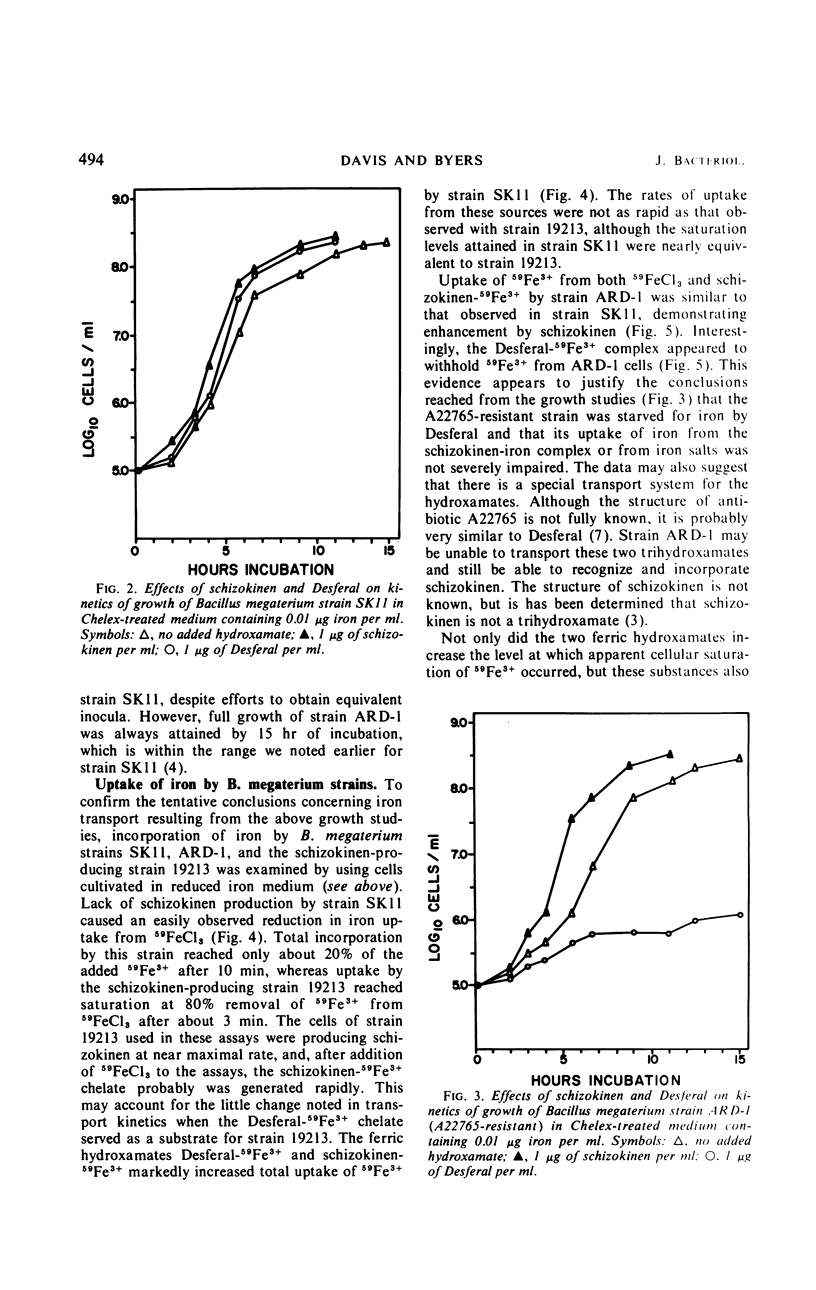
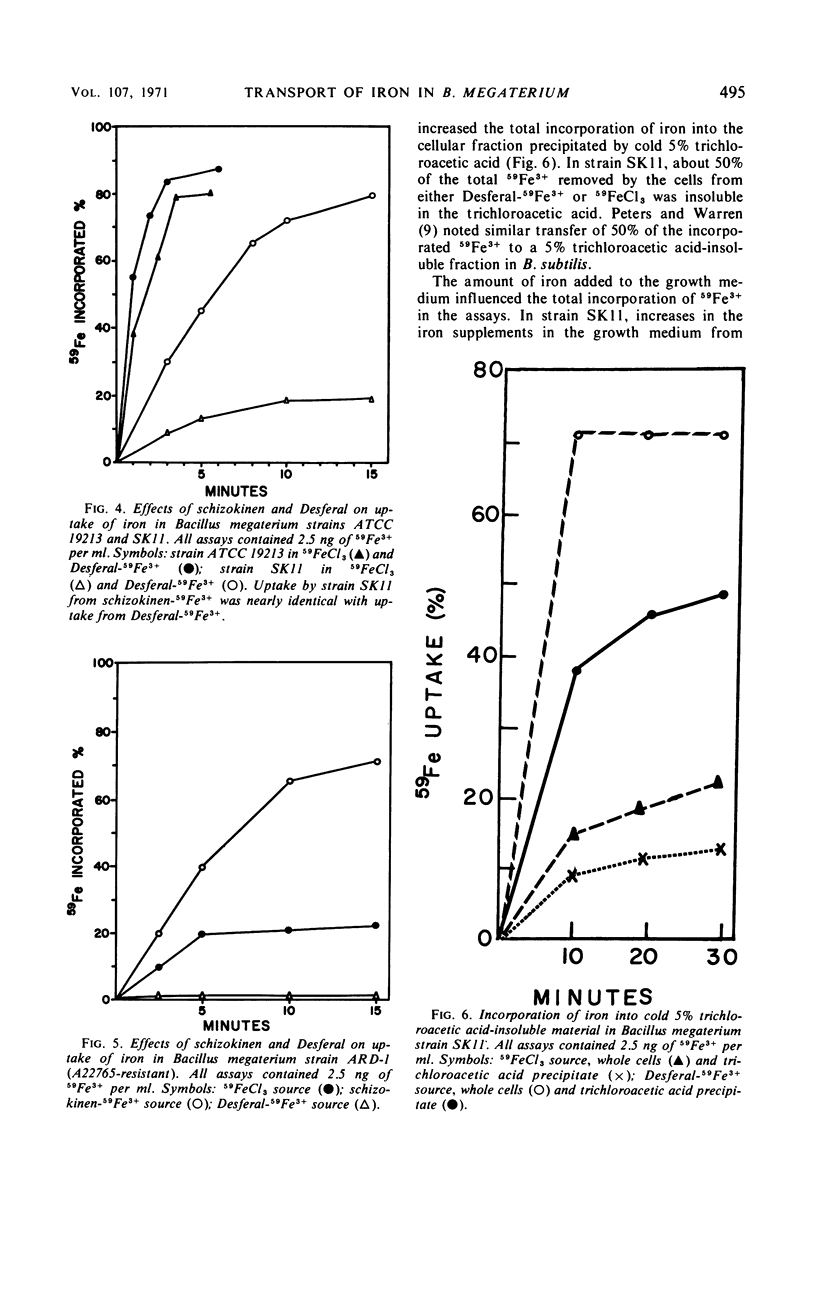
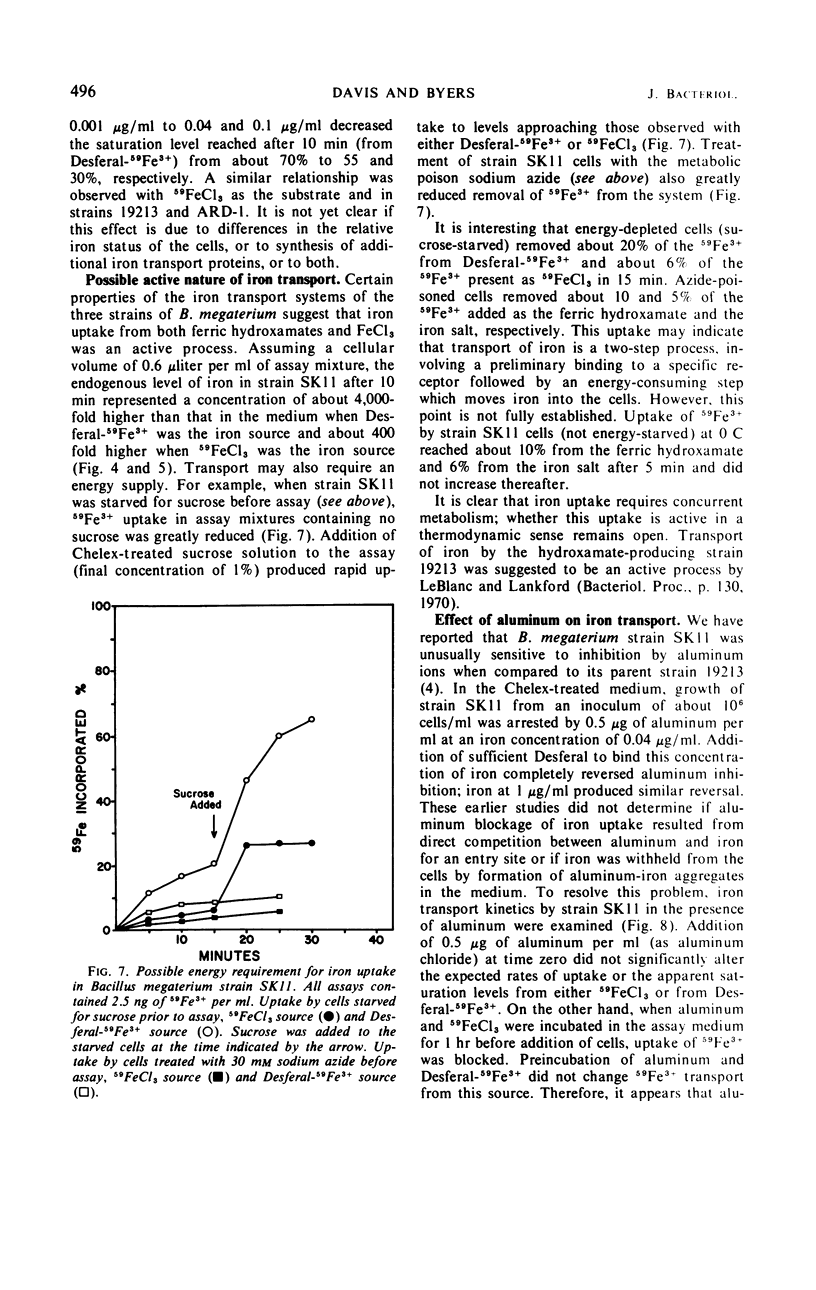
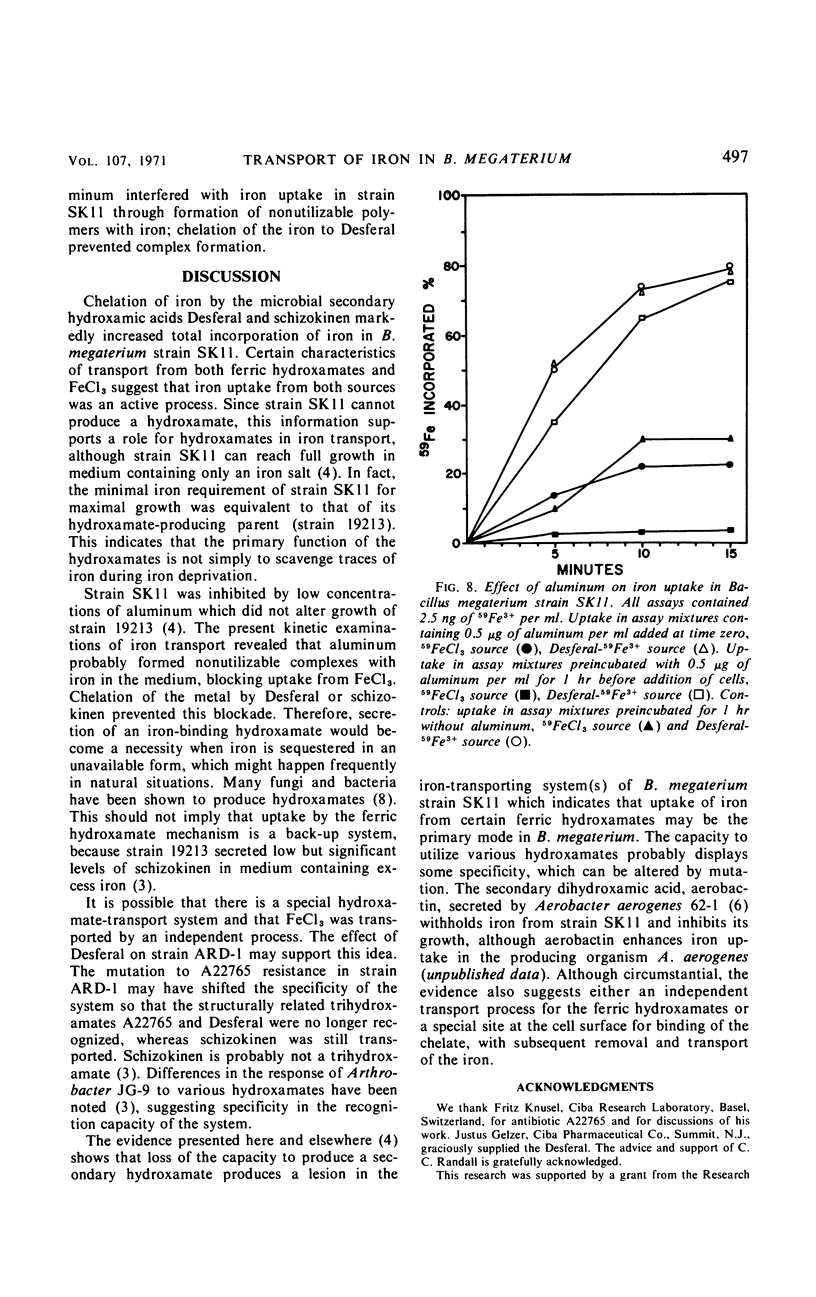
Abstract
Kinetics of radioactive iron transport were examined in three strains of Bacillus megaterium. In strain ATCC 19213, which secretes the ferric-chelating secondary hydroxamic acid schizokinen, 59Fe3+ uptake from 59FeCl3 or the ferric hydroxamate Desferal-59Fe3+ was rapid and reached saturation within 3 min. In strain SK11, which does not secrete schizokinen, transport from 59FeCl3 was markedly reduced; the two ferric hydroxamates Desferal-59Fe3+ or schizokinen-59Fe3+ increased both total 59Fe3+ uptake and the 59Fe3+ appearing in a cellular trichloroacetic acid-insoluble fraction, although 10 min was required to reach saturation. Certain characteristics of transport from both ferric hydroxamates and FeCl3 suggest that iron uptake was an active process. The