Abstract
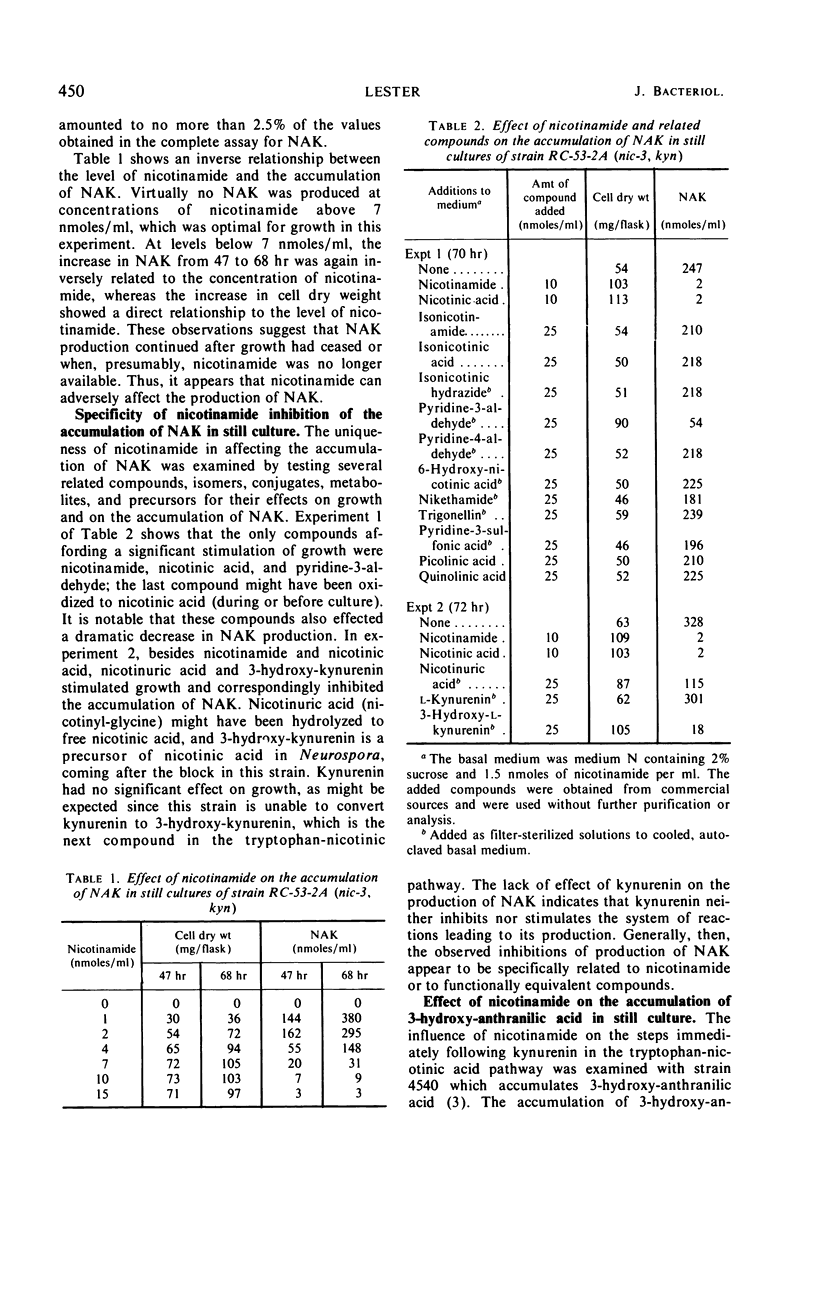
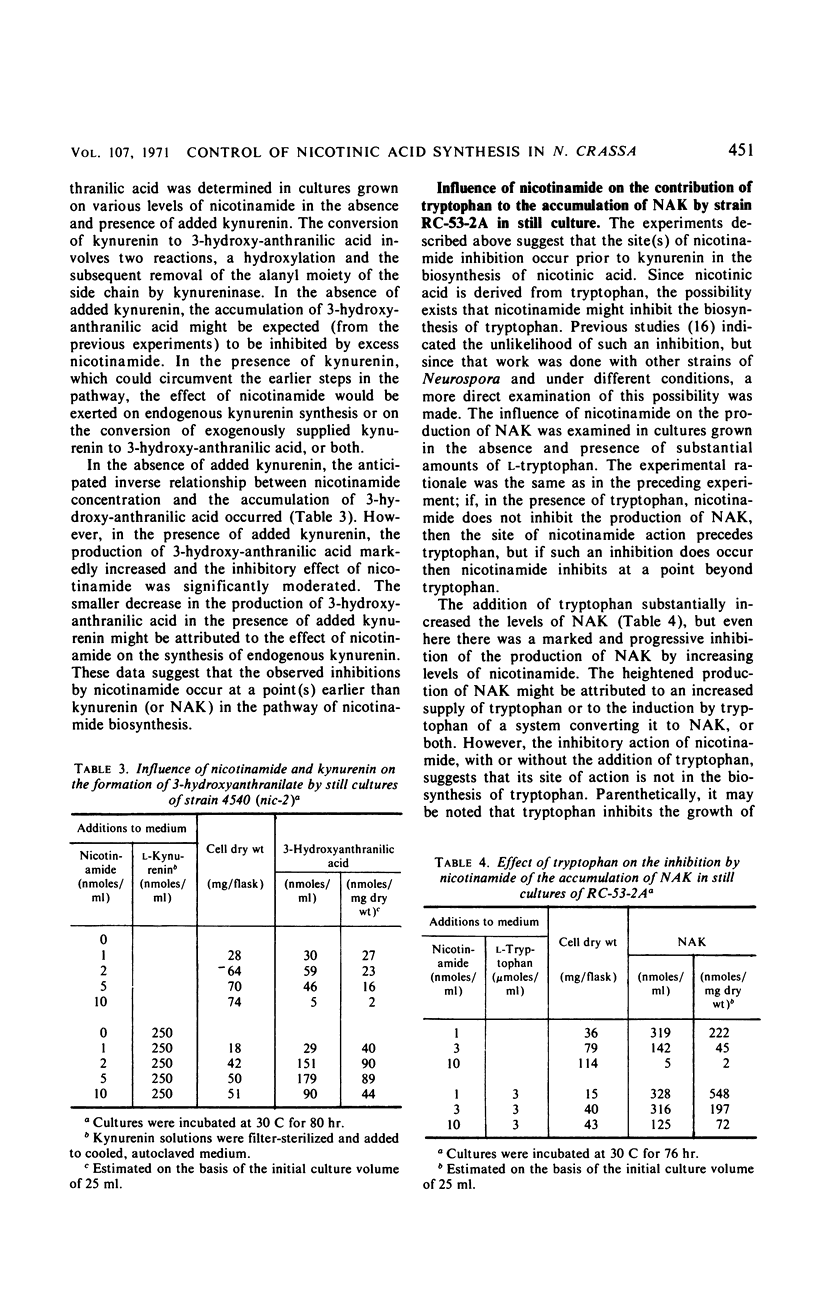
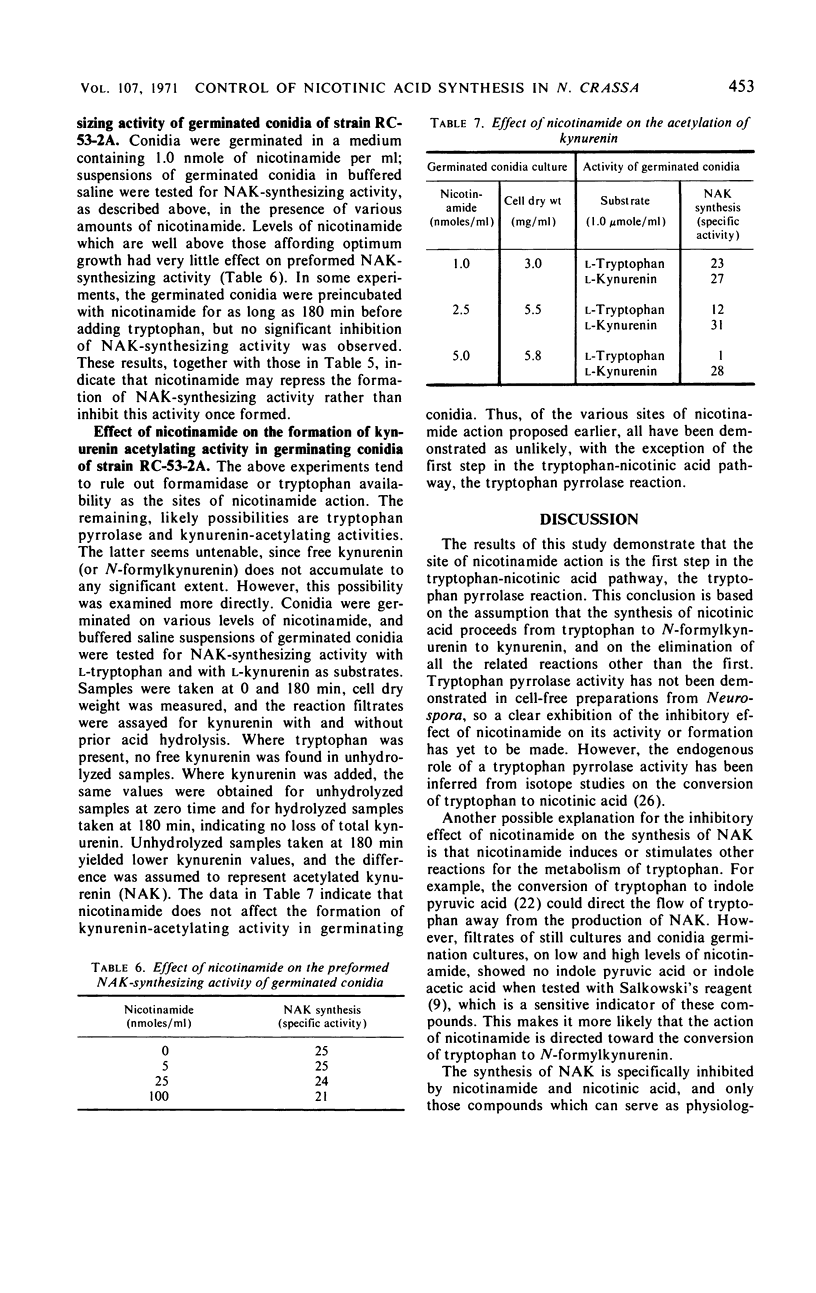
The regulation of the tryptophan-nicotinic acid pathway in Neurospora crassa was examined with mutants (nic-2, nic-3) which require nicotinamide for growth. The accumulation of N-acetylkynurenin and 3-hydroxyanthranilic acid by these mutants served to estimate the level of function of the early reactions in the pathway. In still cultures, maximal accumulation occurred with media containing growth-limiting amounts of nicotinamide; the accumulation of intermediates was almost negligible with nicotinamide in excess. Only nicotinamide and closely related compounds which also supported the growth of these mutants inhibited the accumulation of intermediates. The site of inhibition was assessed to be between tryptophan and kynurenin (or N-acetylkynurenin). The synthesis of N-acetylkynurenin was examined in washed germinated conidia suspended in buffer; the level of N-acetylkynurenin-synthesizing activity was inversely related to the concentration of nicotinamide in the germination medium. The addition of large amounts of nicotinamide to suspensions of germinated conidia did not affect their N-acetylkynurenin-synthesizing activity. Formamidase activity, kynurenin-acetylating activity, and gross tryptophan metabolism in germinated conidia was not influenced by the concentration of nicotinamide in the germination medium. The results obtained indicate that the site of inhibition by nicotinamide is the first step in the pathway, the tryptophan pyrrolase reaction. The data are interpreted as nicotinamide or a product thereof, such as nicotinamide adenine dinucleotide, acting as a repressor of the formation of tryptophan pyrrolase in N. crassa.