Abstract
We tested whether severe congestive heart failure (CHF), a condition associated with excess free-water retention, is accompanied by altered regulation of the vasopressin-regulated water channel, aquaporin-2 (AQP2), in the renal collecting duct. CHF was induced by left coronary artery ligation. Compared with sham-operated animals, rats with CHF had severe heart failure with elevated left ventricular end-diastolic pressures (LVEDP): 26.9 ± 3.4 vs. 4.1 ± 0.3 mmHg, and reduced plasma sodium concentrations (142.2 ± 1.6 vs. 149.1 ± 1.1 mEq/liter). Quantitative immunoblotting of total kidney membrane fractions revealed a significant increase in AQP2 expression in animals with CHF (267 ± 53%, n = 12) relative to sham-operated controls (100 ± 13%, n = 14). In contrast, immunoblotting demonstrated a lack of an increase in expression of AQP1 and AQP3 water channel expression, indicating that the effect on AQP2 was selective. Furthermore, postinfarction animals without LVEDP elevation or plasma Na reduction showed no increase in AQP2 expression (121 ± 28% of sham levels, n = 6). Immunocytochemistry and immunoelectron microscopy demonstrated very abundant labeling of the apical plasma membrane and relatively little labeling of intracellular vesicles in collecting duct cells from rats with severe CHF, consistent with enhanced trafficking of AQP2 to the apical plasma membrane. The selective increase in AQP2 expression and enhanced plasma membrane targeting provide an explanation for the development of water retention and hyponatremia in severe CHF.
Keywords: hyponatremia, water retention
Severe heart failure is generally associated with marked defects in renal handling of sodium and water resulting in extracellular fluid expansion and hyponatremia. The renal water retention is thought to be mediated in part by increased baroreceptor-mediated vasopressin release (for a recent review, see ref. 1). Vasopressin binds to V2 receptors in the basolateral plasma membrane in kidney collecting duct principal cells. V2 receptors activate adenylyl cyclase through heterotrimeric–GTP binding proteins, increasing cAMP levels which in turn increase water reabsorption. Increased water reabsorption presumably contributes to the development of hyponatremia. However, the renal mechanisms involved in the increased water reabsorption are poorly defined. The present study is aimed at analyzing the role of renal aquaporins (AQPs) in the development of renal water retention in congestive heart failure (CHF).
The AQPs are a family of membrane water channels that mediate rapid water transport across cell membranes (2). AQP2 (3) was found to be the predominant vasopressin-regulated water channel of the kidney collecting duct (for recent reviews, see refs. 4 and 5). In principal cells of the collecting duct, AQP2 is localized in the apical plasma membrane and in cytoplasmic vesicles (6). Water reabsorption in the collecting duct is regulated both by short-term and long-term mechanisms, both of which have been shown to depend critically on AQP2. Short-term regulation occurs as a result of exocytic insertion of AQP2 water channels into the apical plasma membrane in response to vasopressin (7–10). In addition, long-term regulation of AQP2 expression has recently been shown to play a major role for regulation of collecting duct water reabsorption. It has been shown that thirsting or water loading for 24 hr or more produces marked changes in AQP2 expression in collecting duct principal cells (6). Moreover, chronic vasopressin infusion in Brattleboro rats, which manifest central diabetes insipidus due to a defect in the neurophysin/vasopressin gene, induces a substantial increase in AQP2 expression (11). This increase in AQP2 is paralleled by a comparable increase in osmotic water permeability (11), demonstrating a direct role of vasopressin in the long-term regulation of AQP2.
It has been recently demonstrated that defective long-term regulation of AQP2 plays a key role in several water balance disorders. Thus, it has been shown that down-regulation of AQP2 in response to lithium treatment, hypokalemia or release of ureteral obstruction, is associated with development of polyuria in proportion to the decrease in AQP2 (12–14). Therefore, down-regulation of AQP2 appears to be a general mechanism in the development of commonly acquired forms of nephrogenic diabetes insipidus. Recently it has also been shown that kidneys of rats with CCl4-induced liver cirrhosis and acites have an increased expression of AQP2 (15). This suggests that AQP2 also has a role in the development of water retention. In the present study we demonstrate that defective short- and long-term regulation of AQP2 are associated with severe CHF.
METHODS
Experimental Animals.
Wistar rats (Møllegard Breeding Centre, Eiby, Denmark) were a fed commercial rat chow (no. 1310; Altromin, Lage, Germany) and were allowed free access to water.
Surgical Preparation.
