Abstract
Amyloid plaques in Alzheimer disease are primarily aggregates of Aβ peptides that are derived from the amyloid precursor protein (APP). Neurotransmitter agonists that activate phosphatidylinositol hydrolysis and protein kinase C stimulate APP processing and generate soluble, non-amyloidogenic APP (APPs). Elevations in cAMP oppose this stimulatory effect and lead to the accumulation of cell-associated APP holoprotein containing amyloidogenic Aβ peptides. We now report that cAMP signaling can also increase cellular levels of APP holoprotein by stimulating APP gene expression in astrocytes. Treatment of astrocytes with norepinephrine or isoproterenol for 24 h increased both APP mRNA and holoprotein levels, and these increases were blocked by the β-adrenergic antagonist propranolol. Treatment with 8-bromo-adenosine 3′,5′-cyclic monophosphate or forskolin for 24 h similarly increased APP holoprotein levels; astrocytes were also transformed into process-bearing cells expressing increased amounts of glial fibrillary acidic protein, suggesting that these cells resemble reactive astrocytes. The increases in APP mRNA and holoprotein in astrocytes caused by cAMP stimulation were inhibited by the immunosuppressant cyclosporin A. Our study suggests that APP overexpression by reactive astrocytes during neuronal injury may contribute to Alzheimer disease neuropathology, and that immunosuppressants can inhibit cAMP activation of APP gene transcription.
Keywords: cyclosporin A, inflammation, gliosis, astrocytes, Alzheimer
Amyloid (Aβ) plaques accumulate in the vicinity of dystrophic neurons, reactive microglia, and astrocytes in Alzheimer disease (AD) (1, 2). The amyloid precursor protein (APP) is a large transmembrane protein expressed in virtually all mammalian cells (3). The Aβ peptide is anchored within the cell membrane, between the extracellular N terminus and the shorter cytoplasmic C terminus of APP. Constitutive APP processing cleaves the Aβ domain, thus disrupting Aβ formation and releasing the soluble nonamyloidogenic N terminus of APP (APPs) into the extracellular medium (4).
Increased APP gene dosage in Down syndrome/trisomy 21 is associated with amyloid plaques and a high incidence of AD at an early age (5). Overexpression of APP in cell cultures can cause aberrant APP processing, neurodegeneration, and production of Aβ or neurotoxic derivatives (6). Transgenic mice overexpressing APP reportedly develop behavioral and neuropathological abnormalities resembling those seen in AD, for example, amyloid deposition, dystrophic neurites, and cognitive deficits (7–9). Reactive astrocytes that are highly immunoreactive for glial fibrillary acidic protein (GFAP) are typically associated with amyloid plaques and with sites of neurodegeneration in the brains of these transgenic mice and of patients with AD (2, 9).
Although general increases in APP expression have not been reported in AD, transcriptional activation of the APP gene may occur in brain regions that are particularly susceptible to damage. Several APP isoforms, ranging in size from 695 to 770 aa, are derived by differential splicing of a primary transcript (10, 11). Of the three major APP isoforms, APP695 is predominantly expressed by neurons; APP751 and APP770, which harbor an additional Kunitz-type protease inhibitor (KPI) insert at the N terminus, are found in astrocytes. Neuronal loss may explain the decreased amounts of APP695 in postmortem AD brains. However increased amounts of APP751 have been detected in regions of neurodegeneration (12, 13), suggesting that overexpression of KPI-containing APP by astrocytes can contribute to AD neuropathology. Brain injury also induces rapid GFAP expression (14) and persistent increases in astrocytic APP immunoreactivity (15).
Activation of neurotransmitter receptors that increase phosphatidylinositol (PI) hydrolysis or protein kinase C (PKC) signaling can promote constitutive APP processing and decrease the levels of cell-associated APP (16, 17). We have shown that in primary cultures of both astrocytes and hippocampal neurons, increases in APPs secretion caused by activating PKC or metabotropic glutamate receptors can be suppressed by short-term (1-h) exposure to forskolin or to the membrane-permeant dibutyryl cAMP (18, 19). Similarly, constitutive or phorbol ester-stimulated APPs secretion in C6 cells transfected with APP751 cDNA can be suppressed by elevations in intracellular cAMP levels (20). Thus, increased cAMP signaling might be expected to reduce APPs secretion and promote the cellular accumulation of APP holoprotein harboring Aβ peptides.