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad F., Moat A. G. Nicotinic acid biosynthesis in prototrophs and tryptophan auxotrophs of Saccharomyces cerevisiae. J Biol Chem. 1966 Feb 25;241(4):775–780. [PubMed] [Google Scholar]
- Beadle G. W., Mitchell H. K., Nyc J. F. Kynurenine as an Intermediate in the Formation of Nicotinic Acid from Tryptophane by Neurospora. Proc Natl Acad Sci U S A. 1947 Jun;33(6):155–158. doi: 10.1073/pnas.33.6.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner D. The Identification of a Natural Precursor of Nicotinic Acid. Proc Natl Acad Sci U S A. 1948 Jan;34(1):5–9. doi: 10.1073/pnas.34.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. T., Wagner C. Regulation of enzymes involved in the conversion of tryptophan to nicotinamide adenine dinucleotide in a colorless strain of Xanthomonas pruni. J Bacteriol. 1970 Feb;101(2):456–463. doi: 10.1128/jb.101.2.456-463.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho-Chung Y. S., Pitot H. C. Feedback control of rat liver tryptophan pyrrolase. I. End product inhibition of trytophan pyrrolase activity. J Biol Chem. 1967 Mar 25;242(6):1192–1198. [PubMed] [Google Scholar]
- DAVIS D., HENDERSON L. M., POWELL D. The niacin-tryptophan relationship in the metabolism of Xanthomonas pruni. J Biol Chem. 1951 Apr;189(2):543–549. [PubMed] [Google Scholar]