Rats were anaesthetized in an inhalation chamber with 4% halothane in 1:1 N2O/O2 gas mixture. After insertion of an endotracheal tube the animal was artificially ventilated with 1% halothane in a 1:1 N2O/O2 gas mixture. Tidal volume and respiratory rate were adjusted to maintain arterial pH between 7.35 and 7.45. During surgery the animal was placed on a heated table that maintained rectal temperature at 37–38°C. Left coronary artery ligation, used to produce CHF (16–18), was performed via a parasternal thoracotomy and a 6-0 silk suture was placed between the pulmonary trunk and the left auricle. Sham-operation was performed without ligating the left coronary artery. To minimize postsurgical pain, rats were treated postoperatively with buprenorphine 0.2 mg/kg s.c. (Anorfin, Bie & Berntsen, Rødovre, Denmark) for 2 days. Two weeks after heart surgery permanent Tygon catheters (Bie & Berntsen) were inserted into the femoral artery and vein. To prevent coagulation, catheters were filled with 50% dextrose with 1,000 units heparin/ml and 10,000 units streptokinase/ml.
Experimental Protocol.
Physiological examinations were performed 3 weeks after heart surgery since the functional deterioration after left coronary artery ligation is generally maximal at this time (16, 17). Measurements of arterial pressure were performed in the conscious, unrestrained state in the rats home cage. The arterial catheter was connected via a swivel system to a Baxter Uniflow transducer and mean arterial pressure was displayed continuously on a Grass model 7D polygraph. After a 2-hr recording period, a 300-μl arterial blood sample was drawn for measurements of plasma sodium and potassium. The rat was then anaesthetized with halothane in 1:1 N2O/O2, incubated and artificially ventilated, and a Tygon catheter was inserted into the left ventricle via the right carotid artery for measurement of left ventricular end-diastolic pressure (LVEDP). Accurate measurement of LVEDP was performed by adjusting the concentration of halothane to maintain after-load during anaesthesia at the same level as mean arterial pressure recorded in the conscious state.
Membrane Fractionation for Immunoblotting.
The kidneys were homogenized (0.3 M sucrose/25 mM imidazole/1 mM EDTA, pH 7.2/8.5 μM leupeptin/1 mM phenylmethylsulfonyl fluoride) and the homogenate was centrifuged in a Beckman L8M centrifuge at 4,000 × g for 15 min. The supernatant was then centrifuged at 200,000 × g for 1 hr to produce a pellet containing both plasma membrane and intracellular vesicle fractions (8, 12). Gel samples were prepared using Laemmli sample buffer containing 2% SDS.
Electrophoresis and Immunoblotting.
Samples of membrane fractions from total kidney (20 μg/lane) were run on 12% polyacrylamide minigels (Bio-Rad Mini Protean II). For each gel an identical gel was run in parallel and subjected to Coomassie staining to assure identical loading (19). The other gel was subjected to immunoblotting. Blots were blocked with 5% milk in PBS-T (80 mM Na2HPO4/20 mM NaH2PO4/100 mM NaCl/0.1% Tween 20, pH 7.5) for 1 hr, and incubated with affinity purified anti-AQP2 [40 ng IgG/μl IgG (6–8, 11)] or with AQP1 immune serum [diluted 1:2000; generously provided by Peter Agre (20)], or with affinity purified anti-AQP3 [0.5 μg/ml (21)]. The labeling was visualized with horseradish peroxidase-conjugated secondary antibody (P448; Dako; diluted 1:3,000) using enhanced chemiluminescence system (Amersham). Controls were made with replacement of primary antibody with antibody pre-absorbed with immunizing peptide IgG), or with nonimmune IgG.
Quantitation of AQP2, AQP1, and AQP3 Expression.
ECL films with bands within the linear ranged were scanned (8) using a Hewlett–Packard Scanjet scanner. For AQP2 and AQP1 both the 29-kDa and the 35- to 50-kDa band [corresponding to nonglycosylated and the glycosylated species (22)] were scanned as described (12–14, 19). For AQP3, the 27-kDa and the 33- to 40-kDa bands (corresponding to nonglycosylated and the glycosylated species) were scanned as described (19, 21). The labeling density was quantitated (12, 13) of blots where samples from CHF kidneys were run on each gel with control kidneys from sham-operated animals. AQP labeling in the samples from the experimental animals was calculated as a fraction of the mean sham control value for that gel. Comparisons between groups were made by unpaired t test. P values < 0.05 were considered significant.
Preparation of Tissue for Immunocytochemistry.
The kidneys from additional four CHF animals and four sham animals were fixed with 4% paraformaldehyde in 0.1 M Na cacodylate buffer, pH 7.4 (6, 11, 20). Tissue blocks were infiltrated for 30 min with 2.3 M sucrose containing 2% paraformaldehyde, mounted on holders, and rapidly frozen in liquid nitrogen (20). For light microscopy, the frozen tissue blocks were cryosectioned (0.8 μm, Reichert Ultracut S Cryoultramicrotome) and sections were incubated with affinity purified anti-AQP2 antibodies (LL127AP or LL358AP) and the labeling was visualized with horseradish peroxidase-conjugated secondary antibody (P448 1:100; Dako). For immunoelectron microscopy frozen blocks were freeze-substituted and embedded in Lowicryl HM20 (23) (Reichert automatic freeze substitution system). Ultrathin Lowicryl HM20 sections (40–60 nm) were incubated with affinity purified anti-AQP2 (LL127AP) and the labeling was visualized with goat-anti-rabbit IgG conjugated to 10 nm colloidal gold particles (GAR.EM10; BioCell Research Laboratories).