It is not known whether neurotransmitters can also regulate the synthesis and cellular levels of APP by transcriptional activation of the APP gene. We now show that activation of cell-surface receptors coupled to increased cAMP formation stimulates APP synthesis in cultured cortical astrocytes; prolonged (24 h) activation of adrenergic receptors with l-norepinephrine bitartrate (NE) or isoproterenol up-regulated levels of both APP mRNA and APP holoprotein. Treatment with 8-bromo-adenosine 3′,5′-cyclic monophosphate (8Br-cAMP) or forskolin also stimulated APP synthesis and increased GFAP expression in cultured astrocytes. Parts of this work have been presented as an abstract (21).
MATERIALS AND METHODS
Cortical Astrocyte Cultures.
Dissociated astrocytes were cultured from cortices of postnatal day 1–3 rat pups as described (22) with minor modifications. Briefly, cortices were incubated with 0.25% trypsin in minimum essential medium (MEM; GIBCO). The cell pellet obtained by centrifugation was resuspended in MEM containing 10% horse serum/5% fetal bovine serum and plated onto poly-l-lysine-coated dishes (≈104–105 cells per dish). Unattached cells were aspirated after 2 h, and fresh MEM containing d-valine (GIBCO) was added to prevent fibroblast proliferation. Twenty-four hours after plating, all dishes were shaken vigorously to remove microglia and debris. Astrocytes were kept at 37°C in a humidified 5% CO2/95% air incubator; media were changed twice weekly. Immunocytochemical staining with antibodies against GFAP or phosphorylation-independent tau (a gift from K. Kosik, Harvard Medical School, Cambridge, MA) revealed that >90% of cultured cells were astrocytes and <5% were neurons.
Pharmacological Manipulations.
Confluent astrocytes (≈7–10 days in vitro) were used for pharmacological studies. NE, R(−)-isoproterenol (+)-bitartrate, and S(−)-propranolol hydrochloride were purchased from Research Biochemicals; forskolin and 8Br-cAMP were from Calbiochem; cyclosporin A was from Alexis Biochemicals (San Diego); and dexnorfenfluramine hydrochloride was obtained from Technologie Servier (Neuilly-sur-Seine, France).
Western Blot Analysis of Proteins.
To detect APPs, cell media from astrocytes grown on 35-mm dishes were collected at the end of the incubation period (1 or 24 h). After centrifugation to remove debris, the media were desalted by filtration through Sephadex G-25 columns. The eluates were frozen on dry ice, dried by vacuum centrifugation, and resuspended in gel loading buffer. Cell-associated APP or GFAP was collected by scraping astrocytes in lysis buffer (60 mM Tris⋅HCl/4% SDS/20% glycerol/1 mM DTT) and ultrasonicating and boiling the samples for 5 min.
The total amount of cell protein per dish, estimated using the bicinchoninic acid assay, was not altered by pharmacological treatments. Bromphenol blue (0.1%) was added to each sample, and equal amounts of protein were loaded on SDS/10% polyacrylamide gels (≈75 μg per lane). Proteins were electroblotted onto Immobilon-P transfer membranes (Millipore) that were incubated with 5% powdered milk in Tris-buffered saline containing 0.15% Tween to block nonspecific binding, and were incubated overnight with the mAbs GFAP or 22C11 (both from Boehringer Mannheim), or with antisera R98 or R37 (gift of F. Kametani, Tokyo Institute of Psychiatry, Tokyo). The blots were rinsed, reincubated with an anti-mouse or anti-rabbit secondary antibody linked to peroxidase (Amersham), and developed on Kodak X-AR films using the chemiluminescence method (New England Nuclear).
Immunoreactive or autoradiograph bands were analyzed by densitometry using a LKB Ultrascan laser. All values were normalized to the control group within the same blot. ANOVA and Student’s t tests were used to compare differences between groups (significance level, P < 0.05).
Northern Blot Analysis and mRNA Extraction.
Total RNA from astrocytes grown on 100-mm dishes was extracted by the acid guanidium thiocynate-phenol-chloroform method (23). RNA concentration and purity were estimated using data from spectroscopy at 260 nm and 280 nm. Equal amounts of RNA (≈20 μg per lane) were fractionated by electrophoresis on 2.2 M formaldehyde/1.2% agarose gel and transferred onto a Hybond-N nylon membrane (Amersham).
