Abstract
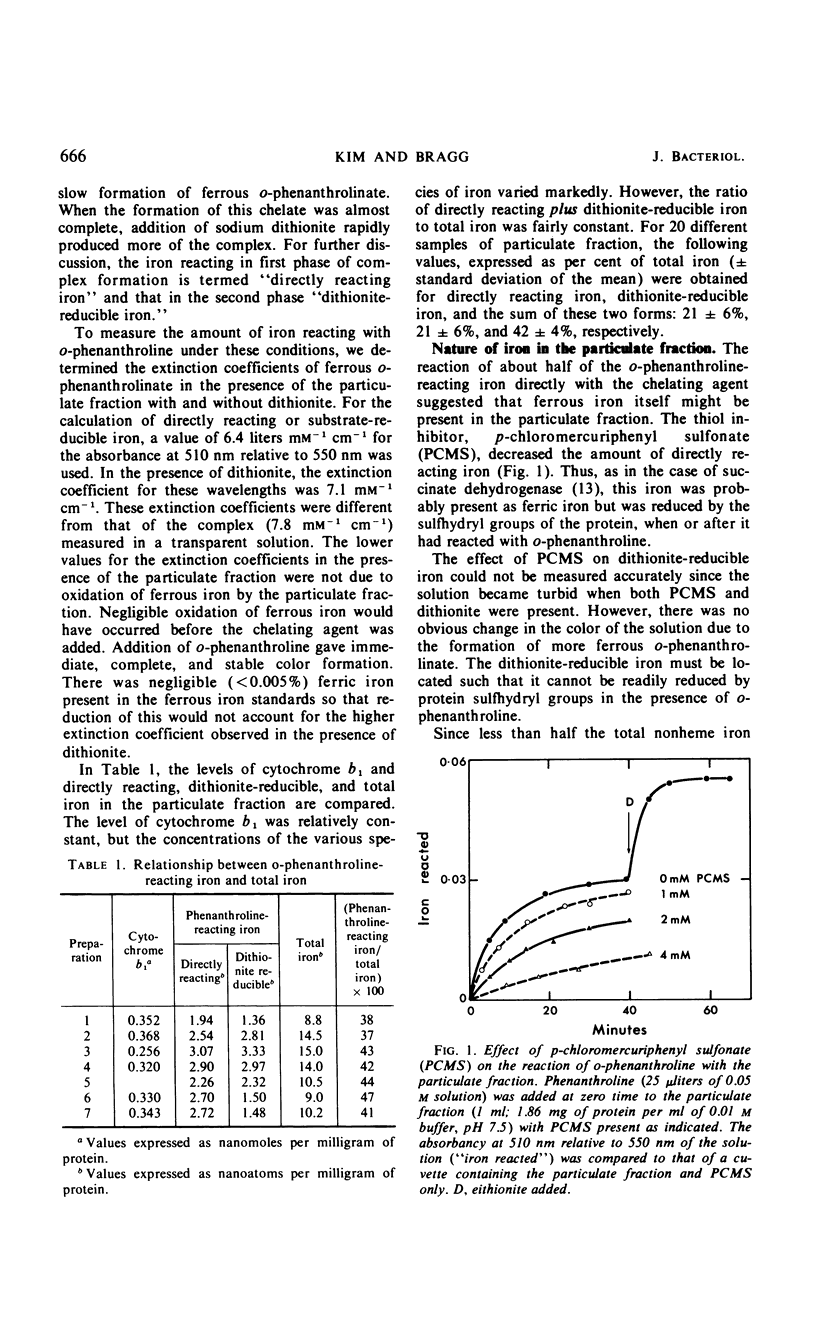
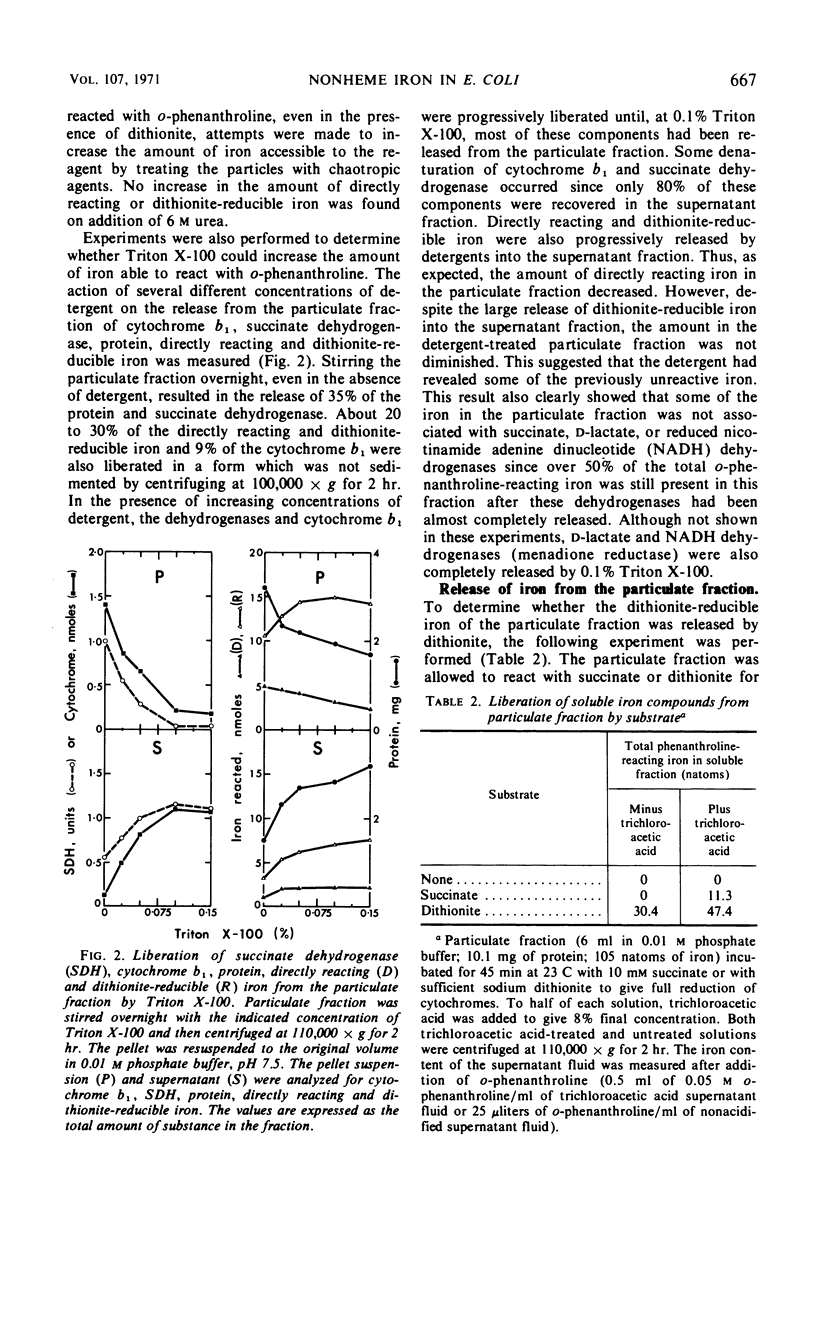
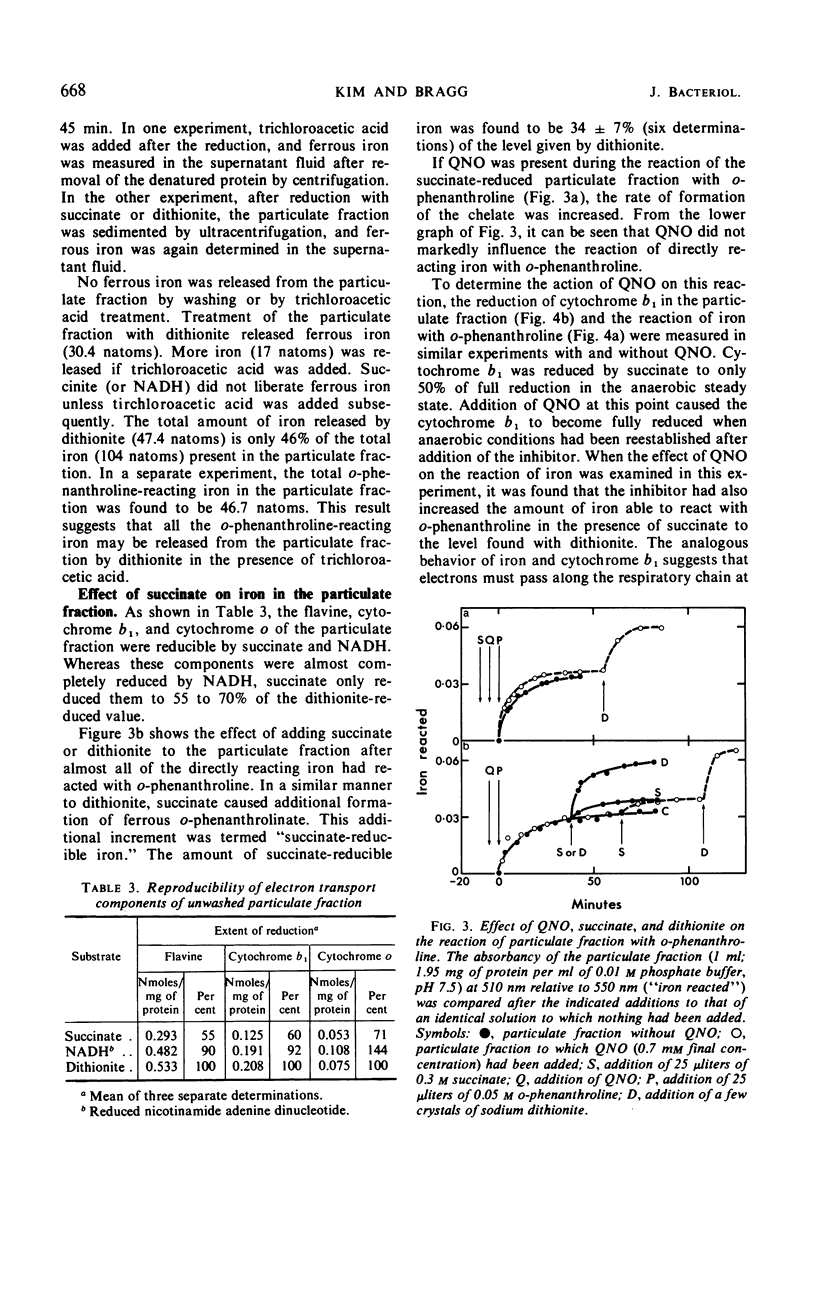
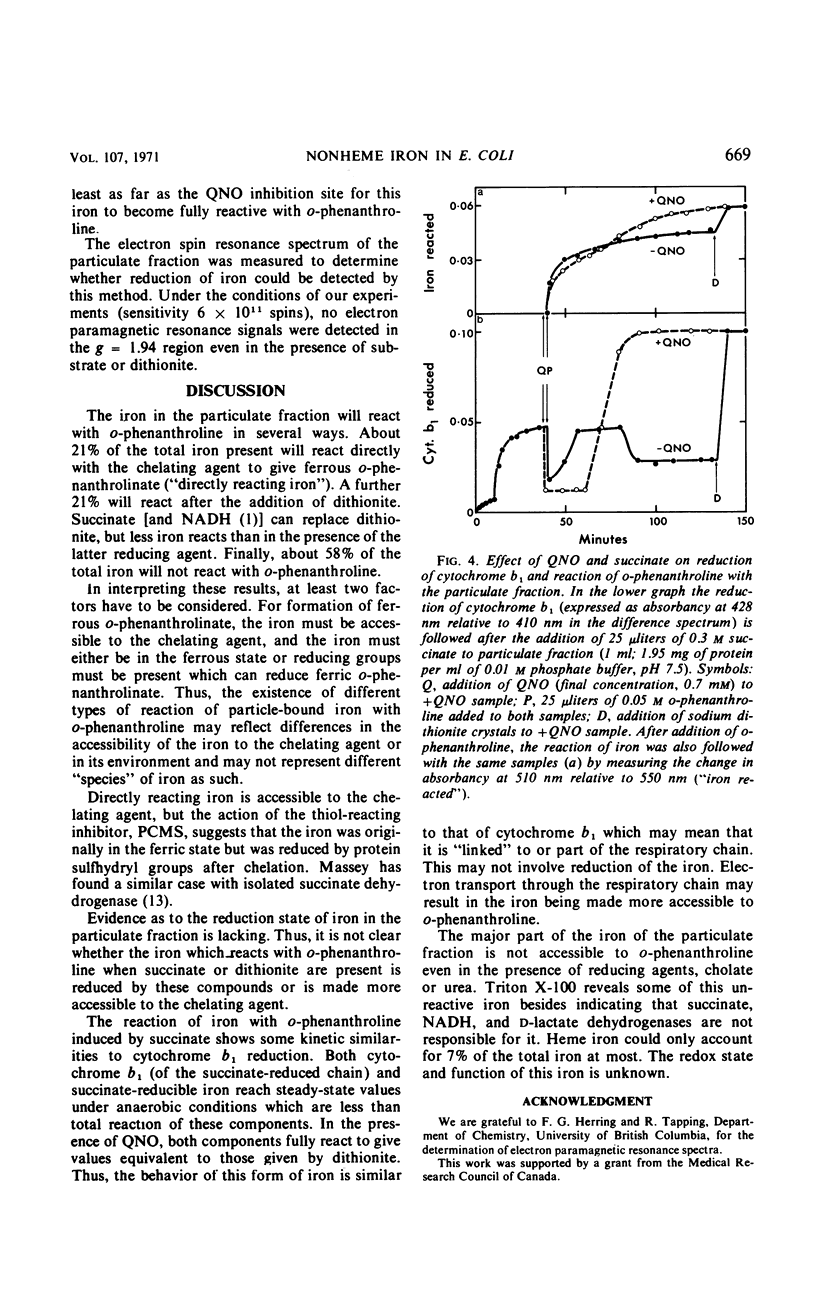
The reaction with o-phenanthroline of nonheme iron found in cell envelope fractions from Escherichia coli has been investigated. About 20% of the total nonheme iron reacts directly with o-phenanthroline. This iron appears to be in the ferric state but is reducible by protein sulfhydryl groups in the presence of the chelating agent. A further 20% of the nonheme iron will react with o-phenanthroline only in the presence of dithionite. Succinate can replace dithionite but only produces about 35% of the reaction given by dithionite. The reduction of cytochrome b1 of the respiratory chain by succinate shows similar behavior to the reaction of iron with o-phenanthroline in the presence of succinate. Both of these components react completely only in the presence of 2-heptyl-4-hydroxyquinoline-N-oxide. The remaining 60% of the nonheme iron of the cell envelope will not react with