RESULTS
Increase in LVEDP and Reduced Plasma Sodium Concentration in Rats with Severe CHF.
Three weeks after left coronary artery ligation most rats developed a dramatic increase in LVEDP, as described (16–18). Based on the characterization of this model by others, rats with LVEDP > 15 mmHg were considered having severe CHF. As demonstrated in Table 1, LVEDP was 4.1 ± 0.3 mmHg in sham controls (n = 14) and was 26.9 ± 3.4 in the group with high LVEDP (n = 12). Importantly this group of rats also had a significant reduction in plasma Na+ concentration from 149.1 ± 1.1 to 142.2 ± 1.2 mEq/liter. Thus, this group had CHF with reduced plasma sodium concentration. The second group of rats with infarction in absence of CHF were selected based on LVEDP values lower than 15 mmHg (on average 7.2 ± 3.9, n = 6) compatible with mild compensated heart failure. In contrast to the group with high LVEDP plasma sodium concentrations were unchanged in this group (Table 1).
Table 1.
Physiological and pathophysiological parameters in sham-operated animals (Sham) and rats with heart failure (congested or compensated)
| Sham | Heart failure
|
|||
|---|---|---|---|---|
| Congestive | Compensated | All | ||
| LVEDP, mm Hg | 4.1 ± 0.3 | 26.9 ± 3.4* | 7.2 ± 3.9 | 20.3 ± 2.8* |
| Serum Na+, mEq/liter | 149.1 ± 1.1 | 142.2 ± 1.6* | 146.5 ± 1.3 | 143.8 ± 1.4* |
| Heart weight, mg | 787 ± 16 | 1,005 ± 54* | 878 ± 52 | 963 ± 40* |
| Mean arterial pressure, mm Hg | 102 ± 2 | 95 ± 4 | 100 ± 2 | 97 ± 3 |
| Kidney weight, mg | 897 ± 22 | 938 ± 34 | 869 ± 35 | 915 ± 22 |
| Rat weight, g | 227 ± 5 | 223 ± 6 | 232 ± 2 | 226 ± 4 |
| Rats, n | 14 | 12 | 6 | 18 |
The animals with heart failure were divided into two groups, one with CHF and one with compensated heart failure. The group termed heart failure-all represent all animals that were subjected to left coronary artery ligation 4 weeks earlier—i.e., the two groups combined. Values are expressed as mean ± SE.
P < 0.05 when compared with the sham-operated group.
Increase of AQP2 Expression in Rats with Severe CHF.
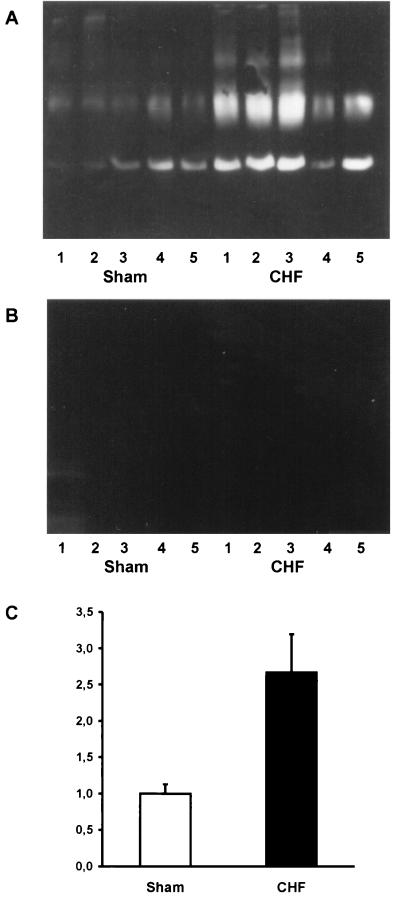
As previously shown, the affinity purified anti-AQP2 antibody recognizes the 29-kDa and the 35- to 50-kDa band (Fig. 1; refs. 13 and 19), corresponding to nonglycosylated and glycosylated AQP2 (22). The AQP2 bands were ablated by use of antibody previously absorbed with immunizing peptide (Fig. 1B) as also previously demonstrated (6, 11, 12). As shown in Fig. 1, a significant increase of both the 29-kDa and the 35- to 55-kDa AQP2 bands was observed in rats with CHF (Fig. 1A). Densitometry of all samples from rats with CHF revealed a marked increase in AQP2 expression from 100 ± 13% (n = 14) in sham-operated controls to 267 ± 53% (n = 12). In contrast no increase in AQP2 expression was noted in the group having compensated heart failure (Table 2), indicating a role of increased AQP2 expression for the development of increased water retention (as indicated by the decrease in plasma sodium concentration).
