Abstract
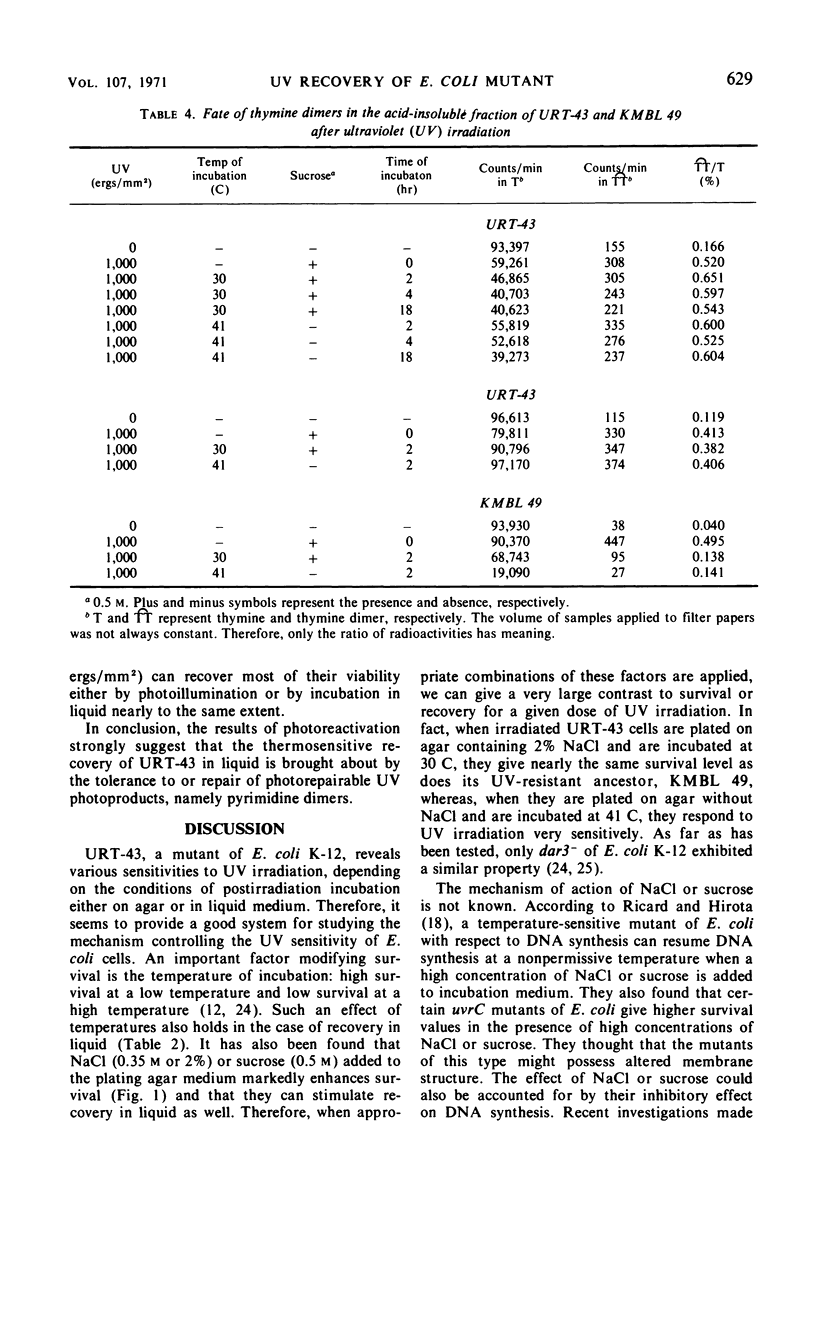
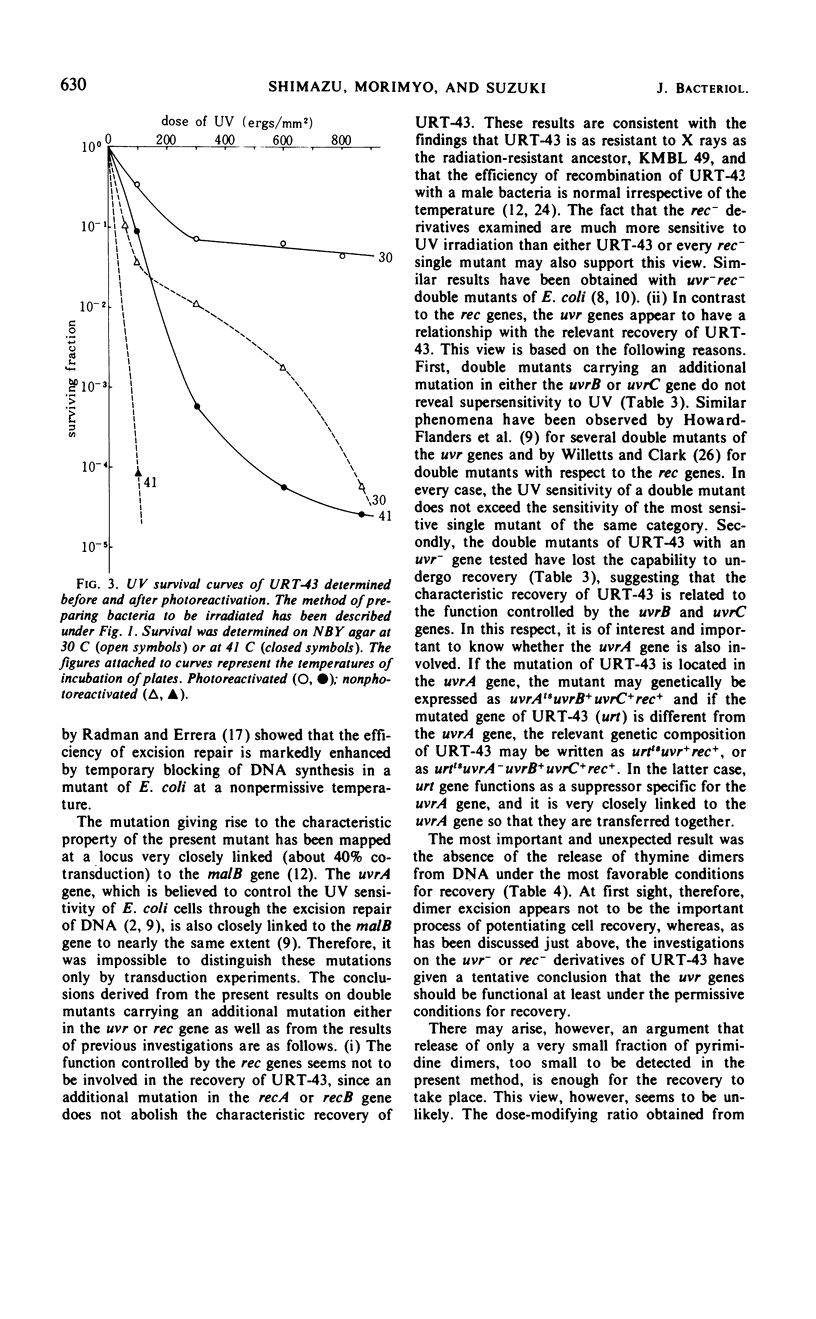
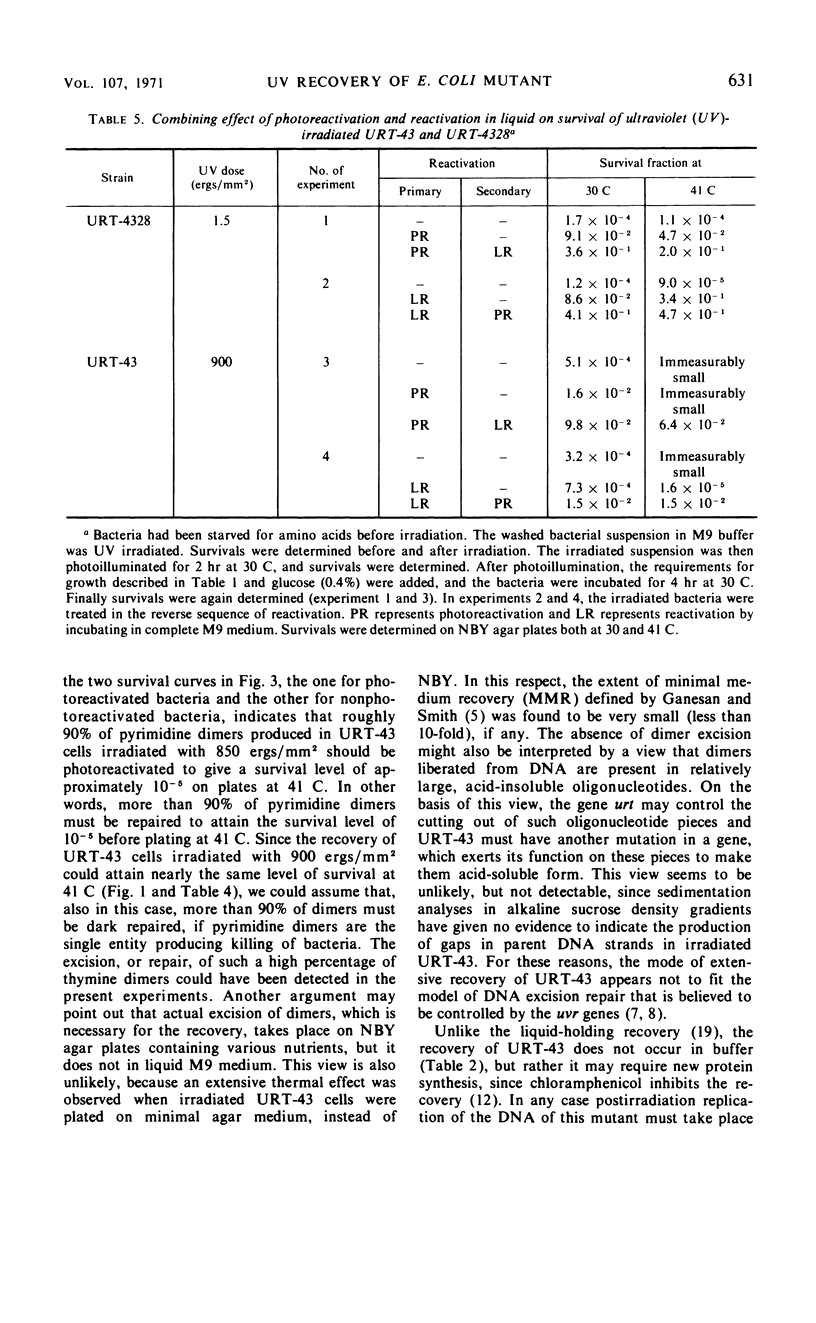
URT-43 is a mutant of Escherichia coli K-12 which gives a much larger number of survivors when ultraviolet (UV)-irradiated bacteria are incubated on agar medium at 30 C than when they are incubated on the medium at 41 C, although in both cases the number of survivors is fewer than that given by its wild-type ancestor. The UV sensitivity of this mutant was found to be markedly influenced by the presence of a high concentration of NaCl or sucrose in the plating medium. Thus, when irradiated bacteria were plated on agar medium containing 2% NaCl or 0.5 m sucrose at 30 C, they exhibited a resistance similar to that of their wild-type ancestor. At 30 C, there was also an extensive recovery in liquid M9 medium supplemented with all of the nutrients required for growth and NaCl or sucrose. At 41 C, however, the recovery was greatly inhibited. Direct chemical analysis of thymine dimers has revealed that no significant amount of the dimer was released from deoxyribonucleic acid during the period of extensive recovery. It was concluded, therefore, that the temperature-sensitive recovery of URT-43 does not accompany excision of the bulk of pyrimidine dimers. To learn the gene function involved in the recovery, double mutants carrying an additional mutation either in a uvr or a rec gene have been investigated for their UV sensitivities and recovery in liquid medium. It was found that recA− and recB− derivatives retain the ability of undergoing an efficient recovery at a low temperature, whereas uvrB− and uvrC− derivatives have completely lost this ability. For these reasons, it was concluded that the mechanism responsible for the recovery of URT-43 involves the function controlled by the uvr genes. The results of photoreactivation suggested that most of the entities dealt with during recovery were pyrimidine dimers.