o-phenanthroline even in the presence of dithionite or 6 m urea. Triton X-100 with dithionite will permit a small part (10%) of this iron to react with o-phenanthroline. The iron which does not react with o-phenanthroline is not associated with succinate, reduced nicotinamide adenine dinucleotide, or d-lactate dehydrogenases.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beinert H., Palmer G. Contributions of EPR spectroscopy to our knowledge of oxidative enzymes. Adv Enzymol Relat Areas Mol Biol. 1965;27:105–198. doi: 10.1002/9780470122723.ch3. [DOI] [PubMed] [Google Scholar]
- Bragg P. D., Hou C. Reduced nicotinamide adenine dinucleotide oxidation in Escherichia coli particles. I. Properties and cleavage of the electron transport chain. Arch Biochem Biophys. 1967 Mar;119(1):194–201. doi: 10.1016/0003-9861(67)90446-8. [DOI] [PubMed] [Google Scholar]
- Bragg P. D. Reduction of nonheme iron in the respiratory chain of Escherichia coli. Can J Biochem. 1970 Jul;48(7):777–783. doi: 10.1139/o70-121. [DOI] [PubMed] [Google Scholar]
- Cox G. B., Gibson F., Luke R. K., Newton N. A., O'Brien I. G., Rosenberg H. Mutations affecting iron transport in Escherichia coli. J Bacteriol. 1970 Oct;104(1):219–226. doi: 10.1128/jb.104.1.219-226.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dervartanian D. V., Veeger C., Orme-Johnson W. H., Beinert H. Kinetic studies of succinate dehydrogenase by electron paramagnetic resonance spectroscopy. Biochim Biophys Acta. 1969 Sep 30;191(1):22–37. doi: 10.1016/0005-2744(69)90311-8. [DOI] [PubMed] [Google Scholar]
- Gutman M., Schejter A., Avi-Dor Y. The preparation and properties of the membranal DPNH dehydrogenase from Escherichia coli. Biochim Biophys Acta. 1968 Nov 26;162(4):506–517. doi: 10.1016/0005-2728(68)90057-1. [DOI] [PubMed] [Google Scholar]
- Hamilton J. A., Cox G. B., Looney F. D., Gibson F. Ubisemiquinone in membranes from Escherichia coli. Biochem J. 1970 Jan;116(2):319–320. doi: 10.1042/bj1160319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurup C. K., Brodie A. F. Oxidative phosphorylation in fractionated bacterial