitor selective for src kinase, srcKI (derived from the primary sequence of amino acids 137 to 157 in the regulatory domain of pp60vsrc, ref. 13). SrcKI (at 70 μM) also attenuated GABA-induced steady-state inhibition when applied intracellularly via the recording pipette (Fig. 1 C and D).
Several control experiments addressed the specificity of the tyrosine kinase inhibitors. The inactive genistein analog, daidzein, was ineffective when bath-applied at 100 μM (Fig. 1C). Similarly, bath application of staurosporine (a broad-spectrum inhibitor of serine/threonine kinases) and a peptide inhibitor of protein kinase C (PKCI 19–31) were also ineffective against GABA-induced inhibition (ref. 7; data not shown).
As a further test of the specificity of tyrosine kinase inhibition, we examined the effect of genistein on another transmitter that modulates N currents. NE, acting through α2-adrenergic receptors, inhibits Ca2+ current through a mechanism that is biophysically similar to that produced by GABA (including both kinetic slowing and steady-state inhibition), but is biochemically different in that it involves protein kinase C-dependent phosphorylation (ref. 7; Fig. 2 ). As seen in Fig. 1C, genistein, at concentrations effective against the GABA-mediated inhibition, does not attenuate inhibition produced by NE. These controls rule out possible nonspecific actions of the tyrosine kinase inhibitors at all concentrations used here and, together with the inhibitor data above, argue that GABA-induced steady-state inhibition requires the activation of a src-like tyrosine kinase.
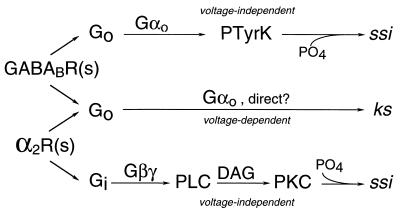
Figure 2.
G protein-coupled inhibitory pathways. Summary of modulatory pathways showing that GABAB receptors [GABABR(s)] and α2-adrenergic receptors [α2R(s)] couple both to a common pathway producing voltage-dependent kinetic slowing and to separate kinase-dependent pathways producing voltage-independent steady-state inhibition. These latter pathways also involve different G protein subunits. PTyrK, protein tyrosine kinase; PLC, phospholipase C; DAG, diacylglycerol; PKC, protein kinase C; ssi, steady-state inhibition; ks, kinetic slowing.
N Current Inhibition Involves Tyrosine Phosphorylation.
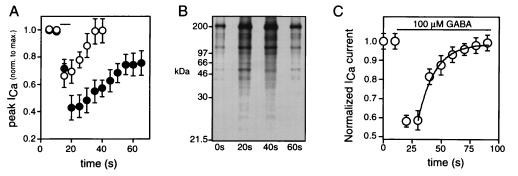
Recovery from GABA-induced inhibition is rapid under control conditions, subsiding to half in <10 s after wash-out of GABA (Fig. 3A). In the presence of 250 μM sodium pervanadate (an inhibitor of tyrosine phosphatases, ref. 14), the peak inhibition was increased by 70%, and the time course of recovery was prolonged ≈2-fold (Fig. 3A, n = 5). Our previous results demonstrated that all GABA-mediated N current inhibition is blocked by pertussis toxin (15); together, the results suggest that, in these cells, a G protein-coupled pathway activates tyrosine kinase-dependent phosphorylation to bring about N channel inhibition.
Figure 3.
GABA-mediated inhibition involves tyrosine phosphorylation. (A) Time course of Ca2+ current inhibition by 100 μM GABA (applied for time indicated by horizontal bar) in control cells (○) or in cells bathed in 250 μM sodium pervanadate (•). (B) Autoradiogram of 32P-labeled proteins from sensory neurons. Cells were labeled metabolically with 32Pi, treated with 100 μM GABA, solubilized, and immunoprecipitated with 4G10 as described in Materials and Methods. GABA applied for times indicated below each lane. (C) Time course for inhibition of N current during prolonged exposure to 100 μM GABA (horizontal bar). Solid line is least-squares fit to single-exponential function (τ = 14 s).