Astrocytic mRNA was detected by hybridization with a ≈1.8-kb human APP cDNA (a gift of Dennis Selkoe, Brigham and Women’s Hospital, Harvard Medical School, Cambridge, MA), human β-actin cDNA, or human glyceraldehyde-3-phosphate dehyrogenase (hG3PDH) cDNA (both from CLONTECH). Blots were prehybridized in hybridization buffer (50% formamide/200 μg/ml salmon sperm DNA/1× Denhardt’s buffer/0.2% SDS/5× SSC/20 mM sodium phosphate, pH 6.5) for 1 h at 42°C, and hybridized overnight at 42°C with cDNA probes labeled with [32P]dCTP using random primed extension (Amersham Megaprime DNA labeling kit). The membrane was washed at room temperature in 2× SSC/0.1% SDS and again at 50°C in 0.1× SSC/0.1% SDS. Autoradiographs were obtained by exposing the membrane to Kodak X-AR films with intensifying screens at −80°C for 1–2 days. The membrane was stripped of 32P-labeled probes in 95°C water and reprobed with another cDNA.
Relative amounts of APP, β-actin, and G3PDH mRNA were estimated by densitometric analysis of autoradiographs. G3PDH labeling, used as an internal control for minor variations in the amount of mRNA loaded per lane, did not change noticeably between treatments. The amounts of mRNA coding for APP or for β-actin were corrected for the level of G3PDH mRNA and expressed as the ratio to mRNA levels obtained from untreated cells.
PI Hydrolysis.
Cells were labeled overnight with 1 μCi (1 Ci = 37 GBq) of myo-[2-3H]inositol in 2 ml of serum-free medium. Cells were rinsed and incubated with serum-free MEM/10 μM LiCl for 30 min before drug treatments. The treatment medium was aspirated after 1 h. Cells were lysed in 1 ml of ice-cold methanol and collected into tubes containing 1 ml of chloroform and 0.5 ml of water; the resulting suspensions were mixed vigorously and centrifuged at 6,000 × g for 10 min to increase phase separation. One milliliter of each sample was loaded onto AG1-X8 anion-exchange resin (Bio-Rad), and [3H]inositol phosphates were eluted with 4 ml of 1 M ammonium sulfate in 0.1 M formic acid. PI hydrolysis was estimated from measurements of [3H]inositol phosphates using liquid scintillation spectrometry.
cAMP Assay.
Levels of cAMP in astrocytes were measured with a [8-3H]cAMP assay kit (Amersham, TRK 432). Briefly, media were aspirated and cells were scraped in 0.8 ml of ethanol after rinsing with ice-cold PBS. After sonication, cell mixtures were left standing at room temperature for 5 min. The supernatant fluids were collected after centrifugation, dried in a rotary evaporator, and resuspended in 120 μl of Tris/EDTA buffer. Binding protein and [8-3H]adenosine 3′,5′-cyclic phosphate tracer were added to duplicate samples (50 μl each). After mixing, the samples were kept cold (2–8°C) for 2 h. A charcoal suspension (100 μl) was added to the samples before centrifugation at 12,000 × g for 10 min. A 200-μl supernatant fraction for each sample was removed for scintillation counting. The amount of cAMP (in picomoles) was estimated by comparing the radioactivity for each sample with that of known standards.
RESULTS
Adrenergic Receptors Coupled to cAMP Formation Stimulate APP Holoprotein and mRNA Production.
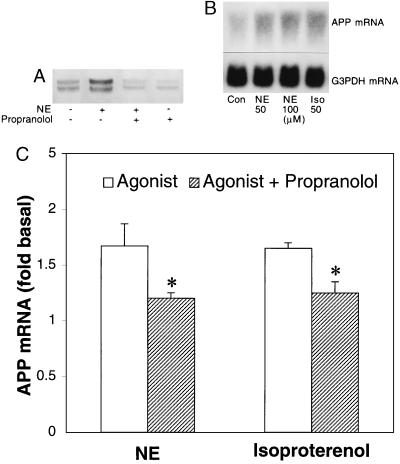
Norepinephrine (50 μM) treatment for 24 h increased the amount of cell-associated APP holoprotein in astrocytes by ≈1.7-fold relative to those in untreated cells (Fig. 1A), as measured using Western blots. This effect of NE was inhibited by the β-adrenergic antagonist propranolol (50 μM), which, on its own, had no significant effect on basal APP protein levels (P > 0.05). Treatment with NE (50 or 100 μM) or with the β-adrenergic agonist isoproterenol (50 μM) for 24 h also produced APP mRNA levels about 1.7- and 1.6-fold higher than those of untreated cells (Fig. 1B), as measured by Northern blotting. However, levels of APP mRNA or of holoprotein from astrocytes did not increase linearly with increasing NE concentrations (50–400 μM). Propranolol (50 μM) abolished the increases in APP mRNA caused by exposure to NE or to isoproterenol (Fig. 1C). Propranolol also inhibited the stimulatory effect of NE or isoproterenol (both 50 μM) on cAMP formation in astrocytes treated for 1 h (P < 0.05) but had no significant effect on the stimulated increase in PI hydrolysis (P > 0.05) (Fig. 2).
