Abstract
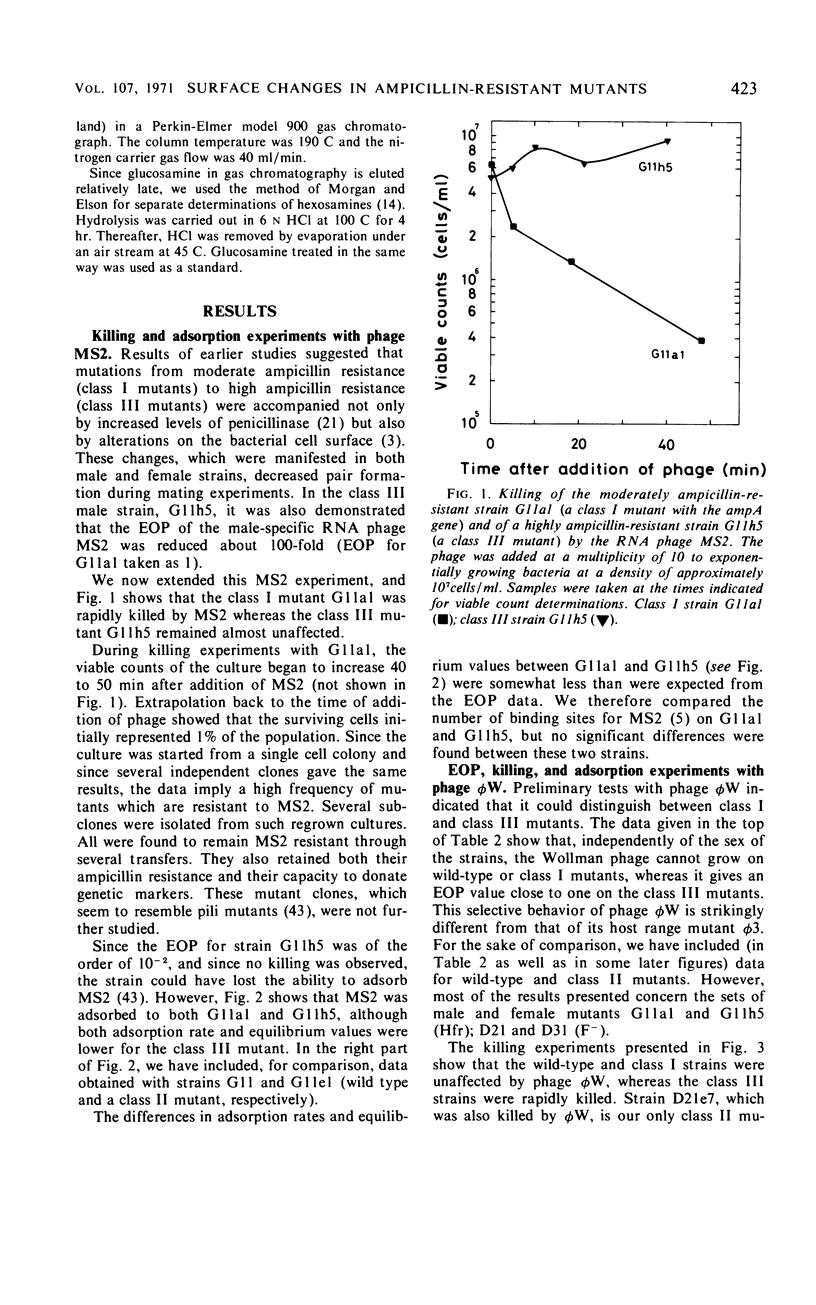
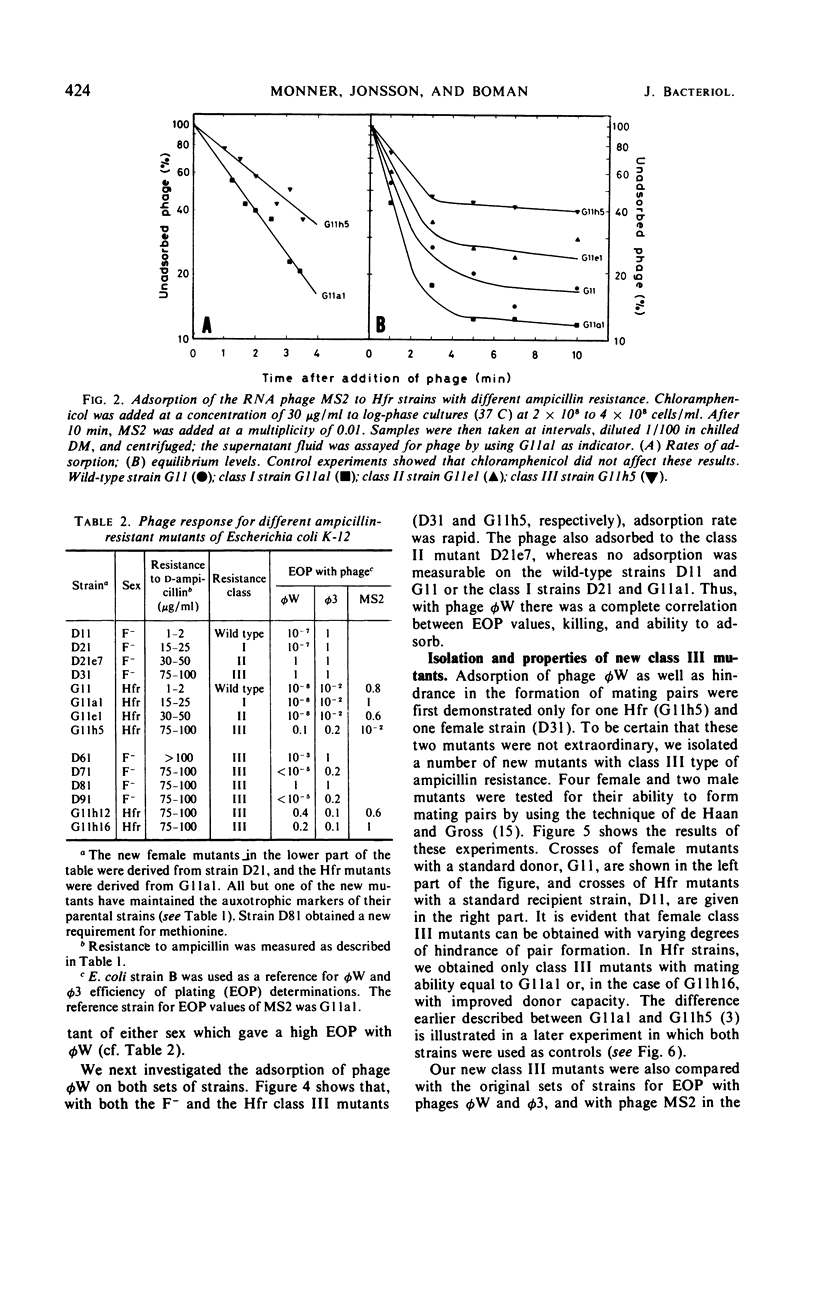
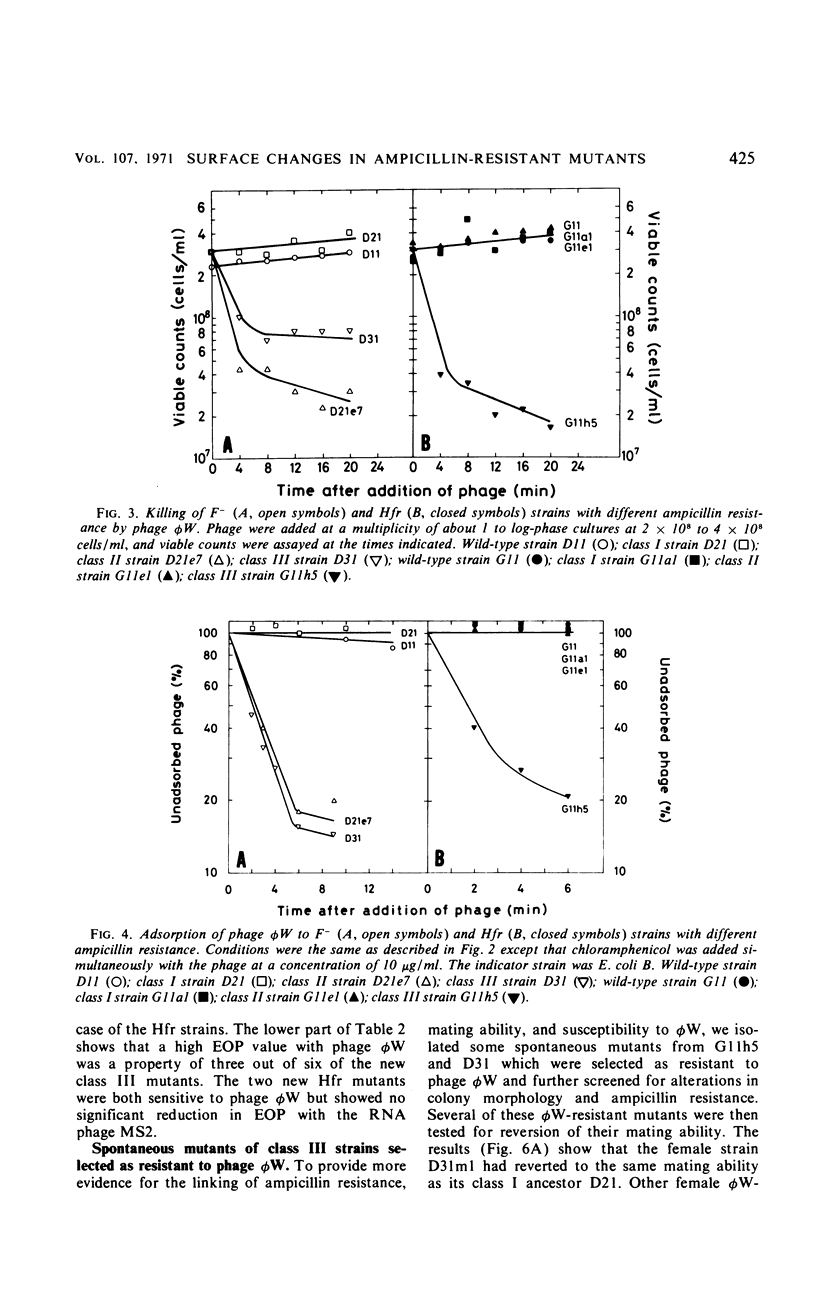
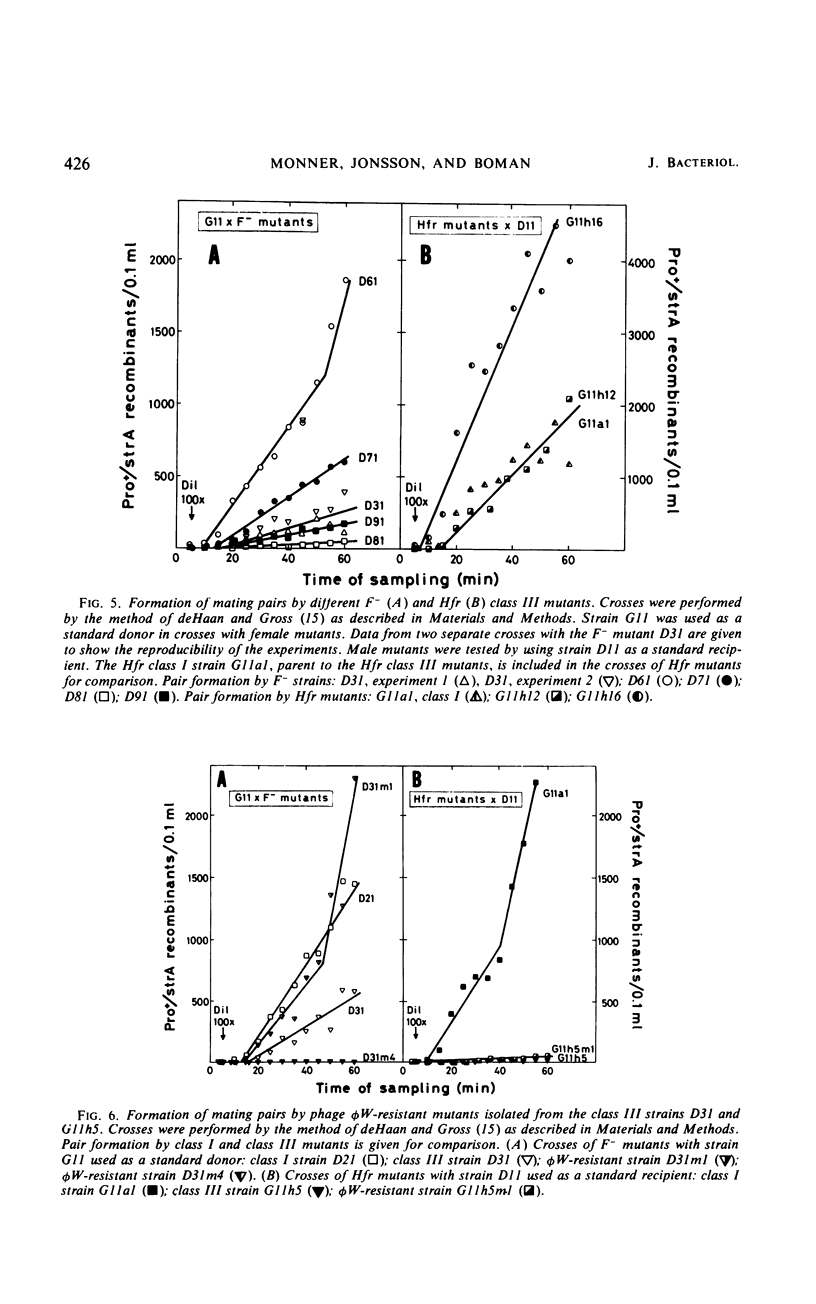
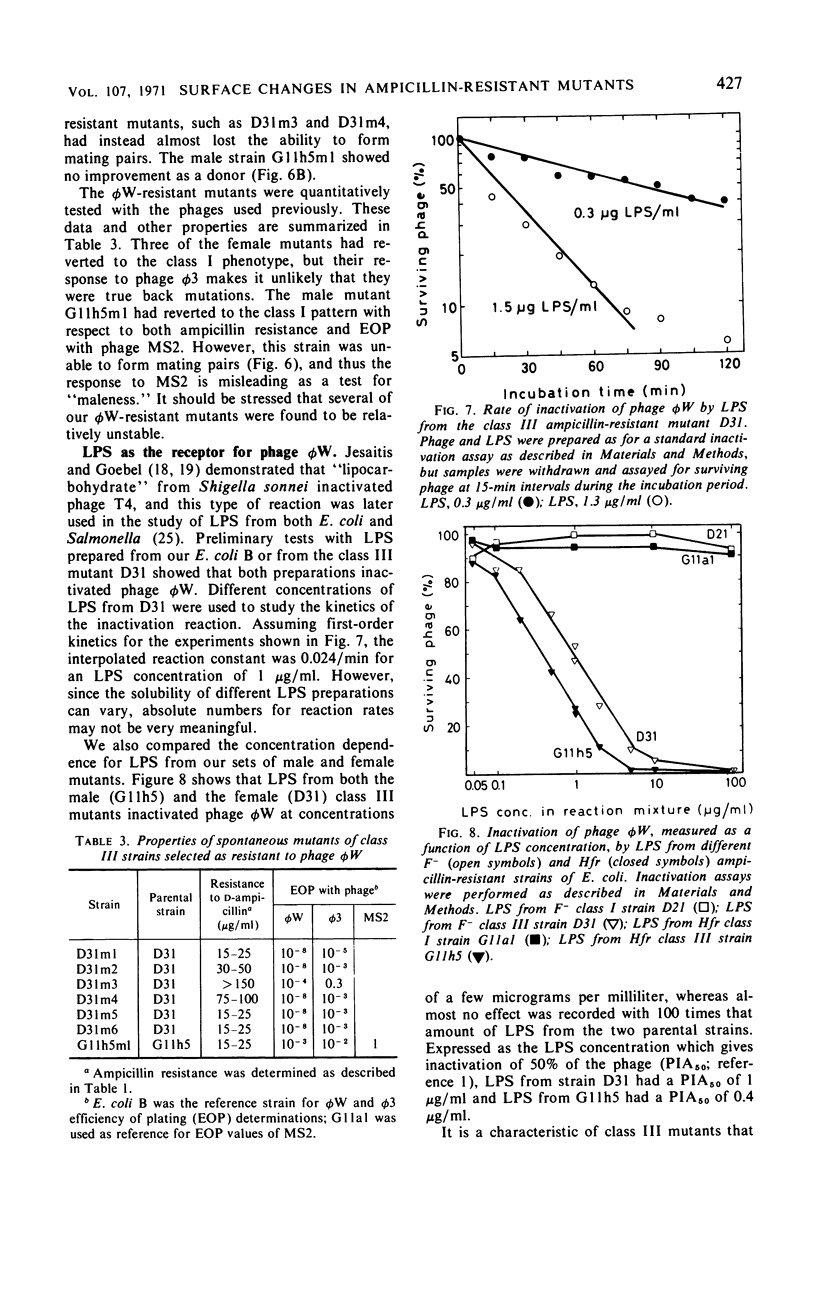
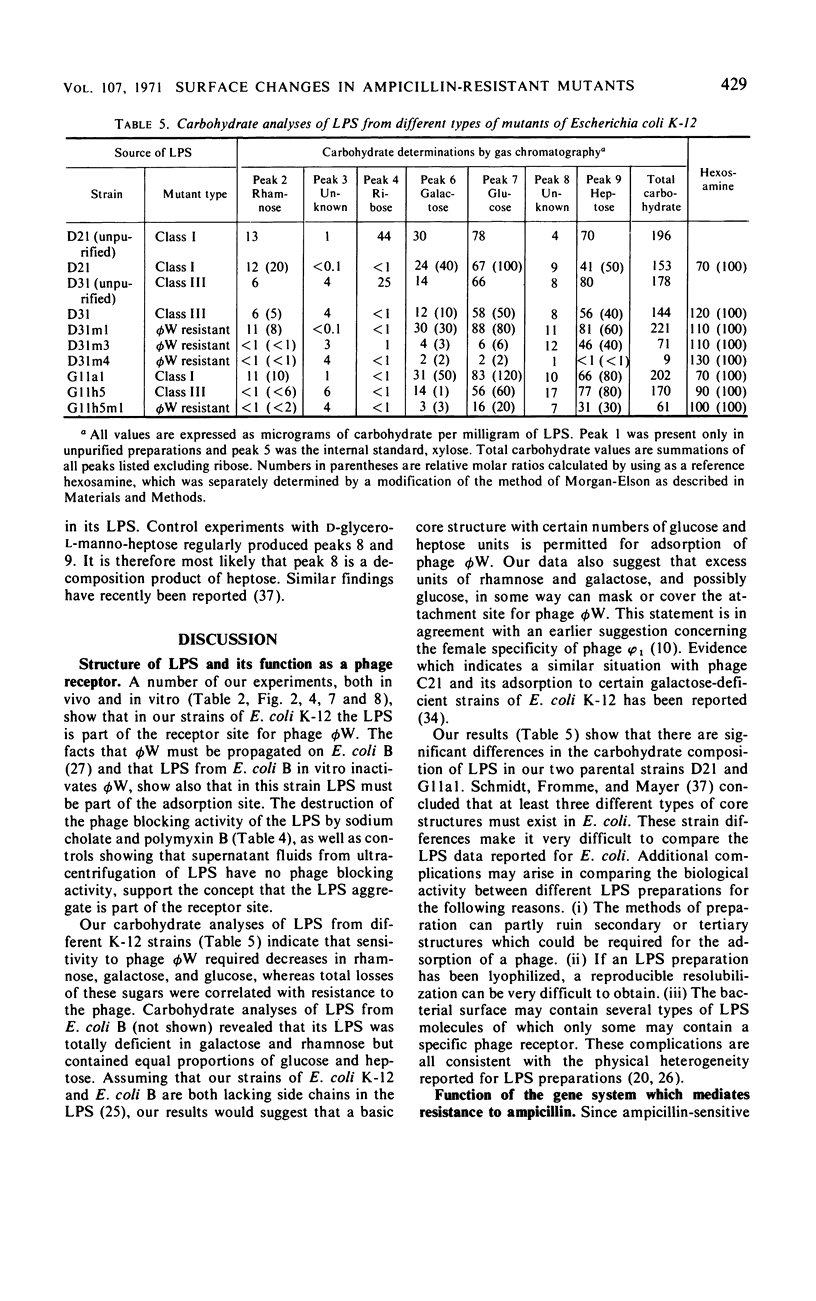
Mutations from moderate (class I) to high (class III) ampicillin resistance in a male and a female strain of Escherichia coli K-12 have been found to be accompanied by surface alterations, first demonstrated as hindrance in the formation of mating pairs. These changes have now been studied with the ribonucleic acid phage MS2, and especially with the “female-specific” phage φW. Several class III mutations in male and female strains were found to make the cells susceptible to phage φW and to reduce their abilities to form mating pairs. Spontaneous phage φW-resistant mutants isolated from class III strains were found also to have acquired changes in ampicillin resistance and ability to form mating pairs. One mutant had reverted to parental class I type in all three properties. Lipopolysaccharides (LPS) prepared from φW-sensitive class III strains inactivated the phage in vitro, whereas LPS from phage-resistant strains had no effect. Carbohydrate analyses of LPS preparations showed that two class III mutants, compared to their