Figure 1.
Immunoblots of membrane fractions (20 μg/lane) from total rat kidney prepared from sham-operated rats (S) and experimental rats with CHF. (A) The immunoblot was reacted with affinity purified anti-AQP2 and reveal 29-kDa and 35- to 50-kDa AQP2 bands. A more prominent labeling is seen in CHF rat kidneys. (B) Immunoblotting control. (C) Densitometry performed on all sham-operated animals (n = 14) and all animals with severe CHF (n = 12). A highly significant increase in AQP2 expression is seen in CHF animals.
Table 2.
Expression of AQP2, AQP1, and AQP3 expressed as percentage of the level in sham-operated animals (Sham)
| Sham | Heart failure
|
|||
|---|---|---|---|---|
| Congestive | Compensated | All | ||
| AQP2 expression | 100 ± 13 | 267 ± 53*† | 121 ± 28 | 218 ± 41* |
| AQP1 expression | 100 ± 14 | 119 ± 18 | 95 ± 8 | 109 ± 10 |
| AQP3 expression | 100 ± 6 | 72 ± 11* | 112 ± 8 | 93 ± 8 |
| Rats; n | 14 | 12 | 6 | 18 |
The animals with heart failure were divided into two groups, one with CHF and one with compensated heart failure, based on determinations of LVEDP (see Table 1 and Methods). Heart failure-all represent all animals that were subjected to left coronary artery ligation 4 weeks earlier—i.e., the two groups combined. Values are expressed as mean ± SE.
P < 0.05 when group was compared to sham.
P < 0.05 when compared to the group with compensated heart failure.
Immunocytochemical Observations.
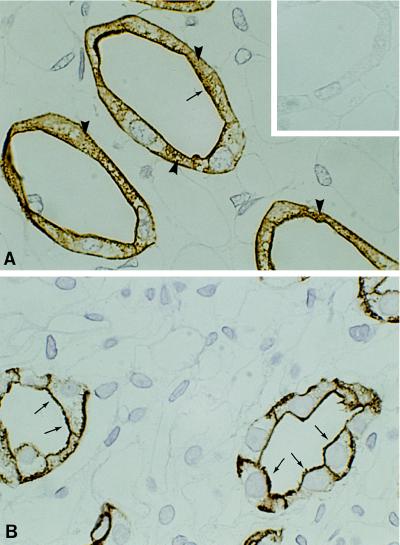
Kidneys from rats with CHF (having LVEDP > 15 mmHg) were fixed and processed for immunocytochemistry. AQP2 labeling was seen exclusively in collecting duct principal cells (Fig. 2). There was a prominent difference in the subcellular localization of AQP2 in kidneys from CHF and sham-operated rats. In CHF rats, AQP2 labeling was seen predominantly localized to the plasma membrane domains with abundant labeling of the apical plasma membrane and only marginal labeling of cytoplasmic vesicles (Fig. 2B). In contrast, AQP2 labeling in kidneys of sham-operated control animals showed a substantial labeling of vesicles throughout the cytoplasm and less prominent plasma membrane labeling (Fig. 2A). Some labeling was also present in the basolateral plasma membranes, as previously documented (6–8, 12).
Figure 2.
Immunohistochemical localization of AQP-2 water channels in kidney inner medulla of rat kidney in sham-operated rats (A) and rats with CHF (B). (A) Sham-operated controls. Abundant labeling is seen exclusively in collecting duct principal cells. The labeling is found scattered both in the plasma membrane domains (arrow) and in cytoplasmic domains corresponding to intracellular vesicles (arrowheads). (Inset) Immunolabeling control. (B) In rats with CHF strong AQP2 labeling is associated with the apical plasma membrane (arrows) and with the basolateral plasma membrane. In contrast only little labeling of cytoplasmic vesicles is seen. (×840.)