Figure 1.
Effects of NE and propranolol on the expression of cell-associated APP. Confluent monolayers of astrocytes were incubated for 24 h in serum-free media containing 50 μM NE. (A) Representative immunoblot with mAb 22C11 shows that astrocytes incubated with NE (50 μM) contained significantly more cell-associated APP than untreated cells, and that this increase was inhibited by propranolol (Prop, 50 μM). This experiment was replicated with similar results. (B) Representative Northern blot indicates that either 50 or 100 μM NE was equally effective in stimulating APP mRNA levels above those of controls (Con), and that this effect was mimicked by isoproterenol (Iso, 50 μM). G3PDH mRNA, used as a control for RNA loading, was not affected by drug treatments. (C) The stimulatory effect of NE or isoproterenol on APP mRNA synthesis was significantly inhibited by propranolol (∗, P < 0.05). The graph represents means and SEM obtained from three independent experiments.
Figure 2.
Effects of propranolol on the changes in PI hydrolysis and cAMP formation caused by NE treatment. PI hydrolysis and cAMP formation were significantly increased by 1-h treatment with NE (50 μM). Coincubation with propranolol (50 μM) inhibited the increase in cAMP formation caused by NE (∗, P < 0.05) but had no effect on the increase in PI hydrolysis. The graph represents means and SEM obtained from three independent experiments.
Cyclosporin A Inhibits APP Synthesis Stimulated by 8Br-cAMP or Forskolin.
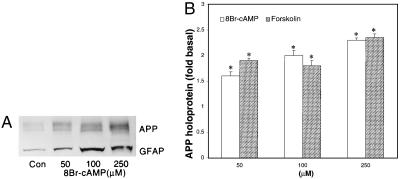
Exposure of astrocytes to the membrane-permeant 8Br-cAMP or to the adenylate cyclase activator forskolin for 24 h significantly increased their levels of APP holoprotein (P < 0.05), as analyzed using antibody 22C11 or antiserum R37 directed against the N terminus or C terminus of APP, respectively (Fig. 3). Use of antiserum R98, which recognizes an epitope of the KPI domain (24), indicated that KPI-containing APP isoforms in cortical astrocytes were similarly increased by treatments that elevated cAMP levels.
Figure 3.
Effects of 8Br-cAMP on cell-associated APP and GFAP from cultured astrocytes. Confluent astrocytes were treated with 8Br-cAMP for 24 h. (A) Representative immunoblot with R37 antiserum and GFAP mAb revealed increases in APP (≈120 kDa) and GFAP (≈50 kDa), respectively, with increasing concentrations of 8Br-cAMP. Similar results were obtained from three independent experiments. (B) Treatment for 24 h with increasing concentrations of 8Br-cAMP or forskolin (50, 100, or 250 μM) increased the amounts of cell-associated APP, as detected by antiserum R37 or mAb 22C11 (∗, P < 0.05). The graph represents data accumulated from three independent experiments.
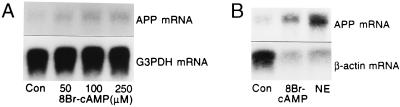
Northern blot analyzes showed that treatment with 8Br-cAMP (250 μM) also increased astrocytic APP mRNA levels to ≈1.8-fold those of untreated cells (Fig. 4A), indicating that prolonged (≈24 h) cAMP signaling in cultured astrocytes can increase APP expression by transcriptional activation. Exposure to lower concentrations (50 or 100 μM) of 8Br-cAMP occasionally increased APP mRNA but these effects were not consistent (P > 0.05), nor did 8Br-cAMP (250 μM) increase APP mRNA after shorter exposures (6 or 12 h).
Figure 4.
Effects of 8Br-cAMP or NE on β-actin and APP mRNA. Astrocytes were prepared as in Fig. 1. (A) Representative Northern blot indicates an increase in APP mRNA with increasing concentrations (50, 100, or 250 μM) of 8Br-cAMP. G3PDH mRNA was unaffected by 8Br-cAMP treatments. Subsequent experiments showed that 250 μM was the most effective and reliable 8Br-cAMP concentration for stimulating APP synthesis. (B) Representative Northern blot shows that 8Br-cAMP (250 μM) or NE (50 μM) treatment for 24 h increased APP mRNA and decreased β-actin mRNA levels. Three independent experiments produced similar effects.