parental strains, had lost significant parts of the rhamnose, galactose, and glucose from the LPS. One of the phage φW-resistant mutants showed a partial restoration of its carbohydrate composition. Other φW-resistant mutants showed, instead, further losses of carbohydrates in their LPS. It is suggested that genes exist which simultaneously mediate a female-specific mating site, ampicillin resistance, and the receptors for phage φW.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERTANI G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951 Sep;62(3):293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boman H. G., Jonsson S., Monner D., Normark S., Bloom G. D. Cell-surface alterations in Escherichia coli K-12 with chromosmal mutations changing ampicillin resistance. Ann N Y Acad Sci. 1971 Jun 11;182:342–357. doi: 10.1111/j.1749-6632.1971.tb30670.x. [DOI] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Charamella L. J., Stallions D. R., Mays J. A. Parental functions during conjugation in Escherichia coli K-12. Bacteriol Rev. 1968 Dec;32(4 Pt 1):320–348. [PMC free article] [PubMed] [Google Scholar]
- Cuzin F. Un bactériophage spécifique du type sexuel F- d'Escherichia coli K 12. C R Acad Sci Hebd Seances Acad Sci D. 1965 Jun 14;260(24):6482–6485. [PubMed] [Google Scholar]
- Eriksson-Grennberg K. G., Boman H. G., Jansson J. A., Thorén S. Resistance of Escherichia coli to Penicillins I. Genetic Study of Some Ampicillin-Resistant Mutants. J Bacteriol. 1965 Jul;90(1):54–62. doi: 10.1128/jb.90.1.54-62.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson-Grennberg K. G. Resistance of Escherichia coli to penicillins. II. An improved mapping of the ampA gene. Genet Res. 1968 Oct;12(2):147–156. doi: 10.1017/s0016672300011769. [DOI] [PubMed] [Google Scholar]
- Hamilton-Miller J. M. Damaging effects of ethylenediaminetetra-acetate and penicillins on permeability barriers in Gram-negative bacteria. Biochem J. 1966 Sep;100(3):675–682. doi: 10.1042/bj1000675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JESAITIS M. A., GOEBEL W. F. Lysis of T4 phage by the specific lipocarbohydrate of phase II Shigella sonnei. J Exp Med. 1955 Dec 1;102(6):733–752. doi: 10.1084/jem.102.6.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JESAITIS M. A., GOEBEL W. F. Mechanism of phage action. Nature. 1953 Oct 3;172(4379):622–623. doi: 10.1038/172622a0. [DOI] [PubMed] [Google Scholar]
- Leive L., Shovlin V. K., Mergenhagen S. E. Physical, chemical, and immunological properties of lipopolysaccharide released from Escherichia coli by ethylenediaminetetraacetate. J Biol Chem. 1968 Dec 25;243(24):6384–6391. [PubMed] [Google Scholar]