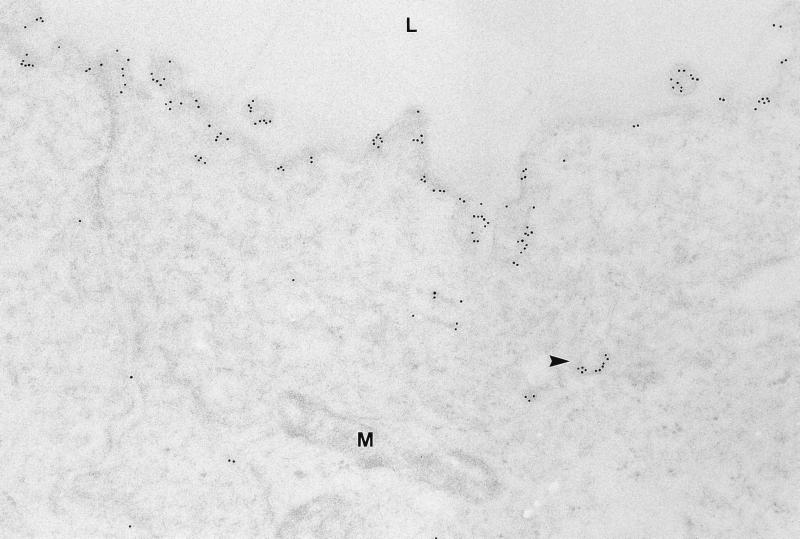
Immunoelectron microscopy was used to examine if the extensive labeling of AQP2 in the apical region of collecting duct principal cells was associated with the plasma membrane in rats with CHF. This was done to assure that the targeting of AQP2 to the apical plasma membrane was intact in these animals. Immunogold labeling revealed very strong labeling of the apical plasma membrane (Fig. 3) and only little labeling was associated with subapical vesicles (Fig. 3). Immunolabeling controls were negative (data not shown).
Figure 3.
Electron micrograph of ultrathin Lowicryl section of inner medullary collecting duct principal cells from CHF rat. Abundant AQP2 immunogold labeling is associated with the apical plasma membrane and only modest labeling is seen of intracellular vesicles (arrowhead). M, mitochondria. (×75,000.)
AQP2 but Not AQP1 or AQP3 Expression Is Up-Regulated.
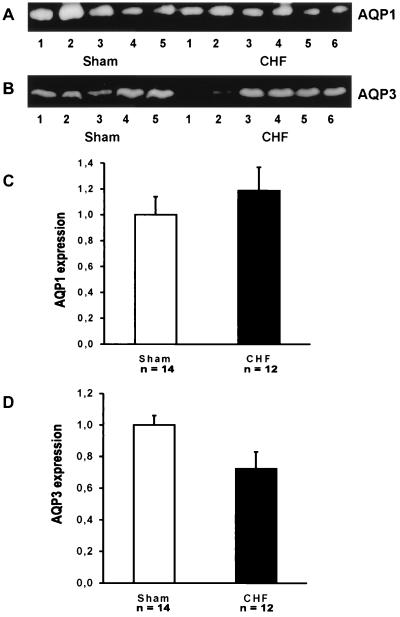
The expression of two other water channels AQP1 and AQP3 was examined. This was done to test if there is a selective up regulation of AQP2 in CHF or a more general increase in water channel expression to facilitate water reabsorption. AQP1 (24, 25) is expressed in the proximal tubule and in descending thin limbs of the loop of Henle (20) and the expression of this water channel is not altered in response to changes in body water balance (19). As shown in Fig. 4 A and C, there is no increase in AQP1 expression in rats with CHF. The expression is 100 ± 14% (n = 14) and 119 ± 18% (n = 12) in sham controls and in rats with CHF, respectively (Table 2). AQP3 (26–28) is expressed in the basolateral plasma membrane of collecting duct principal cells (21). Quantitative immunoblotting revealed no increase in AQP3 expression (Fig. 4 and Table 2). Indeed a modest decrease in expression was noted in the same membrane fraction used in parallel to examine AQP2 as well as AQP1 and AQP3 expression (Table 2). Thus, there is a selective increase in AQP2 expression associated with CHF with no increase in AQP1 or AQP3 expression.
Figure 4.
(A and B) Immunoblots of total kidney membrane fractions (20 μg/lane) from sham-operated control animals and CHF animals. In A, the immunoblot was reacted with anti-AQP1 serum and the 29-kDa AQP1 band is shown. In B, the immunoblot was reacted with affinity purified anti-AQP3 and the 27-kDa AQP3 band is shown. (C) Densitometry of AQP1 expression in sham-operated controls (n = 14) and in rats with CHF (n = 12). No change in AQP1 expression is seen. (D) Densitometry of AQP3 expression in sham-operated controls (n = 14) and in rats with CHF (n = 12). A modest decrease in AQP3 expression is seen.
DISCUSSION
The present results demonstrate that severe CHF is associated with marked dysregulation of the AQP2 water channel in the renal collecting duct. Two modes of dysregulation were revealed: (i) a marked increase in the abundance of AQP2 water channel protein in the collecting duct principal cells, and (ii) a marked redistribution of AQP2 water channels in the principal cells such that most of the channels are present in the apical plasma membrane. Both modes of dysregulation would be expected to be associated with enhancement of collecting duct water permeability and increased water absorption from the collecting duct. Thus, these results provide an explanation for excess free-water retention in severe CHF and for the development of hyponatremia. Both modes of dysregulation are most likely attributable to chronically increased circulating levels of vasopressin.