- FRANK L. H., DEMOSS R. D. Specific enzymic method for the estimation of L-tryptophan. Arch Biochem Biophys. 1957 Apr;67(2):387–397. doi: 10.1016/0003-9861(57)90293-x. [DOI] [PubMed] [Google Scholar]
- Gordon S. A., Weber R. P. COLORIMETRIC ESTIMATION OF INDOLEACETIC ACID. Plant Physiol. 1951 Jan;26(1):192–195. doi: 10.1104/pp.26.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskins F. A., Mitchell H. K. Evidence for a Tryptophane Cycle in Neurospora. Proc Natl Acad Sci U S A. 1949 Sep;35(9):500–506. doi: 10.1073/pnas.35.9.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAKOBY W. B., BONNER D. M. Kynureninase from Neurospora: purification and properties. J Biol Chem. 1953 Dec;205(2):699–707. [PubMed] [Google Scholar]
- JAKOBY W. B. Kynurenine formamidase from Neurospora. J Biol Chem. 1954 Apr;207(2):657–663. [PubMed] [Google Scholar]
- Knox W. E. The regulation of tryptophan pyrrolase activity by tryptophan. Adv Enzyme Regul. 1966;4:287–297. doi: 10.1016/0065-2571(66)90023-9. [DOI] [PubMed] [Google Scholar]
- LESTER G. Regulation of early reactions in the biosynthesis of tryptophan in Neurospora crassa. J Bacteriol. 1963 Feb;85:468–475. doi: 10.1128/jb.85.2.468-475.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LESTER G. Repression and inhibition of indole-synthesizing activity in Neurospora crassa. J Bacteriol. 1961 Aug;82:215–223. doi: 10.1128/jb.82.2.215-223.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LESTER G. Some aspects of tryptophan synthetase formation in Neurospora crassa. J Bacteriol. 1961 Jun;81:964–973. doi: 10.1128/jb.81.6.964-973.