Additional support for this conclusion comes from biochemical experiments that demonstrate GABA-mediated increases in protein phosphotyrosine content. Sensory neurons were metabolically labeled with 32Pi, exposed to GABA (in the presence of the GABAA receptor antagonist bicuculline), and homogenized. Protein was immunoprecipitated with antiphosphotyrosine antibody, 4G10 (8), separated on polyacrylamide gels, and analyzed autoradiographically. GABA produced a rapid, transient increase in several 32P-labeled proteins recognized by 4G10 (Fig. 3B). Similar results were seen in six separate experiments. Incorporation was blocked by bath-application of genistein and enhanced by treatment of the cells with sodium pervanadate (data not shown), suggesting significant phosphorylation on tyrosine residues. The time course of GABA-stimulated tyrosine phosphorylation was similar to that for GABA-induced inhibition of Ca2+ channels. The inhibition was maximal by 10 s and, in the continued presence of GABA, desensitized over the course of 1–2 min (Fig. 3C, n = 5). Although direct biochemical investigations will be necessary to identify the tyrosine-phosphorylated substrates that mediate the actions of GABA in chicken sensory neurons, our results demonstrate that GABA, via a GABAB receptor, promotes tyrosine phosphorylation following a time course consistent with that described for its inhibition of N channels.
Steady-State Inhibition Is Mediated by Gαo.
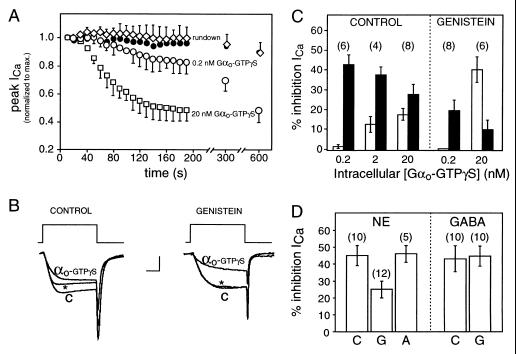
It is possible to trigger the downstream effects of GABA (bypassing the GABA receptor) by introducing into sensory neurons recombinant Gαo, activated with GTPγS. This produced a time- and concentration-dependent inhibition of the current that mimicked that produced by GABA, evoking both kinetic slowing and steady-state inhibition (Fig. 4B). At 20 nM, the highest concentration tested, Gαo-GTPγS inhibited Ca2+ current by 46 ± 5%, achieving maximal inhibition at ≈2.5 min (Fig. 4A). Approximately 60% of the effect was associated with steady-state inhibition (Fig. 4 B and C). At lower concentrations of Gαo-GTPγS (200 pM), the inhibition was evoked without associated kinetic slowing (Fig. 4C) or voltage-dependent reversal, suggesting that the kinetic slowing pathway is less sensitive to Gαo-GTPγS than is steady-state inhibition. Previous results have similarly demonstrated that kinetic slowing is evoked only at higher transmitter concentrations (5).
Figure 4.
GABA-induced steady-state inhibition is mediated by Gαo. (A) Time course of Ca2+ current during intracellular application of Gαo-GTPγS (0.2 nM, ○, n = 6; 20 nM, □, n = 8), Gαo-GDP (20 nM, •, n = 3), or control internal solution without Gαo (“rundown,” ⋄, n = 4). Data plotted as means ± SEM; one-sided error bars are plotted for clarity. (B) Three superimposed current traces evoked by 10-ms step depolarizations to 0 mV from a holding potential of −80 mV. Shown are the relative proportions of kinetic slowing and steady-state inhibition produced by 20 nM intracellular Gαo-GTPγS in control cell (Left) and cell exposed to 100 μM extracellular genistein (Right). Control currents (C) were taken within 20 s of achieving whole cell access (before Gαo-GTPγS inhibited the current). Inhibited currents (measured after 5 min of exposure to intracellular solution containing Gαo-GTPγS) were evoked either without (αo-GTPγS) or with (∗) a depolarizing conditioning pulse (not shown) to reverse kinetic slowing. Calibration: 0.75 nA (Left), 1 nA (Right), 5 ms. (C) Mean inhibition of Ca2+ current produced by intracellular application of recombinant Gαo-GTPγS at the concentrations indicated on abscissa; kinetic slowing (empty bars) and steady-state inhibition (filled bars) in control cells or cells exposed to 100 μM genistein in the bath (as indicated). (D) Mean inhibition of calcium current produced by 100 μM GABA or NE in control cells (C) or in cells exposed to 100 μM intracellular βγ-binding peptide G (G) or peptide A (A) of the same length but without βγ binding activity. Errors represent SEM in all panels (n in parentheses).