systems. XXIX. The involvement of nonheme iron in the respiratory pathways of Mycobacterium phlei. J Biol Chem. 1967 Dec 25;242(24):5830–5837. [PubMed] [Google Scholar]
- Kurup C. K., Brodie A. F. Oxidative phosphorylation in fractionated bacterial systems. XXVII. The nature of nonheme iron in Mycobacterium phlei. J Biol Chem. 1967 Jun 25;242(12):2909–2916. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MASSEY V. Studies on succinic dehydrogenase. VII. Valency state of the iron in beef heart succinic dehydrogenase. J Biol Chem. 1957 Dec;229(2):763–770. [PubMed] [Google Scholar]
- MASSEY V. The role of iron in beefheart succinic dehydrogenase. Biochim Biophys Acta. 1958 Dec;30(3):500–509. doi: 10.1016/0006-3002(58)90095-7. [DOI] [PubMed] [Google Scholar]
- RIESKE J. S., HANSEN R. E., ZAUGG W. S. STUDIES ON THE ELECTRON TRANSFER SYSTEM. 58. PROPERTIES OF A NEW OXIDATION-REDUCTION COMPONENT OF THE RESPIRATORY CHAIN AS STUDIED BY ELECTRON PARAMAGNETIC RESONANCE SPECTROSCOPY. J Biol Chem. 1964 Sep;239:3017–3022. [PubMed] [Google Scholar]