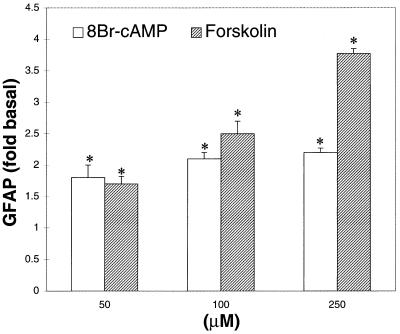
The increases in APP mRNA caused by 8Br-cAMP (250 μM) or NE (50 μM) were associated with decreases in levels of mRNA for β-actin (Fig. 4B). GFAP expression in cultured astrocytes was also increased by forskolin or 8Br-cAMP (Figs. 3A and 5), both of which significantly increased cAMP levels compared with those of untreated cells (P < 0.05). APPs levels in astrocytes treated with or without 8Br-cAMP (250 μM for 24 h) did not differ significantly (P > 0.05).
Figure 5.
Effects of 8Br-cAMP or forskolin on GFAP expression in cultured astrocytes. Astrocytes were prepared as in Fig. 1. 8Br-cAMP or forskolin (both at 50, 100, or 250 μM) caused significant increases in GFAP protein, as assayed using Western blotting (∗, P < 0.05). The graph represents means and SEM obtained from three independent experiments.
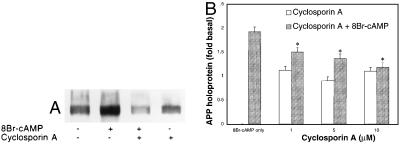
Cyclosporin A (1, 5, or 10 μM) treatment for 24 h inhibited the increases in APP mRNA and APP holoprotein caused by 8Br-cAMP (250 μM for 24 h) treatment (Fig. 6A). The inhibitory effects did not exhibit dose-dependence in the range studied (P > 0.05) (Fig. 6B); moreover, cyclosporin A failed to affect basal APP holoprotein levels, APPs secretion, or GFAP expression (P > 0.05).
Figure 6.
Effect of cyclosporin A on APPs secretion and on the increase in cell-associated APP caused by 8Br-cAMP. Astrocytes were prepared as in Fig. 1. (A) Representative immunoblot shows that the increase in cell-associated APP caused by 24-h treatment with 8Br-cAMP (250 μM) was suppressed by cotreatment with 1 μM cyclosporin A, which, by itself, had no effect on basal APP levels. Although astrocytes treated with 8Br-cAMP and cyclosporin A appeared to have slightly lower APP levels than untreated cells, this effect was not significant and was not reproduced in subsequent experiments. (B) The increase in cell-associated APP caused by 24-h treatment with 8Br-cAMP (250 μM) was significantly suppressed (∗, P < 0.05) by the three doses of cyclosporin A (1, 5, or 10 μM) tested. Thr graph represents means and SEM of pooled data obtained from four independent experiments.
Activation of PKC or Hydrolysis of PI Stimulates APPs Secretion but Not APP Synthesis.
Phorbol 12-myristate 13-acetate (5 μM) or activation of serotoninergic receptors by dexnorfenfluramine (100 μM), which stimulated PI hydrolysis by ≈2.6-fold relative to rates in untreated astrocytes (P < 0.05), did not increase APP mRNA or holoprotein levels (P > 0.05). Treatment for 1 h with phorbol 12-myristate 13-acetate (5 μM) or dexnorfenfluramine (100 μM) did increase APPs secretion to ≈3.0- and ≈2.2-fold, respectively, that of untreated cells (P < 0.05).
DISCUSSION
Our data demonstrate that activation of adrenergic receptors coupled to increased cAMP formation stimulates APP overexpression in astrocyte cultures. The increases in APP mRNA and APP holoprotein caused by treatment with NE (50, 100, or 250 μM for 24 h) were inhibited by the β-adrenergic antagonist propranolol. Since propranolol inhibited the increase in cAMP formation but not the increase in PI hydrolysis caused by NE, the stimulatory effect of NE on APP synthesis is probably mediated by increased cAMP formation. Treatment of astrocytes with the β-adrenergic agonist isoproterenol (50 μM for 24 h), which stimulated cAMP formation, similarly increased APP mRNA and holoprotein. Lower concentrations of NE or isoproterenol (both 10 μM) did not consistently stimulate APP production, as analyzed on Western blots (unpublished data). Furthermore, we recently reported that treatment of cultured astrocytes for 24 h with prostaglandin E2 (10 μM) increased both cAMP production, and the levels of APP holoprotein and mRNA (25). Thus, our results show that cAMP elevations can promote the accumulation of cell-associated APP holoprotein by activating APP gene expression.
