Abstract
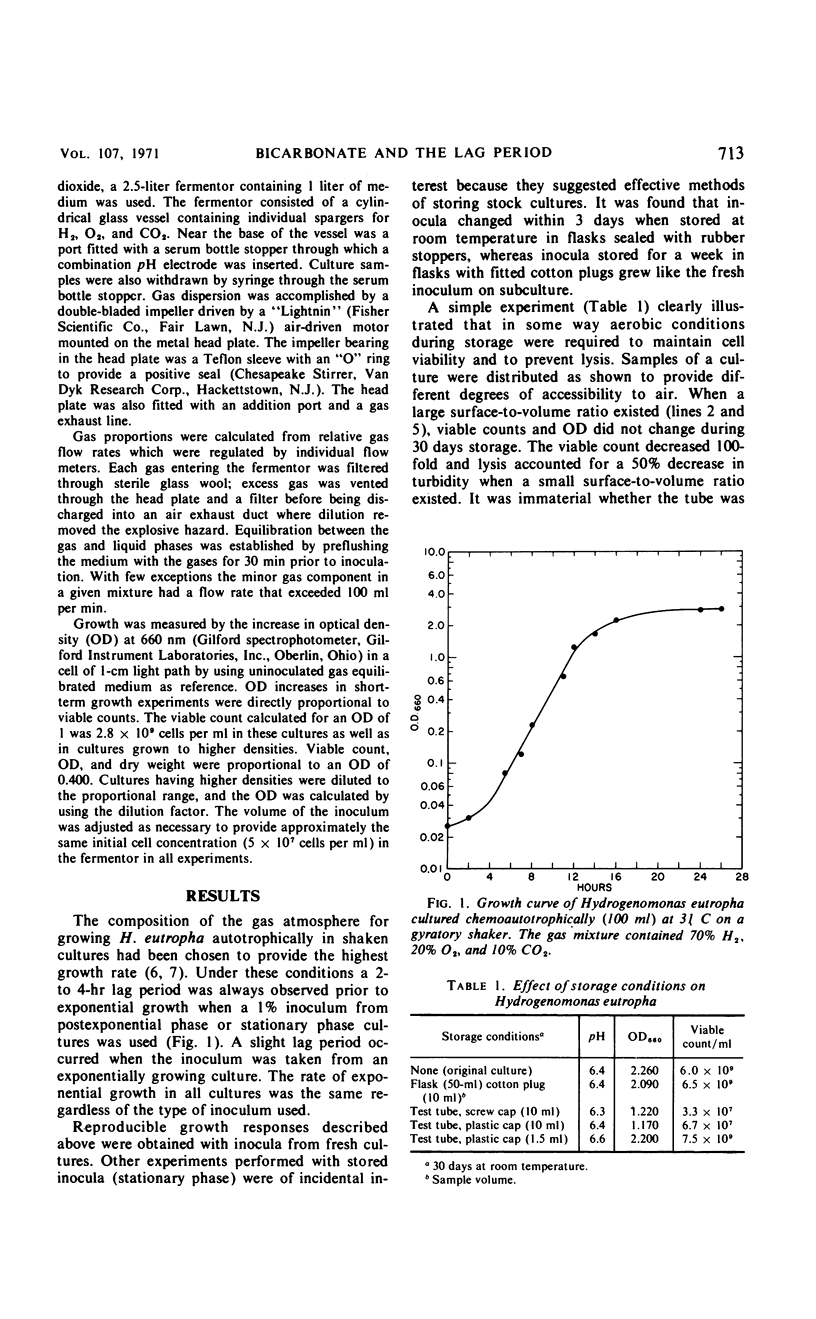
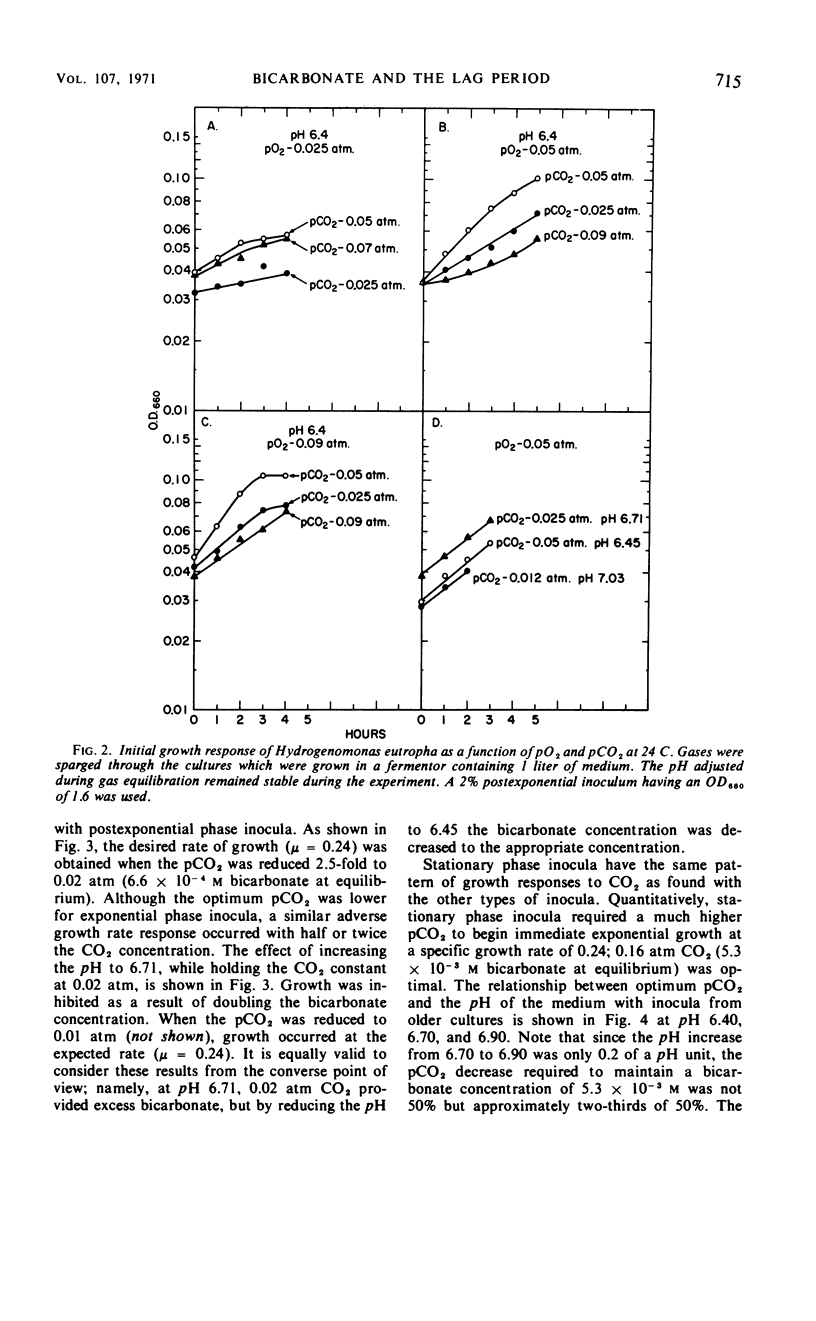
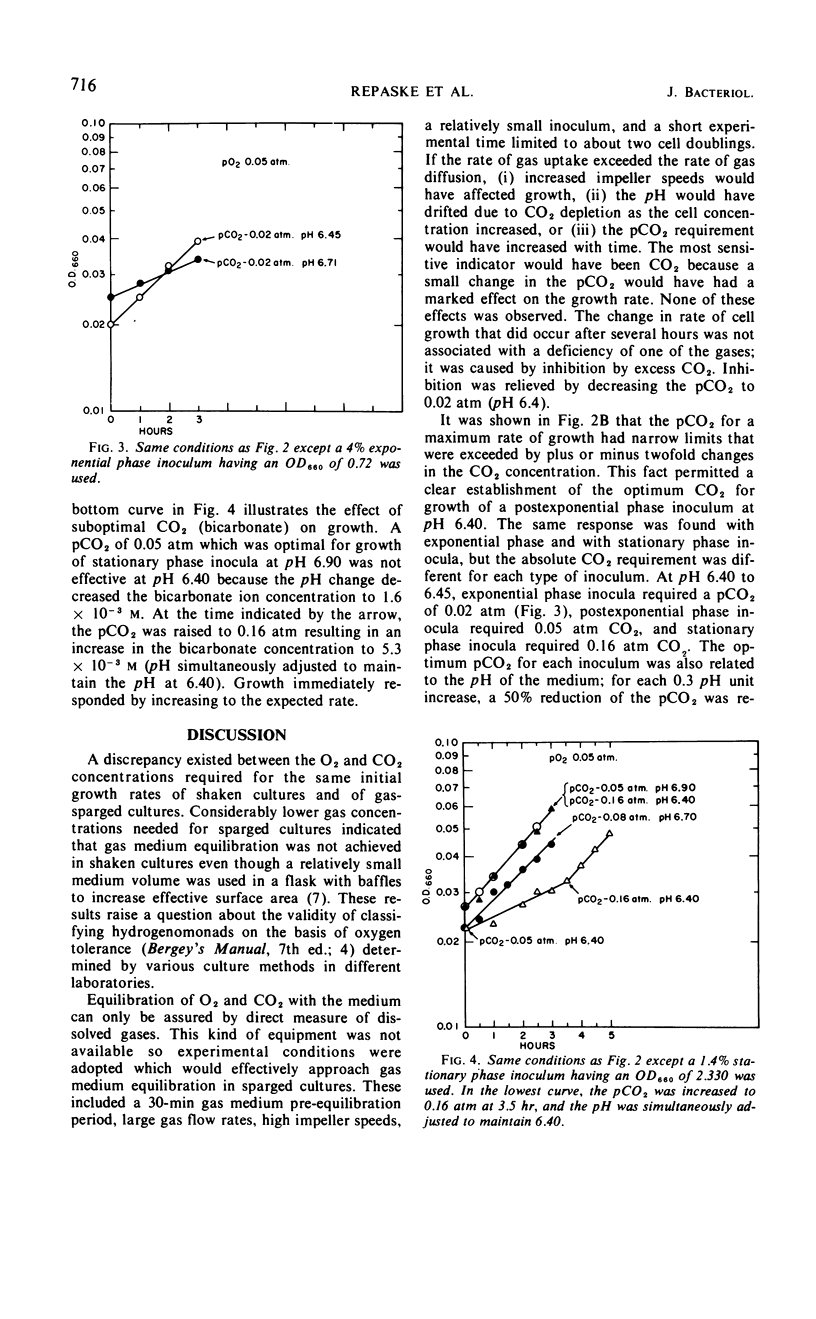
Carbon dioxide and oxygen concentrations have a profound effect on the lag period of chemoautotrophically grown Hydrogenomonas eutropha. Minimum lag periods and high growth rates were obtained in shaken flask cultures with a prepared gas mixture containing 70% H2, 20% O2, and 10% CO2. However, excessively long lag periods resulted when the same gas mixture was sparged through the culture. The lag period was shortened in sparged cultures by decreasing both the pO2 and the pCO2, indicating that gas medium equilibration had not occurred in shaken cultures. The lag period was completely eliminated at certain concentrations of O2 and CO2. The optimum pO2 was 0.05 atm, but the optimum pCO2 varied according to the pH of