Physiological studies have demonstrated two general mechanisms by which vasopressin increases water permeability in the renal collecting duct. The first, short-term regulation, is manifest within a few minutes after a rise in circulating vasopressin levels and is due to trafficking of AQP2 water channel vesicles to the apical plasma membrane (7). The second, long-term regulation, is manifest only after many hours or days of increased vasopressin levels and is due to an increase in the abundance of the AQP2 protein in collecting duct cells (6, 11, 19). The latter mode of regulation is also associated with a rise in AQP2 mRNA levels and is most likely due to increased transcription of the AQP2 gene (reviewed in ref. 5). The results of the present study make it clear that the water retention associated with severe CHF is due, at least in part, to marked augmentation of both the short- and long-term regulatory processes.
Defects in the long-term regulation of AQP2 expression have previously been implicated in a wide range of water balance disorders including central diabetes insipidus (11, 29) and acquired diabetes insipidus due to lithium intoxication (12), to prolonged hypokalemia (13), to ureteral obstruction (14), and to purine amino nucleoside toxicity (30). Recently, a study of CCl4-induced hepatic cirrhosis with water retention by Saito and coworkers (15) has extended this list to include also disorders associated with free-water retention and hyponatremia. The present study demonstrated that in rats with CHF, a condition known clinically to be associated with severe alteration in water balance, AQP2 expression is significantly increased suggesting a role in the development of free water retention. This conclusion is supported by the evidence presented here that AQP2 expression was only increased in severe CHF with reduced plasma sodium concentrations, but was not increased in rats with mild heart failure and no decrease in plasma sodium concentrations. Recently, similar observations demonstrating increased AQP2 levels in kidneys of rats with chronic heart failure have been made in a preliminary report (31).
Our studies are consistent with the view that the observed reduction in plasma sodium is secondary to the increase in AQP2 expression. This is supported by the observation that rats with mild heart failure with LVEDP < 15 mmHg showed no increase in AQP2 expression nor did they show any reduction in plasma sodium concentrations (Tables 1 and 2). The reduction in plasma sodium concentration in rats with severe CHF can be speculated to be related to the severity of the disease and may only occur as a terminal event.
Abundant AQP2 in Apical Plasma Membrane in CHF.
A striking finding was the extraordinary redistribution of AQP2 within the collecting duct principal cells of rats with severe CHF. Both immunocytochemistry and immunoelectron microscopy demonstrated that AQP2 was almost exclusively localized to the plasma membranes whereas AQP2 labeling was reduced in cytoplasmic vesicles. This labeling pattern in the CHF rat kidneys is almost identical to the labeling pattern observed in rats treated with dDAVP (1-desamino-8-d-arginine-vasopressin) for 30 min (8). Furthermore, the pattern is similar to the one found in Brattleboro rat kidneys after vasopressin treatment (9, 11). Since vasopressin acutely regulates the apical plasma membrane water permeability by shuttling of AQP2 from an intracellular compartment in vesicles to the apical plasma membrane (7–10), the marked changes in the subcellular distribution in kidneys of CHF rats is fully consistent with a significant effect of vasopressin, known to be elevated in CHF (1). The findings that AQP2 is very efficiently targeted to the plasma membrane in rats with CHF strongly support the view that enhanced AQP2 shuttling to the apical plasma membrane contributes to the free water retention seen in CHF. Thus, excessive activation of the short-term mechanism of AQP2 regulation in CHF augments the effect of over-expression of AQP2 protein.
Signaling Pathways Potentially Involved in Modulating AQP2 Expression and Targeting in CHF.
Several studies have demonstrated a highly significant increase in vasopressin levels in CHF both in patients and in experimental models of CHF (1, 32–35). Diminished circulatory perfusion due to CHF is believed to activate arterial baroreceptors residing in the carotid sinus and aortic arch. This may produce activation of the sympathetic nervous system, renin–angiotensin system, and the nonosmotic release of AVP (arginine-vasopressin) (1). Because vasopressin treatment induces the expression of AQP2 (11), it is very likely that the increased AQP2 expression in the CHF rats (Table 2) is a direct result of increased vasopressin levels. However, contribution of other regulatory factors cannot be totally excluded at this stage.
Increase in AQP2 but Not in AQP1 or AQP3 Expression in CHF.