As with GABA, steady-state inhibition produced by Gαo-GTPγS was sensitive to genistein but not to the protein kinase C inhibitor staurosporine. At concentrations of Gαo-GTPγS (200 pM) that evoked steady-state inhibition without kinetic slowing, bath application of genistein reduced the inhibition to less than half of its control level (Fig. 4 B and C). At 20 nM Gαo-GTPγS (a concentration sufficient to evoke kinetic slowing as well), genistein was found to be selective for the steady-state component. In fact, in the presence of genistein, the total inhibition remained about the same as in control, but the proportion of steady-state inhibition decreased (from 61% to 20%) with a corresponding increase in the proportion of kinetic slowing. Staurosporine (at 10 μM, a concentration sufficient to block NE effects on calcium current) was without effect on the Gαo-mediated inhibition (47 ± 7% in control and 49 ± 6% in the presence of staurosporine, n = 4).
As GTPγS can directly activate inhibitory G proteins (1, 2), it was necessary to control for possible effects of unbound GTPγS as well as complications arising from the presence of detergent in the Gαo preparation. The following control experiments were performed and results summarized: (i) Nonactivated (GDP-bound) Gαo was without effect at 20 nM (n = 3, Fig. 4A); (ii) Gαi2, in the same detergent and activated similarly, was ineffective (n = 6); and (iii) heating the complete reaction mixture (80°C for 30 min) eliminated its efficacy (n = 4); heating a solution of 100 μM GTPγS (>5 times the nucleotide concentration present in the Gαo solutions) did not eliminate the ability of GTPγS itself to inhibit Ca2+ currents (n = 4). These control experiments rule out the possibility that Ca2+ current inhibition resulted from an action of GTPγS and/or the detergent-containing vehicle and point to Gαo-GTPγS as the active subunit. Results argue that GABA brings about steady-state inhibition via activated Gαo. This is further supported by the finding that intracellular application of peptide G, a Gβγ-binding protein derived from βARK2 (which blocks NE-mediated inhibition, ref. 16), was ineffective against the GABA response (Fig. 4D).
Models for G Protein-Induced Inhibition of N Current.
The involvement of Gαo in voltage-dependent kinetic slowing comes as a surprise in light of recent studies demonstrating Gβγ as the active subunit in the inhibition of mammalian N- and P/Q-type Ca2+ channels (17–21). The apparent conflict with the data reported here may arise from the existence of multiple modulatory mechanisms and their differential expression among tissues. Heterogeneity in pathways that inhibit N channels is well known (1, 2), but their differential expression has been little explored. Resolution of this issue will require interspecies and intercellular comparisons made under identical conditions. It is also possible, however, that overexpression of G protein subunits might lead to forms of Ca2+ channel inhibition not observed in the native environment. As G protein signaling pathways are evoked through relatively low-affinity molecular interactions, their selective activation is a sensitive function of expression levels. For this reason, studies of primary cells, where signaling molecules are expressed at physiologically appropriate levels, are important complements to heterologous expression approaches.
Based on the differential sensitivity to Gαo of the two modulatory components, it is likely that kinetic slowing and steady-state inhibition are evoked via separate effectors. The presence of multiple variants of Gαo in sensory neurons that could be separated by isoelectric focusing (unpublished observations) points to the possibility that each pathway is governed by a separate G protein subunit as well. Isotype-specific coupling between Gαo and effectors has not been demonstrated, however; thus, testing whether different Gαos mediate kinetic slowing and steady-state inhibition will be an important future challenge. Alternatively, the two forms of inhibition could be mediated by a single type of Gαo that interacts with other proteins that confer specificity through spatial localization of the signaling molecules (22).
A Novel Tyrosine Kinase Pathway.