Although PKC reportedly increases APP promoter activity and APP gene transcription in some cells (26), the production of APP mRNA by astrocytes in our study was not increased when PKC was activated with phorbol ester. Consistent with findings of other studies (16–20) was our observation that exposure to phorbol ester increased APPs secretion in cortical astrocytes. That this effect resulted from accelerated APP metabolism, not synthesis, is suggested by the fact that stimulation of 5-HT2a or 5-HT2c serotoninergic receptors by dexnorfenfluramine, which increased PI hydrolysis (27), failed to increase astrocytic APP mRNA even though this treatment did increase APPs secretion.
Further evidence that adrenergic stimulation of APP synthesis is mediated by cAMP production but not by PI hydrolysis was provided by our observation that treatment with 8Br-cAMP or with forskolin, which activates adenylate cyclase, also increased APP holoprotein as detected by antibody 22C11 (28) or antisera R37 (24) directed at the N and C termini of APP, respectively. Because APPs secretion from astrocytes was not significantly decreased by 8Br-cAMP or forskolin (both 250 μM) treatments, the increase in cell-associated APP cannot be explained by APP degradation.
Northern blot analysis indicated that the stimulation of APP expression by cAMP can result from transcriptional activation of the APP gene. Treatment with 250 μM 8Br-cAMP for 24 h increased astrocytic APP mRNA levels to about 200% those in untreated cells. Although treatment with 50 or 100 μM 8Br-cAMP did not consistently stimulate APP mRNA production, these concentrations did significantly increase the levels of cell-associated APP in cultured astrocytes. Because elevations in cAMP levels inhibit APP processing (18–20), we suggest that the increase in cell-associated APP with lower concentration (50 or 100 μM) 8Br-cAMP treatment results from decreased APPs secretion, independent of APP mRNA synthesis.
Neurodegeneration in AD is associated with an increase in APP isoforms containing KPI (12, 13). Antiserum R98, which recognizes an epitope of the KPI domain on Western blots (24), confirmed that 8Br-cAMP treatment increased the amount of KPI-containing APP isoforms in cortical astrocytes. Thus, astrocytes can be a significant non-neuronal source of APP. While elevations in cAMP stimulated APP synthesis in serum-deprived astrocytes (our study), it seemed unable to do so in the presence of serum (29).
Examination of postmortem brains has shown that β2-adrenergic receptor density is elevated in frontal cortex and hippocampus in aging and in AD (30). Because astrocytes are the principal cell type in the brain that expresses β2-adrenergic receptors, it has been suggested that this elevation reflects astrocytic proliferation induced by neuronal injury (30). If this is so, overactivation of β2-adrenergic receptors in astrocytes by circulating NE in AD or aging (31), may promote aberrant cAMP signaling, resulting in increased GFAP immunoreactivity and APP overexpression.
Reactive astrocytes in brains of AD patients, or in those of transgenic mice overexpressing APP, are highly immunoreactive for GFAP, an intermediate filament protein that is up-regulated in brain inflammation (9, 14). Our cultured astrocytes responded to 8Br-cAMP by increasing GFAP expression and also by decreasing levels of β-actin mRNA. The down-regulation of actin may be related to the morphological differentiation of astrocytes from flat, polygonal cells to process-bearing astrocytes (32). Therefore, cultured astrocytes treated with 8Br-cAMP resemble the in vivo morphological and biochemical changes of reactive astrocytes associated with brain injury and AD (14, 15).
The APP promoter contains several regulatory regions that can be activated by physiologic stress, cytokines, or growth factors (26). The induction of APP synthesis by cAMP signaling may be mediated by a consensus binding sequence for cAMP response element on the APP promoter. The immunosuppressants cyclosporin A or FK-506 prevented cAMP response element-mediated transcription in pancreatic islet cells that were stimulated by cAMP (33). In our study, the increases in APP mRNA and APP protein caused by 8Br-cAMP was effectively blocked by cyclosporin A, which by itself had no significant effect on basal APP levels or GFAP expression. Hence, immunosuppressants may be useful in preventing aberrant APP expression and, possibly, the production of Aβ or neurotoxic peptides in reactive astrocytes.
