Abstract
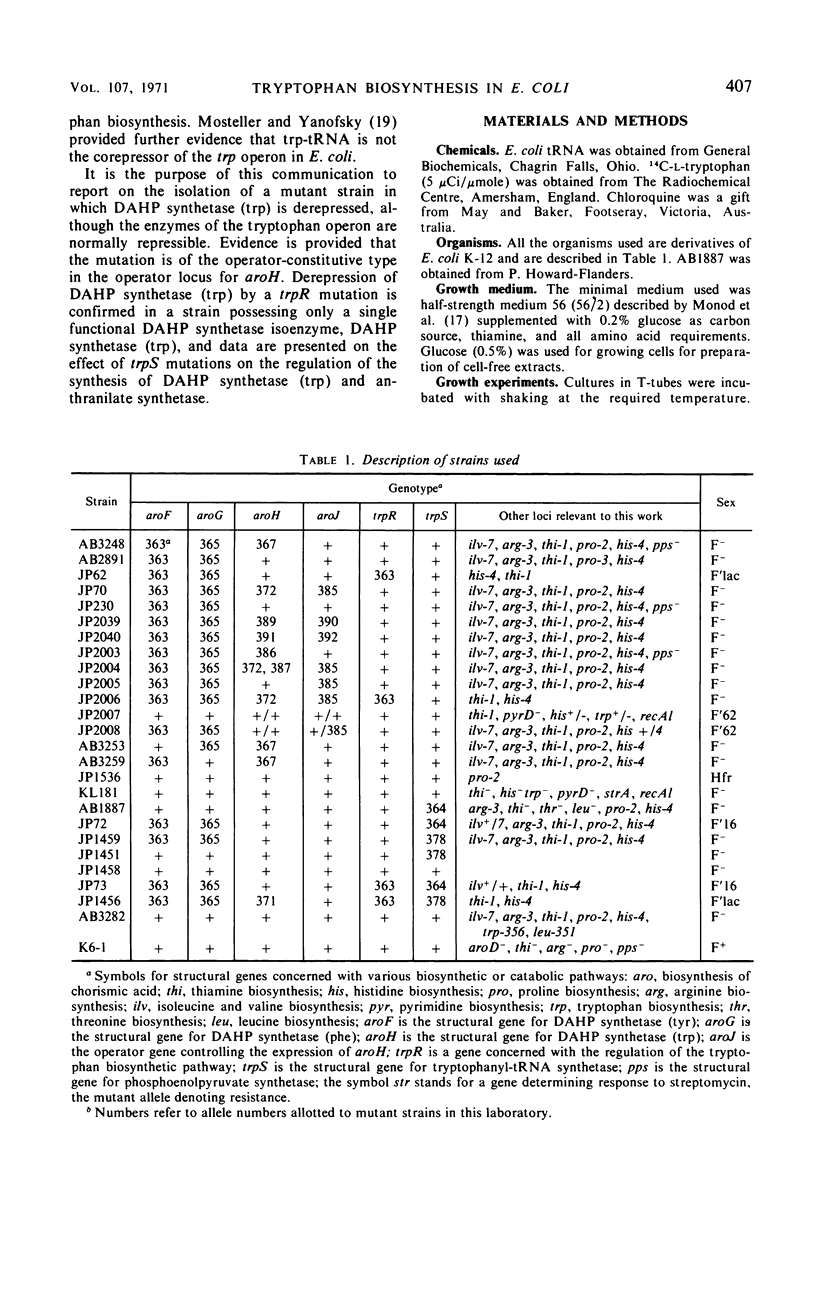
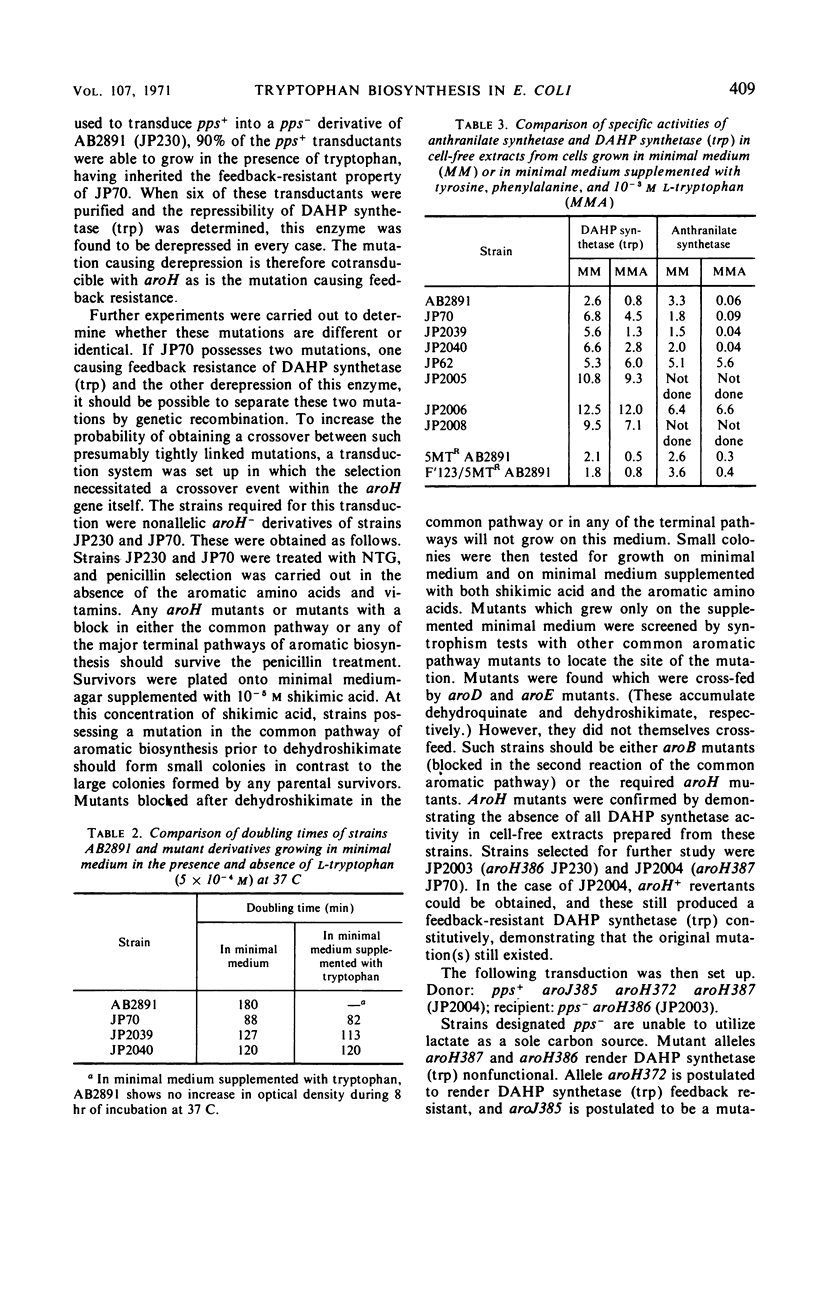
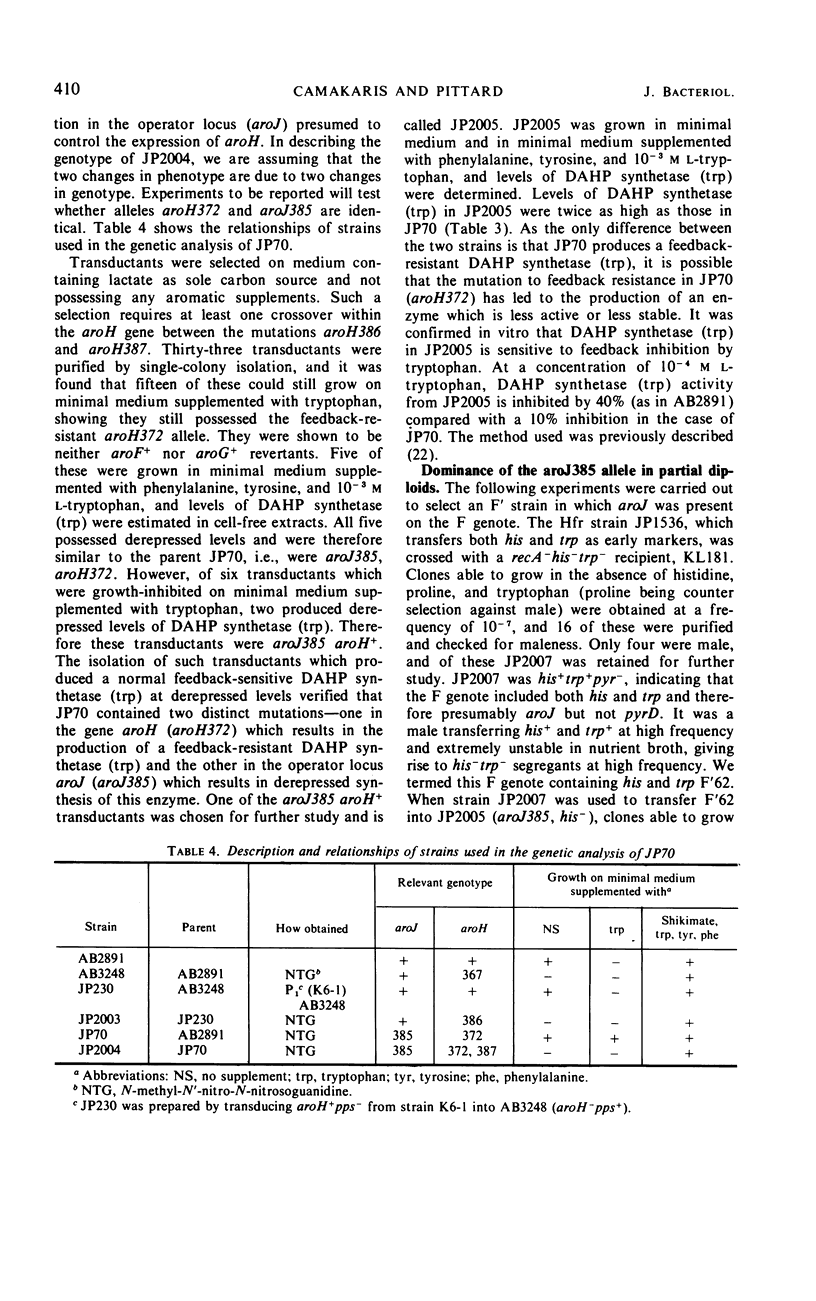
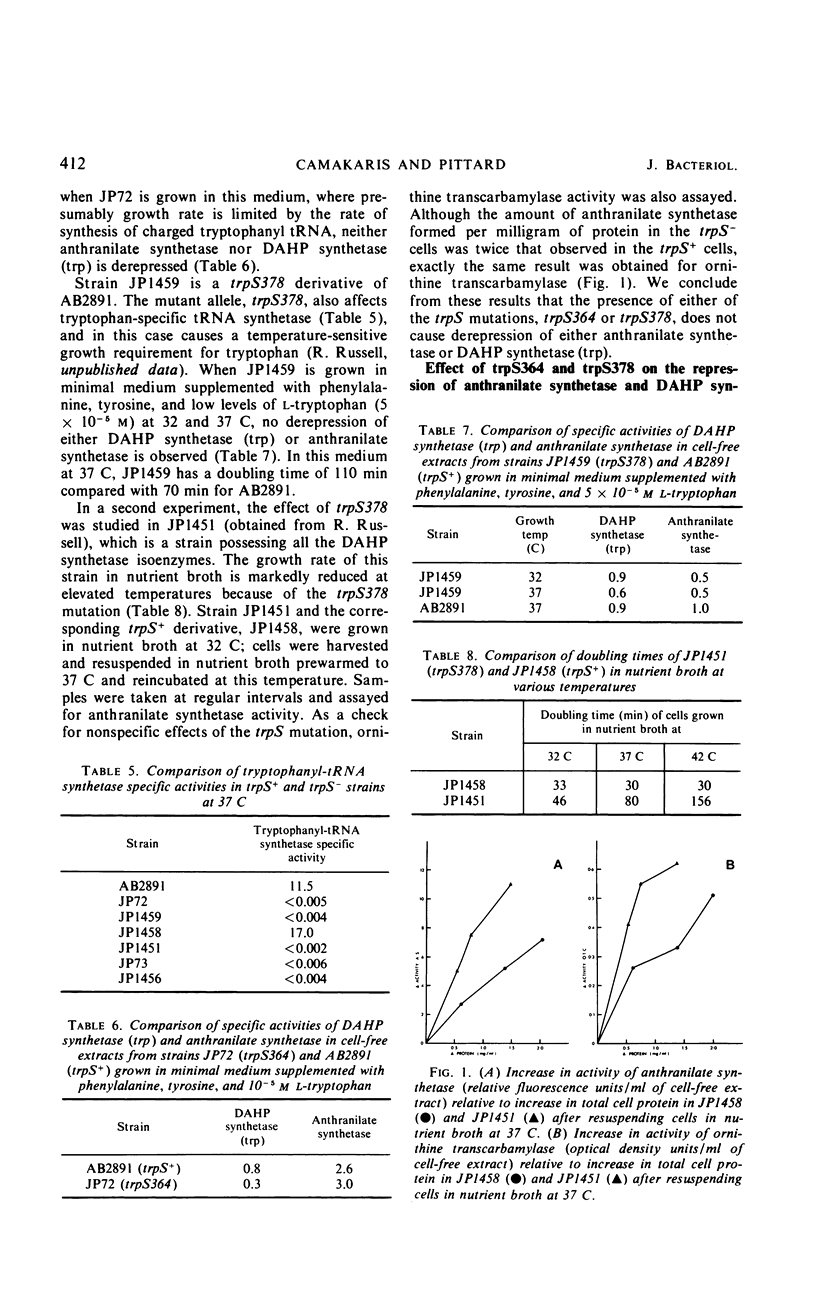
Mutant strains of Escherichia coli K-12 have been isolated in which the synthesis of 3-deoxy-d-arabinoheptulosonic acid-7-phosphate (DAHP) synthetase (trp) is partially constitutive. The mutation causing derepression is closely linked to aroH [the structural gene for DAHP synthetase (trp)] and occurs in a locus designated aroJ. The aroJ mutation is not recessive in an aroJ+/aroJ− diploid strain, as the synthesis of DAHP synthetase (trp) is still derepressed in this strain. On the basis of its close linkage to aroH and its continued expression in an aroJ+/aroJ− diploid, it is postulated that aroJ is an operator locus controlling the expression of the structural gene aroH. In support of this conclusion, the synthesis of anthranilate synthetase is still normally repressible in aroJ− strains, whereas, in trpR− strains, both DAHP synthetase (trp) and anthranilate synthetase are synthesized constitutively. The synthesis of DAHP synthetase (trp) remains repressible in an operator-constitutive mutant of the tryptophan operon. In two trpS mutants which possess defective tryptophanyl transfer ribonucleic acid synthetase enzymes, neither DAHP synthetase (trp) nor anthranilate synthetase derepress under conditions in which the defective synthetase causes a decrease in growth rate. On the other hand, an effect of the trpS mutant alleles on the level of anthranilate synthetase has been observed in strains which are derepressed for the synthesis of this enzyme, because of a mutation in the gene trpR. Possible explanations for this effect are presented.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown K. D. Regulation of aromatic amino acid biosynthesis Escherichia coli K12. Genetics. 1968 Sep;60(1):31–48. doi: 10.1093/genetics/60.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle W. F., Yanofsky C. Mutants of Escherichia coli with an altered tryptophanyl-transfer ribonucleic acid synthetase. J Bacteriol. 1968 Apr;95(4):1283–1294. doi: 10.1128/jb.95.4.1283-1294.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EIDLIC L., NEIDHARDT F. C. ROLE OF VALYL-SRNA SYNTHETASE IN ENZYME REPRESSION. Proc Natl Acad Sci U S A. 1965 Mar;53:539–543. doi: 10.1073/pnas.53.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraga S. Operator mutants of the tryptophan operon in Escherichia coli. J Mol Biol. 1969 Jan 14;39(1):159–179. doi: 10.1016/0022-2836(69)90340-4. [DOI] [PubMed] [Google Scholar]
- Hirota Y. THE EFFECT OF ACRIDINE DYES ON MATING TYPE FACTORS IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1960 Jan;46(1):57–64. doi: 10.1073/pnas.46.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J., Crawford I. P. Regulation of the enzymes of the tryptophan pathway in Escherichia coli. Genetics. 1965 Dec;52(6):1303–1316. doi: 10.1093/genetics/52.6.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Hiraga S., Yura T. Temperature-sensitive repression of the tryptophan operon in Escherichia coli. J Bacteriol. 1969 Jul;99(1):279–286. doi: 10.1128/jb.99.1.279-286.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Hiraga S., Yura T. Tryptophanyl transfer RNA synthetase and expression of the tryptophan operon in the trpS mutants of Escherichia coli. Genetics. 1969 Mar;61(3):521–538. doi: 10.1093/genetics/61.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano Y., Matsushiro A., Shimura Y. Isolation of the novel regulatory mutants of the tryptophan biosynthetic system in Escherichia coli. Mol Gen Genet. 1968;102(1):15–26. doi: 10.1007/BF00341866. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lavallé R., De Hauwer G. Tryptophan messenger translation in Escherichia coli. J Mol Biol. 1970 Jul 28;51(2):435–447. doi: 10.1016/0022-2836(70)90153-1. [DOI] [PubMed] [Google Scholar]