- Linial M., Malamy M. H. Studies with bacteriophage phi II. Events following infection of male and female derivatives of Escherichia coli K-12. J Virol. 1970 Jan;5(1):72–78. doi: 10.1128/jvi.5.1.72-78.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linström E. B., Boman H. G., Steele B. B. Resistance of Escherichia coli to penicillins. VI. Purification and characterization of the chromosomally mediated penicillinase present in ampA-containing strains. J Bacteriol. 1970 Jan;101(1):218–231. doi: 10.1128/jb.101.1.218-231.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes J., Inniss W. E. Electron microscopy of effect of polymyxin on Escherichia coli lipopolysaccharide. J Bacteriol. 1969 Nov;100(2):1128–1129. doi: 10.1128/jb.100.2.1128-1130.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüderitz O. Recent results on the biochemistry of the cell wall lipopolysaccharides of Salmonella bacteria. Angew Chem Int Ed Engl. 1970 Sep;9(9):649–663. doi: 10.1002/anie.197006491. [DOI] [PubMed] [Google Scholar]
- McIntire F. C., Barlow G. H., Sievert H. W., Finley R. A., Yoo A. L. Studies on a lipopolysaccharide from Escherichia coli. Heterogeneity and mechanism of reversible inactivation by sodium deoxycholate. Biochemistry. 1969 Oct;8(10):4063–4067. doi: 10.1021/bi00838a024. [DOI] [PubMed] [Google Scholar]