The increase in AQP2 expression appears to be rather selective. This was documented by using two additional membrane protein markers. AQP1 is very abundant in proximal tubules and descending thin limbs, and this water channel has been shown not to be regulated by vasopressin, by thirsting, or by water loading (19). Consistent with this, no change in AQP1 expression was observed in rats with CHF (Table 2). Moreover, we tested for possible change in AQP3 expression (26–28), which is abundant in the basolateral plasma membrane of collecting duct principal cells (21). There was no increase in AQP3 expression in CHF, rather a slight decrease in expression (Table 2). Thus, the absence of an increase in AQP1 and AQP3 expression strongly supports a selective increase in AQP2 expression. Selective change in AQP2 expression without change in AQP3 expression has also been reported in rats fed a low-protein diet and which have a concentrating defect (36). Like AQP2, AQP3 expression appears to be regulated by long-term mechanisms (19, 21). Further studies will be required to determine the basis at a transcription level of long-term regulation of AQP2 (and of AQP3) in states of water retention or polyuria.
Acknowledgments
We thank Mette Vistisen, Trine Møller, Hanne Weiling, Anette Franck, and Lisette Knoth-Nielsen for expert technical assistance. Support was provided by the Novo Nordisk Foundation, Karen Elise Jensen Foundation, and the Danish Medical Research Council.
ABBREVIATIONS
- CHF
congestive heart failure
- AQP
aquaporin
- LVEDP
left ventricular end-diastolic pressures
References
- 1.Bichet D G, Kluge R, Howard R L, Schrier R W. In: Hyponatraemic States. Seldin D W, Giebisch G, editors. Vol. 2. New York: Raven; 1992. pp. 1727–1751. [Google Scholar]
- 2.Agre P, Preston G M, Smith B L, Jung J S, Raina S, Moon C, Guggino W B, Nielsen S. Am J Physiol. 1993;265:F463–F476. doi: 10.1152/ajprenal.1993.265.4.F463. [DOI] [PubMed] [Google Scholar]
- 3.Fushimi K, Uchida S, Hara Y, Hirata Y, Marumo F, Sasaki S. Nature (London) 1993;361:549–552. doi: 10.1038/361549a0. [DOI] [PubMed] [Google Scholar]
- 4.Knepper M A, Wade J B, Terris J, Ecelbarger C, Marples D, Mandon B, Chou C L, Kishore B K, Nielsen S. Kidney Int. 1996;49:1712–1717. doi: 10.1038/ki.1996.253. [DOI] [PubMed] [Google Scholar]
- 5.Nielsen S, Marples D, Frøkiær J, Knepper M A, Agre P. Kidney Int. 1996;49:1718–1723. doi: 10.1038/ki.1996.254. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen S, DiGiovanni S R, Christensen E I, Knepper M A, Harris H W. Proc Natl Acad Sci USA. 1993;90:11663–11667. doi: 10.1073/pnas.90.24.11663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nielsen S, Chou C L, Marples D, Christensen E I, Kishore B K, Knepper M A. Proc Natl Acad Sci USA. 1995;92:1013–1017. doi: 10.1073/pnas.92.4.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marples D, Knepper M A, Christensen E I, Nielsen S. Am J Physiol. 1995;269:C655–C664. doi: 10.1152/ajpcell.1995.269.3.C655. [DOI] [PubMed] [Google Scholar]
- 9.Sabolic I, Katsura T, Verbavatz J M, Brown D. J Membr Biol. 1995;143:165–175. doi: 10.1007/BF00233445. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto T, Sasaki S, Fushimi K, Ishibashi K, Yaoita E, Kawasaki K, Marumo F, Kihara I. Am J Physiol. 1995;268:C1546–C1551. doi: 10.1152/ajpcell.1995.268.6.C1546. [DOI] [PubMed] [Google Scholar]
- 11.DiGiovanni S R, Nielsen S, Christensen E I, Knepper M A. Proc Natl Acad Sci USA. 1994;91:8984–8988. doi: 10.1073/pnas.91.19.8984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marples D, Christensen S, Christensen E I, Ottosen P D, Nielsen S. J Clin Invest. 1995;95:1838–1845. doi: 10.1172/JCI117863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marples D, Frøkiær J, Dørup J, Knepper M A, Nielsen S. J Clin Invest. 1996;97:1960–1968. doi: 10.1172/JCI118628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frøkiær J, Marples D, Knepper M A, Nielsen S. Am J Physiol. 1996;270:F657–F668. doi: 10.1152/ajprenal.1996.270.4.F657. [DOI] [PubMed] [Google Scholar]