growth-inhibitory effect of aluminum on strain SK11 was probably due to the formation of nonutilizable iron-aluminum complexes which blocked uptake from 59FeCl3. Desferal or schizokinen prevented this blockage. A strain (ARD-1) resistant to the ferric hydroxamate antibiotic A22765 was isolated from strain SK11. Strain ARD-1 failed to grow with Desferal-Fe3+ as an iron source, and it was unable to incorporate 59Fe3+ from this source. Growth and iron uptake in strain ARD-1 were similar to strain SK11 with schizokinen-Fe3+ or the iron salt as sources. It is suggested that the ferric hydroxamates, or the iron they chelate, may be transported by a special system which might be selective for certain ferric hydroxamates. Strain ARD-1 may be unable to recognize both the antibiotic A22765 and the structurally similar chelate Desferal-Fe3+, while retaining its capacity to utilize schizokinen-Fe3+.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arceneaux J. L., Lankford C. E. A schizokinen (siderochrome) auxotroph of Bacillus megaterium induced with N-methyl-N'-nitro-N-nitrosoguanidine. Biochem Biophys Res Commun. 1966 Aug 12;24(3):370–375. doi: 10.1016/0006-291x(66)90166-5. [DOI] [PubMed] [Google Scholar]
- Byers B. R., Powell M. V., Lankford C. E. Iron-chelating hydroxamic acid (schizokinen) active in initiation of cell division in Bacillus megaterium. J Bacteriol. 1967 Jan;93(1):286–294. doi: 10.1128/jb.93.1.286-294.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis W. B., McCauley M. J., Byers B. R. Iron requirements and aluminum sensitivity of an hydroxamic acid-requiring strain of Bacillus megaterium. J Bacteriol. 1971 Feb;105(2):589–594. doi: 10.1128/jb.105.2.589-594.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downer D. N., Davis W. B., Byers B. R. Repression of phenolic acid-synthesizing enzymes and its relation to iron uptake in Bacillus subtilis. J Bacteriol. 1970 Jan;101(1):181–187. doi: 10.1128/jb.101.1.181-187.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson F., Magrath D. I. The isolation and characterization of a hydroxamic acid (aerobactin) formed by Aerobacter aerogenes 62-I. Biochim Biophys Acta. 1969 Nov 18;192(2):175–184. doi: 10.1016/0304-4165(69)90353-5. [DOI] [PubMed] [Google Scholar]
- Neilands J. B. Hydroxamic acids in nature. Science. 1967 Jun 16;156(3781):1443–1447. doi: 10.1126/science.156.3781.1443. [DOI] [PubMed] [Google Scholar]
- Peters W. J., Warren R. A. Phenolic acids and iron transport in Bacillus subtilis. Biochim Biophys Acta. 1968 Sep 3;165(2):225–232. doi: 10.1016/0304-4165(68)90050-0. [DOI] [PubMed] [Google Scholar]
- Zimmermann W., Knüsel F. Permeability of Staphylococcus aureus to the Sideromycin antibiotic A 22,765. Arch Mikrobiol. 1969 Oct;68(2):107–112. doi: 10.1007/BF00413870. [DOI] [PubMed] [Google Scholar]