- Monner D. A., Boman H. G. Female strains of Escherichia coli K12 as selective hosts for the isolation of female specific mutants of phage omega II. Biochem Biophys Res Commun. 1970;39(6):1017–1020. doi: 10.1016/0006-291x(70)90659-5. [DOI] [PubMed] [Google Scholar]
- Monner D., Boman H. G., Jonsson S. Use of sex specific phages for demonstration of cell surface changes mediated by genes for ampicillin resistance in escherichia coli. J Gen Microbiol. 1969 Aug;57(3):xxxi–xxxi. [PubMed] [Google Scholar]
- Nelson B. W., Roantree R. J. Analyses of lipopolysaccharides extracted from penicillin-resistant, serum-sensitive salmonella mutants. J Gen Microbiol. 1967 Aug;48(2):179–188. doi: 10.1099/00221287-48-2-179. [DOI] [PubMed] [Google Scholar]
- Nordström K., Burman L. G., Eriksson-Grennberg K. G. Resistance of Escherichia coli to penicillins. 8. Physiology of a class II ampicillin-resistant mutant. J Bacteriol. 1970 Mar;101(3):659–668. doi: 10.1128/jb.101.3.659-668.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordström K., Eriksson-Grennberg K. G., Boman H. G. Resistance of Escherichia coli to penicillins. 3. AmpB, a locus affecting episomally and chromosomally mediated resistance to ampicillin and chlorampheincol. Genet Res. 1968 Oct;12(2):157–168. doi: 10.1017/s0016672300011770. [DOI] [PubMed] [Google Scholar]