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lester G. Genetic control of amino acid permeability in Neurospora crassa. J Bacteriol. 1966 Feb;91(2):677–684. doi: 10.1128/jb.91.2.677-684.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester G. In vivo regulation of intermediate reactions in the pathway of tryptophan biosynthesis in Neurospora crassa. J Bacteriol. 1968 Nov;96(5):1768–1773. doi: 10.1128/jb.96.5.1768-1773.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingens F., Vollprecht P. Zur Biosynthese der Nicotinsäure in Streptomyceten, Algen, Phycomyceten und Hefe. Hoppe Seylers Z Physiol Chem. 1964;339(1):64–74. [PubMed] [Google Scholar]
- MATCHETT W. H., DEMOSS J. A. Direct evidence for a trytophan-anthranilic acid cycle in Neurospora. Biochim Biophys Acta. 1963 Jun 4;71:632–642. doi: 10.1016/0006-3002(63)91136-3. [DOI] [PubMed] [Google Scholar]
- Matchett W. H. The utilization of tryptophan by neurospora. Biochim Biophys Acta. 1965 Sep 13;107(2):222–231. doi: 10.1016/0304-4165(65)90129-7. [DOI] [PubMed] [Google Scholar]
- NISHIZUKA Y., HAYAISHI O. STUDIES ON THE BIOSYNTHESIS OF NICOTINAMIDE ADENINE DINUCLEOTIDE. I. ENZYMIC SYNTHESIS OF NIACIN RIBONUCLEOTIDES FROM 3-HYDROXYANTHRANILIC ACID IN MAMMALIAN TISSUES. J Biol Chem. 1963 Oct;238:3369–3377. [PubMed] [Google Scholar]
- PALLERONI N. J., STANIER R. Y. REGULATORY MECHANISMS GOVERNING SYNTHESIS OF THE ENZYMES FOR TRYPTOPHAN OXIDATION BY PSEUDOMONAS FLUORESCENS. J Gen Microbiol. 1964 May;35:319–334. doi: 10.1099/00221287-35-2-319. [DOI] [PubMed] [Google Scholar]