- MONOD J., COHEN-BAZIRE G., COHN M. Sur la biosynthèse de la beta-galactosidase (lactase) chez Escherichia coli; la spécificité de l'induction. Biochim Biophys Acta. 1951 Nov;7(4):585–599. doi: 10.1016/0006-3002(51)90072-8. [DOI] [PubMed] [Google Scholar]
- Morse D. E., Yanofsky C. Amber mutants of the trpR regulatory gene. J Mol Biol. 1969 Aug 28;44(1):185–193. doi: 10.1016/0022-2836(69)90413-6. [DOI] [PubMed] [Google Scholar]
- Mosteller R. D., Yanofsky C. Evidence that tryptophanyl transfer ribonucleic acid is not the corepressor of the tryptophan operon of Escherichia coli. J Bacteriol. 1971 Jan;105(1):268–275. doi: 10.1128/jb.105.1.268-275.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nass G. Regulation of histidine biosynthetic enzymes in a mutant of Escherichia coli with an altered histidyl-tRNA synthetase. Mol Gen Genet. 1967;100(2):216–224. doi: 10.1007/BF00333608. [DOI] [PubMed] [Google Scholar]
- Pittard J., Camakaris J., Wallace B. J. Inhibition of 3-deoxy-d-arabinoheptulosonic acid-7-phosphate synthetase (trp) in Escherichia coli. J Bacteriol. 1969 Mar;97(3):1242–1247. doi: 10.1128/jb.97.3.1242-1247.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittard J., Wallace B. J. Distribution and function of genes concerned with aromatic biosynthesis in Escherichia coli. J Bacteriol. 1966 Apr;91(4):1494–1508. doi: 10.1128/jb.91.4.1494-1508.