- Normark S. Genetics of a chain-forming mutant of Escherichia coli. Transduction and dominance of the envA gene mediating increased penetration to some antibacterial agents. Genet Res. 1970 Aug;16(1):63–78. doi: 10.1017/s0016672300002287. [DOI] [PubMed] [Google Scholar]
- Rapin A. M., Kalckar H. M., Alberico L. The metabolic basis for masking of receptor-sites on E. coli K-12 for C21, a lipopolysaccharide core-specific phage. Arch Biochem Biophys. 1968 Oct;128(1):95–105. doi: 10.1016/0003-9861(68)90011-8. [DOI] [PubMed] [Google Scholar]
- Ribi E., Anacker R. L., Brown R., Haskins W. T., Malmgren B., Milner K. C., Rudbach J. A. Reaction of endotoxin and surfactants. I. Physical and biological properties of endotoxin treated with sodium deoxycholate. J Bacteriol. 1966 Nov;92(5):1493–1509. doi: 10.1128/jb.92.5.1493-1509.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkind D., Palmer J. D. Neutralization of endotoxin toxicity in chick embryos by antibiotics. J Bacteriol. 1966 Oct;92(4):815–819. doi: 10.1128/jb.92.4.815-819.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH J. T. Penicillinase and ampicillin resistance in a strain of Escherichia coli. J Gen Microbiol. 1963 Feb;30:299–306. doi: 10.1099/00221287-30-2-299. [DOI] [PubMed] [Google Scholar]