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYCE R. P., HOWARD-FLANDERS P. RELEASE OF ULTRAVIOLET LIGHT-INDUCED THYMINE DIMERS FROM DNA IN E. COLI K-12. Proc Natl Acad Sci U S A. 1964 Feb;51:293–300. doi: 10.1073/pnas.51.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges B. A., Ashwood-Smith M. J., Munson R. J. On the nature of the lethal and mutagenic action of ultraviolet light on frozen bacteria. Proc R Soc Lond B Biol Sci. 1967 Aug 15;168(1011):203–215. doi: 10.1098/rspb.1967.0061. [DOI] [PubMed] [Google Scholar]
- CLARK A. J., MARGULIES A. D. ISOLATION AND CHARACTERIZATION OF RECOMBINATION-DEFICIENT MUTANTS OF ESCHERICHIA COLI K12. Proc Natl Acad Sci U S A. 1965 Feb;53:451–459. doi: 10.1073/pnas.53.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan A. K., Smith K. C. Dark-recovery processes in Escherichia coli irradiated with ultraviolet light. 3. Effect of rec mutations on recovery of excision-deficient mutants of Escherichia coli K-12. J Bacteriol. 1970 May;102(2):404–410. doi: 10.1128/jb.102.2.404-410.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P. DNA repair and genetic recombination: studies on mutants of Escherichia coli defective in these processes. Radiat Res. 1966;(Suppl):156+–156+. [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P., Theriot L. Three loci in Escherichia coli K-12 that control the excision of pyrimidine dimers and certain other mutagen products from DNA. Genetics. 1966 Jun;53(6):1119–1136. doi: 10.1093/genetics/53.6.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard-Flanders P. Genes that control DNA repair and genetic recombination in Escherichia coli. Adv Biol Med Phys. 1968;12:299–317. doi: 10.1016/b978-1-4831-9928-3.50016-3. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Theriot L., Stedeford J. B. Some properties of excision-defective recombination-deficient mutants of Escherichia coli K-12. J Bacteriol. 1969 Mar;97(3):1134–1141. doi: 10.1128/jb.97.3.1134-1141.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Morimyo M., Shimazu Y., Suzuki K. A mutant of Escherichia coli K12 exhibiting varying ultraviolet sensitivities depending on the temperature of incubation after irradiation. II. Cross-sensitivity, recovery in liquid and genetic analysis. Mutat Res. 1969 Nov-Dec;8(3):467–479. doi: 10.1016/0027-5107(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Ogawa H., Shimada K., Tomizawa J. Studies on radiation-sensitive mutants of E. coli. I. Mutants defective in the repair synthesis. Mol Gen Genet. 1968 May 3;101(3):227–244. doi: 10.1007/BF00271625. [DOI] [PubMed] [Google Scholar]
- PITTARD J., LOUTIT J. S., ADELBERG E. A. GENE TRANSFER BY F' STRAINS OF ESCHERICHIA COLI K-12. I. DELAY IN INITIATION OF CHROMOSOME TRANSFER. J Bacteriol. 1963 Jun;85:1394–1401. doi: 10.1128/jb.85.6.1394-1401.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash L., Strauss B. Repair of alkylation damage: stability of methyl groups in Bacillus subtilis treated with methyl methanesulfonate. J Bacteriol. 1970 Jun;102(3):760–766. doi: 10.1128/jb.102.3.760-766.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radman M., Cordone L., Krsmanovic-Simic D., Errera M. Complementary action of recombination and excision in the repair of ultraviolet irradiation damage to DNA. J Mol Biol. 1970 Apr 14;49(1):203–212. doi: 10.1016/0022-2836(70)90386-4. [DOI] [PubMed] [Google Scholar]
- Radman M., Errera M. Enhanced efficiency of excision repair of non-replicated UV-damaged E. coli DNA. Mutat Res. 1970 Jun;9(6):553–560. doi: 10.1016/0027-5107(70)90100-4. [DOI] [PubMed] [Google Scholar]