Several aspects of the GABA- or Gαo-induced inhibition of Ca2+ current are notable. First, as opposed to the tyrosine kinase pathways reported to inhibit K channels (23, 24) or activate mitogen-activated protein kinase (25, 26) in heterologous expression systems, the effects of GABA and Gαo on sensory neurons are independent of Ca2+ and protein kinase C, suggesting the involvement of a different type of tyrosine kinase. Our experiments have been performed in the presence of 5 mM intracellular 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid, a rapid and effective chelator that buffers cytosolic Ca2+ concentration below 10 nM. Furthermore, inhibitors of protein kinase C fail to alter GABA-mediated inhibition (7). Second, the G protein is Go not Gi as for other G protein pathways reported to activate tyrosine kinase. Although Go has been shown capable of coupling platelet-activating factor receptors to mitogen-activated protein kinase activation in a heterologous expression system, it is likely that these receptors do not signal through Go in platelets due to low-expression Gαo expression levels (27). Third, Ca2+ current inhibition by GABA does not involve a Gβγ-mediated mechanism such as that reported to activate G protein-coupled mitogenic pathways in tumor cells (25) but rather is effected through an action of Gα. Fourth, the GABA-stimulated pathway is at least 60-fold faster (both in its onset and its recovery) than other tyrosine kinase pathways reported to modulate ion channel function. For example, the inhibition of K channels by transmitter-induced tyrosine kinase activation in Xenopus oocytes (23), NG108–15 neuroblastoma cells (23), and PC12 cells (24) develops in minutes after application of transmitter. By contrast, sensory neuron Ca2+ currents are inhibited by GABA with a time constant of less than 2 s.
In summary, our results suggest that primary sensory neurons use a unique src-like protein tyrosine kinase pathway to effect rapid and reversible inhibition of Ca2+ channels. Although the physiological significance of such inhibition is unknown, regulation of Ca2+ influx through somatic N channels (as with L-type Ca2+ channels, ref. 28) is likely to exert long-term influence over Ca2+-dependent enzymatic activity and gene transcription. The presence of a similar pathway in nerve terminals likely would regulate exocytosis acutely. Curiously, tyrosine kinase, known for its prominent role in mitogenesis, is used to a different end in sensory neurons. It is interesting to speculate that postmitotic neurons and dividing cells use similar pathways differently. Perhaps, as neurons lose their ability to divide, the machinery essential for mitogenesis may be left to assume new roles—for example, mediation of short- or long-term changes in neuronal synaptic function.
Acknowledgments
We thank Michael Goy and Michael Mendelsohn for critical comments, Thomas Roberts for anti-phosphotyrosine antibody (4G10), Paul Goldsmith for anti-Gαo antibody, Robert Stoffel and Robert Lefkowitz for the Gβγ-binding peptide, and Margaret Lindorfer and James Garrison for purified, recombinant Gαi2. This work was supported by a Samuel B. Levine Fellowship from the Massachusetts Affiliate of the American Heart Association (M.D.-P.), Jacob Javits Award NS16483 (K.D.), and National Institutes of Health Grant GM39561 (R.R.N.).
ABBREVIATIONS
- GABA
γ-aminobutyric acid
- NE
norepinephrine
- GTPγS
guanosine 3′-[γ-thio]triphosphate
References
- 1.Hille B. Trends Neurosci. 1994;17:531–536. doi: 10.1016/0166-2236(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 2.Dolphin A C. Exp Physiol. 1995;80:1–36. doi: 10.1113/expphysiol.1995.sp003825. [DOI] [PubMed] [Google Scholar]