- STENT G. S., BRENNER S. A genetic locus for the regulation of ribonucleic acid synthesis. Proc Natl Acad Sci U S A. 1961 Dec 15;47:2005–2014. doi: 10.1073/pnas.47.12.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt G., Fromme I., Mayer H. Immunochemical studies on core lipopolysaccharides of Enterobacteriaceae of different genera. Eur J Biochem. 1970 Jun;14(2):357–366. doi: 10.1111/j.1432-1033.1970.tb00297.x. [DOI] [PubMed] [Google Scholar]
- Steele B., Boman H. G. Capsular material and morphology of some ampicillin sensitive and resistant strains of Escherichia coli K12. Acta Pathol Microbiol Scand B Microbiol Immunol. 1970;78(1):59–74. doi: 10.1111/j.1699-0463.1970.tb04270.x. [DOI] [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuber M. Release of the periplasmic penicillinases from Escherichia coli by toluene. Arch Mikrobiol. 1970;73(1):61–64. doi: 10.1007/BF00409952. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Watanabe T., Arai T., Hattori T. Effects of cell wall polysaccharide on the mating ability of Salmonella typhimurium. Nature. 1970 Jan 3;225(5227):70–71. doi: 10.1038/225070a0. [DOI] [PubMed] [Google Scholar]


