Abstract
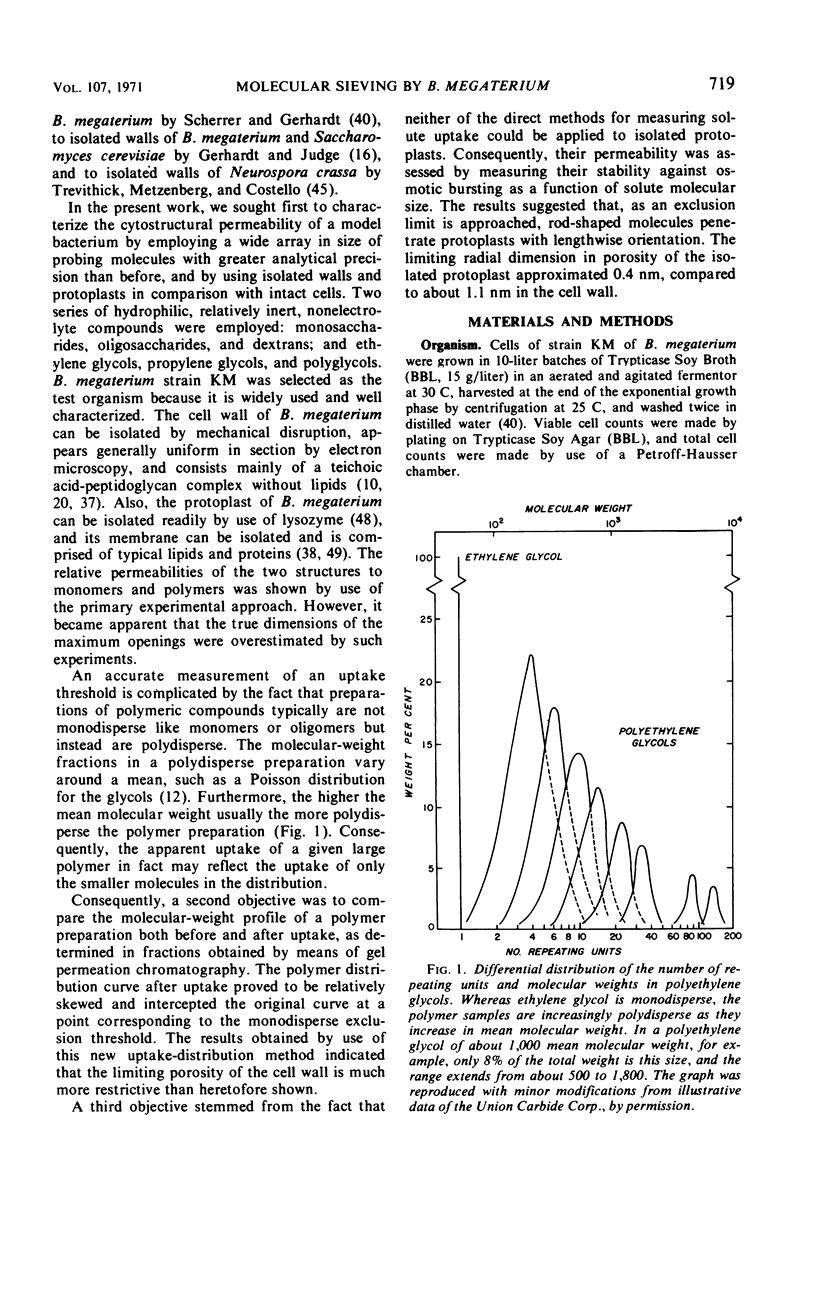
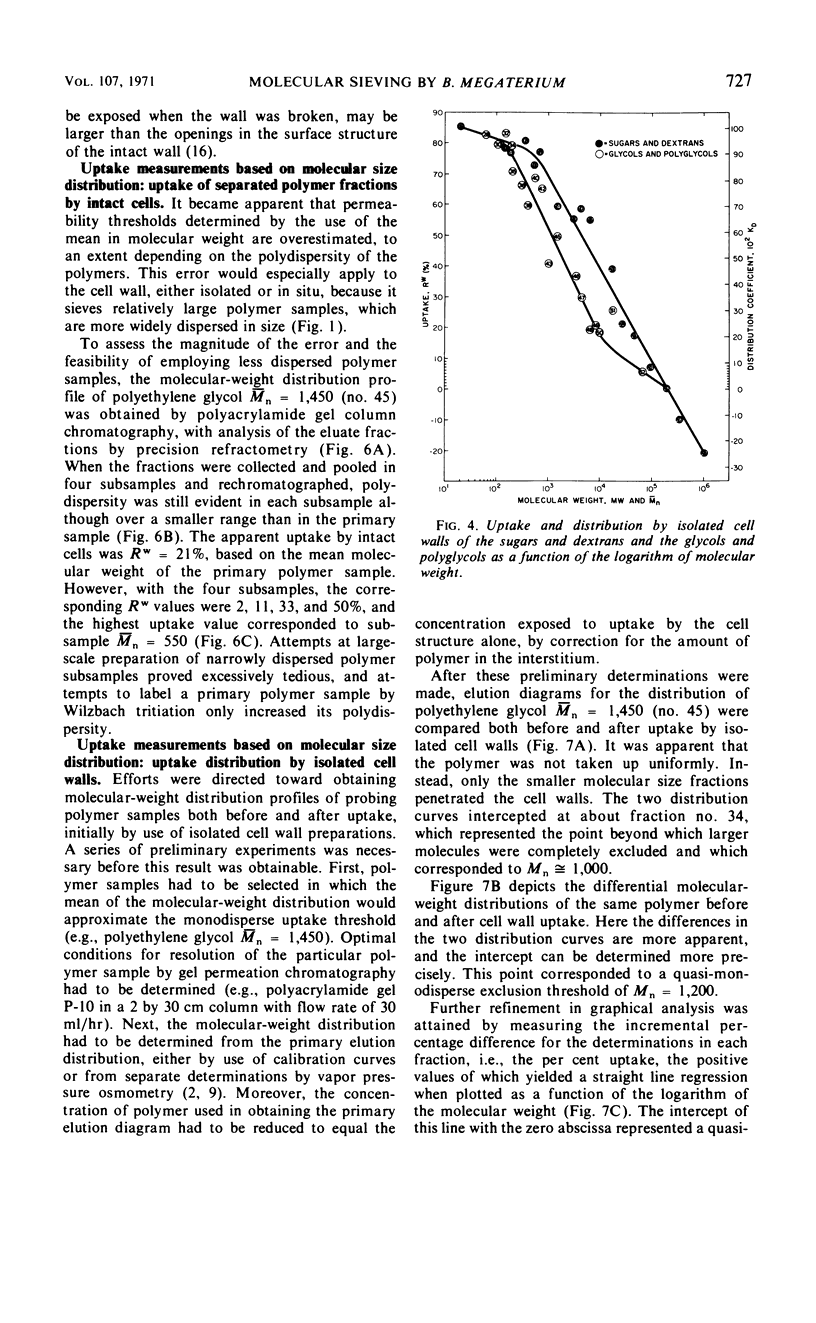
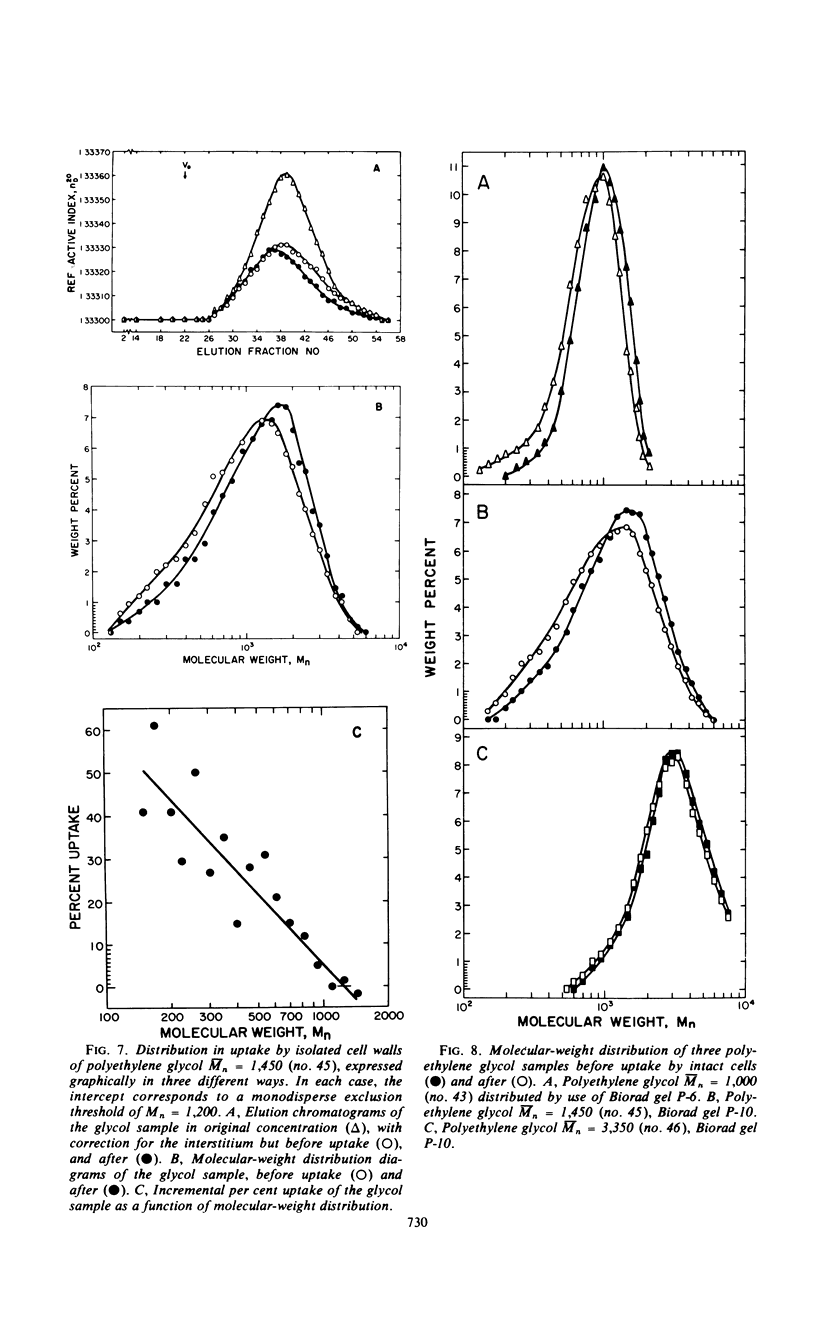
Passive permeabilities of the cell wall and protoplast of Bacillus megaterium strain KM were characterized by use of 50 hydrophilic probing molecules (tritiated water, sugars, dextrans, glycols, and polyglycols) which varied widely in size. Weight per cent uptake values (Rw) were measured at diffusional equilibrium under conditions that negated the influences of adsorption or active transport. Plots of Rw for intact cells as a function of number-average molecular weight (¯Mn) or Einstein-Stokes hydrodynamic radius (¯rES) of the solutes showed three phases: a protoplast uptake phase with a polydisperse exclusion threshold of ¯Mn = 0.6 × 103 to 1.1 × 103, ¯rES = 0.6 to 1.1 nm; a cell wall uptake phase with a polydisperse exclusion threshold of ¯Mn = 0.7 × 105 to 1.2 × 105, ¯rES ≅ 8.3 nm; and a total exclusion phase. Isolated cell walls showed only the latter two phases. However, it became evident that the cell