the medium and physiological age of the inoculum. At pH 6.4, the pCO2 required to obtain immediate growth of exponential, postexponential, and stationary phase inocula at equal specific rates was 0.02, 0.05, and 0.16 atm, respectively. With each 0.3-unit increase in the pH of the medium, a 50% decrease in the CO2 concentration was needed to permit growth to occur at the same rate. The pCO2 changes required to compensate for the pH changes of the medium had the net effect of maintaining a constant bicarbonate ion concentration. Initial growth of H. eutropha was therefore indirectly related to pCO2 and directly dependent upon a constant bicarbonate ion concentration.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davis D. H., Stanier R. Y., Doudoroff M., Mandel M. Taxonomic studies on some gram negative polarly flagellated "hydrogen bacteria" and related species. Arch Mikrobiol. 1970;70(1):1–13. doi: 10.1007/BF00691056. [DOI] [PubMed] [Google Scholar]
- PACKER L., VISHNIAC W. Chemosynthetic fixation of carbon dioxide and characteristics of hydrogenase in resting cell suspensions of Hydrogenomonas ruhlandii nov. spec. J Bacteriol. 1955 Aug;70(2):216–223. doi: 10.1128/jb.70.2.216-223.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REPASKE R. Nutritional requirements for Hydrogenomonas eutropha. J Bacteriol. 1962 Feb;83:418–422. doi: 10.1128/jb.83.2.418-422.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON E., STOUT H. A., POWELSON D., KOFFLER H. Comparative biochemistry of the hydrogen bacteria. I. The simultaneous oxidation of hydrogen and lactate. J Bacteriol. 1953 Mar;65(3):283–287. doi: 10.1128/jb.65.3.283-287.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]