In summary, our studies show that stimulation of adrenergic receptors coupled with increased cAMP formation in astrocytes increases the production of APP mRNA and APP holoprotein. Aging and brain injury are frequently associated with reactive astrocytes and astrocyte proliferation (14, 34). In our astrocyte cultures, elevations in cAMP levels increased GFAP and APP expression, suggesting that the gliotic response of astrocytes in AD (14) includes increased production of cell-associated APP holoprotein. Inasmuch as APP overexpression promotes neurodegeneration, neurite dystrophy, and cognitive dysfunction (6, 8), modulation of cAMP signaling by cell-surface receptors or immunosuppressants may be useful in preventing the progression of AD.
Acknowledgments
We thank Dr. F. Kametani (Tokyo Institute of Psychiatry) for R37 and R98 antisera, and Dr. D. J. Selkoe (Harvard Medical School) for APP cDNA. We are grateful to B. H. J. Tan and U. Ingrid Richardson for technical assistance involving Northern blots and cyclosporin A, respectively. This work was supported by the National Institutes of Health (Grant MH-28783) and The Center for Brain Sciences and Metabolism Charitable Trust.
ABBREVIATIONS
- AD
Alzheimer disease
- APP
amyloid precursor protein
- APPs
soluble, nonamyloidogenic APP
- GFAP
glial fibrillary acidic protein
- KPI
Kunitz-type protease inhibitor
- PI
phosphatidylinositol
- PKC
protein kinase C
- NE
l-norepinephrine bitartate
- 8Br-cAMP
8-bromo-adenosine 3′,5′-cyclic monophosphate
- G3PDH
glyceraldehyde-3-phosphate dehyrogenase
References
- 1.Alzheimer A. Allg Z Psychiatr Psychol-Gerichtl Med. 1907;64:146–148. [Google Scholar]
- 2.Simchowicz T. Histol Histopathol Arb. 1910;4:267–444. [Google Scholar]
- 3.Kang J, Lemaire H-G, Unterbeck A, Salbaum J, Masters C, Grzeschik K-H, Multhaup G, Beyreuther K, Muller-Hill B. Nature (London) 1987;325:733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- 4.Esch F S, Keim P S, Beattie E C, Blacher R W, Culwell A R, Oltersdorf T, McClure D, Ward P. Science. 1990;248:1122–1124. doi: 10.1126/science.2111583. [DOI] [PubMed] [Google Scholar]
- 5.Malamud N. In: Aging and the Brain. Gaitz C M, editor. New York: Plenum; 1972. pp. 63–87. [Google Scholar]
- 6.Yoshikawa K, Aizawa T, Hayashi Y. Nature (London) 1992;7:64–67. doi: 10.1038/359064a0. [DOI] [PubMed] [Google Scholar]
- 7.Moran P M, Higgins L S, Cordell B, Moser P C. Proc Natl Acad Sci USA. 1995;92:5341–5345. doi: 10.1073/pnas.92.12.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Higgins L S, Rodems J M, Catalano R, Quon D, Cordell B. Proc Natl Acad Sci USA. 1995;92:4402–4406. doi: 10.1073/pnas.92.10.4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsiao K K, Borchelt D R, Olson K, Johannsdottir R, Kitt C, Yunis W, Xu S, Eckman C, Younkin S, Price D, Iadecola C, Clark H B, Carlson G. Neuron. 1995;15:1203–1218. doi: 10.1016/0896-6273(95)90107-8. [DOI] [PubMed] [Google Scholar]
- 10.Kitaguchi N, Takahashi Y, Tokushima Y, Shiojiri S, Ito H. Nature (London) 1988;331:530–532. doi: 10.1038/331530a0. [DOI] [PubMed] [Google Scholar]
- 11.Tanzi R E, McClatchey A I, Lamperti E D, Villa-Komaroff L L, Gusella J F, Neve R L. Nature (London) 1988;331:528–530. doi: 10.1038/331528a0. [DOI] [PubMed] [Google Scholar]