- 3.Luebke J I, Dunlap K. Pflügers Arch. 1994;428:499–507. doi: 10.1007/BF00374571. [DOI] [PubMed] [Google Scholar]
- 4.Dunlap K. Brit J Pharmacol. 1981;74:579–585. doi: 10.1111/j.1476-5381.1981.tb10467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diversé-Pierluissi M, Goldsmith P, Dunlap K. Neuron. 1995;14:191–200. doi: 10.1016/0896-6273(95)90254-6. [DOI] [PubMed] [Google Scholar]
- 6.Dolphin A C, McGuirk S M, Scott R H. Br J Pharmacol. 1989;97:263–273. doi: 10.1111/j.1476-5381.1989.tb11950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diversé-Pierluissi M, Dunlap K. Neuron. 1993;10:753–760. doi: 10.1016/0896-6273(93)90175-q. [DOI] [PubMed] [Google Scholar]
- 8.Druker B J, Mamon H J, Roberts T M. N Engl J Med. 1989;321:1383–1391. doi: 10.1056/NEJM198911163212007. [DOI] [PubMed] [Google Scholar]
- 9.Mumby S M, Linder M E. Methods Enzymol. 1994;237:254–268. doi: 10.1016/s0076-6879(94)37067-2. [DOI] [PubMed] [Google Scholar]
- 10.Graber S G, Figler R A, Garrison J C. Methods Enzymol. 1994;237:212–226. doi: 10.1016/s0076-6879(94)37064-8. [DOI] [PubMed] [Google Scholar]
- 11.Boyer J L, Waldo G L, Evans T, Northup J K, Downes C P, Harden T K. J Biol Chem. 1989;264:13917–13922. [PubMed] [Google Scholar]
- 12.Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y. J Biol Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- 13.Sato K, Miki S, Tachibana H, Hayashi F, Akiyama T, Fukami Y. Biochem Biophys Res Commun. 1990;171:1152–1159. doi: 10.1016/0006-291x(90)90805-w. [DOI] [PubMed] [Google Scholar]
- 14.Hunter T. Cell. 1995;80:225–236. doi: 10.1016/0092-8674(95)90405-0. [DOI] [PubMed] [Google Scholar]
- 15.Holz G G, Rane S G, Dunlap K. Nature (London) 1986;319:670–672. doi: 10.1038/319670a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diversé-Pierluissi M, Inglese J, Stoffel R H, Lefkowitz R J, Dunlap K. Neuron. 1996;16:579–585. doi: 10.1016/s0896-6273(00)80077-x. [DOI] [PubMed] [Google Scholar]
- 17.Ikeda S. Nature (London) 1996;380:255–258. doi: 10.1038/380255a0. [DOI] [PubMed] [Google Scholar]
- 18.Herlitze S, Garcia D E, Mackie K, Hille B, Scheuer T, Catterall W A. Nature (London) 1996;380:258–262. doi: 10.1038/380258a0. [DOI] [PubMed] [Google Scholar]
- 19.Zamponi G W, Bourinet E, Nelson D, Nargeot J, Snutch T P. Nature (London) 1997;385:442–446. doi: 10.1038/385442a0. [DOI] [PubMed] [Google Scholar]
- 20.De Waard M, Liu H, Walker D, Scott V E S, Gurnett C A, Campbell K P. Nature (London) 1997;385:446–450. doi: 10.1038/385446a0. [DOI] [PubMed] [Google Scholar]
- 21.Page K M, Stephens G J, Berrow N S, Dolphin A C. J Neurosci. 1997;14:1330–1338. doi: 10.1523/JNEUROSCI.17-04-01330.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neubig R R. FASEB J. 1994;8:939–946. doi: 10.1096/fasebj.8.12.8088459. [DOI] [PubMed] [Google Scholar]
- 23.Huang X-Y, Morielli A D, Peralta E G. Cell. 1993;75:1145–1156. doi: 10.1016/0092-8674(93)90324-j. [DOI] [PubMed] [Google Scholar]
- 24.Lev S, Moreno H, Martinez R, Canoll P, Peles E, Musacchio J M, Plowman G D, Rudy B, Schlessinger J. Nature (London) 1995;376:737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- 25.van Biesen T, Hawes B E, Luttrell D K, Krueger K M, Touhara K, Porfiri E, Sakaue M, Luttrell L M, Lefkowitz R J. Nature (London) 1995;376:781–784. doi: 10.1038/376781a0. [DOI] [PubMed] [Google Scholar]
- 26.van Biesen T, Hawes B E, Raymond J R, Luttrell L M, Lefkowitz R J. J Biol Chem. 1996;271:1266–1269. doi: 10.1074/jbc.271.3.1266. [DOI] [PubMed] [Google Scholar]
- 27.Brass L F, Hoxie J A, Manning D R. Thromb Haemostasis. 1993;70:217–223. [PubMed] [Google Scholar]
- 28.Segal R A, Greenberg M E. Annu Rev Neurosci. 1996;19:463–484. doi: 10.1146/annurev.ne.19.030196.002335. [DOI] [PubMed] [Google Scholar]