- 15.Fujita N, Ishikawa S E, Sasaki S, Fujisawa G, Fushimi K, Marumo F, Saito T. Am J Physiol. 1996;269:F926–F931. doi: 10.1152/ajprenal.1995.269.6.F926. [DOI] [PubMed] [Google Scholar]
- 16.Bech O M, Kahr O, Diamant B, Steiness E. Cardiovasc Res. 1996;23:649–654. doi: 10.1093/cvr/23.8.649. [DOI] [PubMed] [Google Scholar]
- 17.Pfeffer M A, Pfeffer J M, Fishbein M C, Flectcher P J, Spadaro J, Kloner R A, Braunwald E. Circ Res. 1996;44:503–512. doi: 10.1161/01.res.44.4.503. [DOI] [PubMed] [Google Scholar]
- 18.Hostetter T H, Pfeffer J M, Pfeffer M A, Dworkin L D, Braunwald E, Brenner B M. Am J Physiol. 1983;245:H98–H103. doi: 10.1152/ajpheart.1983.245.1.H98. [DOI] [PubMed] [Google Scholar]
- 19.Terris J, Ecelbarger C A, Nielsen S, Knepper M A. Am J Physiol. 1996;271:F414–F422. doi: 10.1152/ajprenal.1996.271.2.F414. [DOI] [PubMed] [Google Scholar]
- 20.Nielsen S, Smith B, Christensen E I, Knepper M A, Agre P. J Cell Biol. 1993;120:371–383. doi: 10.1083/jcb.120.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ecelbarger C, Terris J, Frindt G, Echevarria M, Marples D, Nielsen S, Knepper M A. Am J Physiol. 1995;269:F663–F672. doi: 10.1152/ajprenal.1995.269.5.F663. [DOI] [PubMed] [Google Scholar]
- 22.Sasaki S, Fushimi K, Saito H, Saito F, Uchida S, Ishibashi K, Kuwahara M, Ikeuchi T, Inui K, Nakajima K, Watanabe T X, Marumo F. J Clin Invest. 1994;93:1250–1256. doi: 10.1172/JCI117079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nielsen S, Pallone T L, Smith B L, Christensen E I, Agre P, Maunsbach A B. Am J Physiol. 1995;268:F1023–F1037. doi: 10.1152/ajprenal.1995.268.6.F1023. [DOI] [PubMed] [Google Scholar]
- 24.Preston G M, Carroll T P, Guggino W B, Agre P. Science. 1992;256:385–387. doi: 10.1126/science.256.5055.385. [DOI] [PubMed] [Google Scholar]
- 25.Preston G M, Agre P. Proc Natl Acad Sci USA. 1991;88:11110–11114. doi: 10.1073/pnas.88.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Echevarria M, Windhager E E, Tate S S, Frindt G. Proc Natl Acad Sci USA. 1994;91:10997–11001. doi: 10.1073/pnas.91.23.10997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishibashi K, Sasaki S, Fushimi K, Uchida S, Kuwahara M, Saito H, Furukawa T, Nakajima K, Yamaguchi Y, Gojobori T, Marumo F. Proc Natl Acad Sci USA. 1994;91:6269–6273. doi: 10.1073/pnas.91.14.6269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma T, Frigeri A, Hasegawa H, Verkman A S. J Biol Chem. 1994;269:21845–21849. [PubMed] [Google Scholar]
- 29.Deen P M, Verdijk M A, Knoers N V, Wieringa B, Monnens L A, van-Os C H, van-Oost B A. Science. 1994;264:92–95. doi: 10.1126/science.8140421. [DOI] [PubMed] [Google Scholar]
- 30.Apostol E, Ecelbarger C A, Terris J, Bradford A D, Andrews P, Knepper M A. J Am Soc Nephrol. 1997;8:15–24. doi: 10.1681/ASN.V8115. [DOI] [PubMed] [Google Scholar]
- 31.Xu D-L, Martin P Y, Ohara M, St John J, Pattison T, Schrier R W. J Am Soc Nephrol. 1996;7:1274. (abstr.). [Google Scholar]
- 32.Szatalowicz V L, Arnold P E, Chaimovitz C, Bichet D, Berl T, Schrier R W. N Engl J Med. 1981;305:263–266. doi: 10.1056/NEJM198107303050506. [DOI] [PubMed] [Google Scholar]
- 33.Mettauer B, Rouleau J L, Bichet D, Juneau C, Kortas C, Barjon J N, de Champlain J. Ann Intern Med. 1986;105:161–167. doi: 10.7326/0003-4819-105-2-161. [DOI] [PubMed] [Google Scholar]
- 34.Bichet D G, Kortas C, Mettauer B, Manzini C, Marc Aurele J, Rouleau J L, Schrier R W. Kidney Int. 1986;29:1188–1196. doi: 10.1038/ki.1986.126. [DOI] [PubMed] [Google Scholar]
- 35.Schrier R W. N Engl J Med. 1988;319:1127–1134. doi: 10.1056/NEJM198810273191705. [DOI] [PubMed] [Google Scholar]
- 36.Sands J M, Naruse M, Jacobs J D, Wilcox J N, Klein J D. J Clin Invest. 1996;97:2807–2814. doi: 10.1172/JCI118736. [DOI] [PMC free article] [PubMed] [Google Scholar]