- 12.Golde T E, Estus S, Usiak M, Younkin L H, Younkin S G. Neuron. 1990;4:253–267. doi: 10.1016/0896-6273(90)90100-t. [DOI] [PubMed] [Google Scholar]
- 13.Neve R L, Finch E A, Dawes L R. Neuron. 1988;1:669–677. doi: 10.1016/0896-6273(88)90166-3. [DOI] [PubMed] [Google Scholar]
- 14.Brun A, Liu X, Erikson C. Neurodegeneration. 1995;4:171–177. doi: 10.1006/neur.1995.0021. [DOI] [PubMed] [Google Scholar]
- 15.Siman R, Card J P, Nelson R B, Davis L G. Neuron. 1989;3:275–285. doi: 10.1016/0896-6273(89)90252-3. [DOI] [PubMed] [Google Scholar]
- 16.Nitsch R M, Slack B E, Wurtman R J, Growdon J H. Science. 1992;258:304–307. doi: 10.1126/science.1411529. [DOI] [PubMed] [Google Scholar]
- 17.Slack B E, Nitsch R M, Livneh E, Kunz G M, Jr, Breu J, Eldar H, Wurtman R J. J Biol Chem. 1993;268:21097–21101. [PubMed] [Google Scholar]
- 18.Lee R K K, Jimenez J, Cox A J, Wurtman R J. In: The Neurobiology of Alzheimer’s Disease. Wurtman R J, Corkin S, Growdon J H, Nitsch R, editors. New York: N.Y. Acad. Sci.; 1996. pp. 338–343. [Google Scholar]
- 19.Lee, R. K. K. & Wurtman, R. J. (1997) J. Neurochem., in press. [DOI] [PubMed]
- 20.Efthimiopoulos S, Punj S, Manolopoulos V, Pangalos M, Wang G P, Refelo L M, Robakis N K. J Neurochem. 1996;67:872–875. doi: 10.1046/j.1471-4159.1996.67020872.x. [DOI] [PubMed] [Google Scholar]
- 21.Lee R K K, Araki W, Wurtman R J. Neurobiol Aging. 1996;17:S201. (abstr.). [Google Scholar]
- 22.McCarthy K D, de Vellis J. J Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chomcznski P, Saachi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 24.Kametani F, Tanaka K, Ishii T, Ikeda S, Kennedy H E, Allsop D. Biochem Biophys Res Commun. 1993;191:392–398. doi: 10.1006/bbrc.1993.1230. [DOI] [PubMed] [Google Scholar]
- 25.Lee, R. K., Knapp, S., Zarach, J. & Wurtman, R. J. (1997) J. Neurochem. 69, in press.
- 26.Lahiri D K, Nall C. Mol Brain Res. 1995;32:233–240. doi: 10.1016/0169-328x(95)00078-7. [DOI] [PubMed] [Google Scholar]
- 27.Nitsch R M, Deng M, Growdon J H, Wurtman R J. J Biol Chem. 1996;271:4188–4194. doi: 10.1074/jbc.271.8.4188. [DOI] [PubMed] [Google Scholar]
- 28.Weidemann A, Konig G, Bunke D, Fischer P, Salbaum J M, Masters C L, Beyreuther K. Cell. 1989;57:115–126. doi: 10.1016/0092-8674(89)90177-3. [DOI] [PubMed] [Google Scholar]
- 29.Gegelashvili G, Bock E, Schousboe A, Linnemann D. Mol Brain Res. 1996;37:151–156. doi: 10.1016/0169-328x(95)00302-9. [DOI] [PubMed] [Google Scholar]
- 30.Kalaria R N, Andorn A C, Tabaton M, Whitehouse P J, Harik S I, Unnerstall J R. J Neurochem. 1989;53:1772–1781. doi: 10.1111/j.1471-4159.1989.tb09242.x. [DOI] [PubMed] [Google Scholar]
- 31.Peskind E R, Wingerson D, Murray S, Pascualy M, Dobie D J, Le Corre P, Le Verge R, Veith R C, Raskind M A. Arch Gen Psychiatry. 1995;52:774–782. doi: 10.1001/archpsyc.1995.03950210068012. [DOI] [PubMed] [Google Scholar]
- 32.Ferrier R, Had L, Rabie A, Faivre-Sarrailh C. Cell Motil Cytoskeleton. 1994;28:303–316. doi: 10.1002/cm.970280404. [DOI] [PubMed] [Google Scholar]
- 33.Schwaninger M, Blume R, Kruger M, Lux G, Oetjen E, Knepel W. J Biol Chem. 1995;270:8860–8866. doi: 10.1074/jbc.270.15.8860. [DOI] [PubMed] [Google Scholar]
- 34.Hansen L A, Armstrong D N, Terry R D. Neurobiol Aging. 1987;8:1–6. doi: 10.1016/0197-4580(87)90051-0. [DOI] [PubMed] [Google Scholar]