- PARTRIDGE C. W. H., BONNER D. M., YANOFSKY C. A quantitative study of the relationship between tryptophan and niacin in Neurospora. J Biol Chem. 1952 Jan;194(1):269–278. [PubMed] [Google Scholar]
- Rosenfeld H., Feigelson P. Synergistic and product induction of the enzymes of tryptophan metabolism in Pseudomonas acidovorans. J Bacteriol. 1969 Feb;97(2):697–704. doi: 10.1128/jb.97.2.697-704.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHIMKE R. T., SWEENEY E. W., BERLIN C. M. THE ROLES OF SYNTHESIS AND DEGRADATION IN THE CONTROL OF RAT LIVER TRYPTOPHAN PYRROLASE. J Biol Chem. 1965 Jan;240:322–331. [PubMed] [Google Scholar]
- Turner J. R., Sorsoli W. A., Matchett W. H. Induction of kynureninase in Neurospora. J Bacteriol. 1970 Aug;103(2):364–369. doi: 10.1128/jb.103.2.364-369.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON R. G., HENDERSON L. M. Tryptophan-niacin relationship in Xanthomonas pruni. J Bacteriol. 1963 Jan;85:221–229. doi: 10.1128/jb.85.1.221-229.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner C., Brown A. T. Regulation of tryptophan pyrrolase activity in Xanthomonas pruni. J Bacteriol. 1970 Oct;104(1):90–97. doi: 10.1128/jb.104.1.90-97.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANOFSKY C., BONNER D. M. Studies on the conversion of 3-hydroxyanthranilic acid to niacin in Neurospora. J Biol Chem. 1951 May;190(1):211–218. [PubMed] [Google Scholar]
- Yanofsky C., Bonner D. M. Evidence for the Participation of Kynurenine as a Normal Intermediate in the Biosynthesis of Niacin in Neurospora. Proc Natl Acad Sci U S A. 1950 Mar;36(3):167–176. doi: 10.1073/pnas.36.3.167. [DOI] [PMC free article] [PubMed] [Google Scholar]