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Mosteller R. D., Yanofsky C. Tryptophan messenger ribonucleic acid elongation rates and steady-state levels of tryptophan operon enzymes under various growth conditions. J Mol Biol. 1970 Aug;51(3):541–550. doi: 10.1016/0022-2836(70)90007-0. [DOI] [PubMed] [Google Scholar]
- SCHLESINGER S., MAGASANIK B. EFFECT OF ALPHA-METHYLHISTIDINE ON THE CONTROL OF HISTIDINE SYNTHESIS. J Mol Biol. 1964 Sep;9:670–682. doi: 10.1016/s0022-2836(64)80174-1. [DOI] [PubMed] [Google Scholar]
- Wallace B. J., Pittard J. Chromatography of 3-deoxy-D-arabinoheptulosonic acid-7-phosphate synthetase (trp) on diethylaminoethyl cellulose: a correction. J Bacteriol. 1967 Oct;94(4):1279–1280. doi: 10.1128/jb.94.4.1279-1280.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace B. J., Pittard J. Genetic and biochemical analysis of the isoenzymes concerned in the first reaction of aromatic biosynthesis in Escherichia coli. J Bacteriol. 1967 Jan;93(1):237–244. doi: 10.1128/jb.93.1.237-244.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace B. J., Pittard J. Regulation of 3-deoxy-D-arabino-heptulosonic 7-phosphate acid synthetase activity in relation to the synthesis of the aromatic vitamins in Escherichia coli K-12. J Bacteriol. 1969 Sep;99(3):707–712. doi: 10.1128/jb.99.3.707-712.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]