- Ricard M., Hirota Y. Effet des sels sur le processus de division cellulaire d'E. coli. C R Acad Sci Hebd Seances Acad Sci D. 1969 Mar 3;268(9):1335–1338. [PubMed] [Google Scholar]
- Roberts R. B., Aldous E. RECOVERY FROM ULTRAVIOLET IRRADIATION IN ESCHERICHIA COLI. J Bacteriol. 1949 Mar;57(3):363–375. doi: 10.1128/jb.57.3.363-375.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp W. D., Howard-Flanders P. Discontinuities in the DNA synthesized in an excision-defective strain of Escherichia coli following ultraviolet irradiation. J Mol Biol. 1968 Jan 28;31(2):291–304. doi: 10.1016/0022-2836(68)90445-2. [DOI] [PubMed] [Google Scholar]
- SETLOW R. B., CARRIER W. L., BOLLUM F. J. NUCLEASE-RESISTANT SEQUENCES IN ULTRAVIOLET-IRRADIATED DEOXYRIBONUCLEIC ACID. Biochim Biophys Acta. 1964 Nov 15;91:446–461. doi: 10.1016/0926-6550(64)90075-1. [DOI] [PubMed] [Google Scholar]
- Smith K. C., O'Leary M. E. Photoinduced DNA-protein cross-links and bacterial killing: a correlation at low temperatures. Science. 1967 Feb 24;155(3765):1024–1026. doi: 10.1126/science.155.3765.1024. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Saito E., Morimyo M. A mutant of Escherichia coli K12 exhibiting varying ultraviolet sensitivities depending on the temperature of incubation after irradiation. Photochem Photobiol. 1969 Mar;9(3):259–272. doi: 10.1111/j.1751-1097.1969.tb07290.x. [DOI] [PubMed] [Google Scholar]
- Willetts N. S., Clark A. J. Characteristics of some multiply recombination-deficient strains of Escherichia coli. J Bacteriol. 1969 Oct;100(1):231–239. doi: 10.1128/jb.100.1.231-239.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Putte P., van Sluis C. A., van Dillewijn J., Rörsch A. The location of genes controlling radiation sensitivity in Escherichia coli. Mutat Res. 1965 Apr;2(2):97–110. doi: 10.1016/0027-5107(65)90041-2. [DOI] [PubMed] [Google Scholar]