wall selectively passed only the smallest molecules in a heterodisperse polymer sample. When the molecular-weight distributions of polyglycol samples (¯Mn = 1,000, 1,450, and 3,350) were determined by analytical gel chromatography before and after uptake by intact cells or isolated cell walls, a quasi-monodisperse exclusion threshold was obtained corresponding to Mn = 1,200, rES = 1.1 nm. The permeability of isolated protoplasts was assessed by the relative ability of solutes to effect osmotic stabilization. An indefinite exclusion threshold, evident even with monodisperse sugars, was attributed to lengthwise orientation of the penetrating rod-shaped molecules. Altogether, the best estimate of the limiting equivalent porosity of the protoplast was 0.4 to 0.6 nm in radius and of the cell wall, 1.1 nm.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLACK S. H., GERHARDT P. Permeability of bacterial spores. I. Characterization of glucose uptake. J Bacteriol. 1961 Nov;82:743–749. doi: 10.1128/jb.82.5.743-749.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRITT E. M., GERHARDT P. Bacterial permeability; total uptake of lysine by intact cells, protoplasts, and cell walls of Micrococcus lysodeikticus. J Bacteriol. 1958 Sep;76(3):288–293. doi: 10.1128/jb.76.3.288-293.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corner T. R., Marquis R. E. Why do bacterial protoplasts burst in hypotonic solutions? Biochim Biophys Acta. 1969;183(3):544–558. doi: 10.1016/0005-2736(69)90168-0. [DOI] [PubMed] [Google Scholar]
- DELAMATER E. D., BABCOCK K. L., MAZZANTI G. R. On the leakage of cellular material from Bacillus megaterium. J Bacteriol. 1959 Apr;77(4):513–514. doi: 10.1128/jb.77.4.513-514.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dezélée P., Bricas E. Structure of the peptidoglycan in Escherichia coli B and Bacillus megaterium KM. Stereospecific synthesis of two meso-diaminopimelic acid peptides with the tetrapeptide subunit of bacterial cell wall peptidoglycan. Biochemistry. 1970 Feb 17;9(4):823–831. doi: 10.1021/bi00806a015. [DOI] [PubMed] [Google Scholar]
- GERHARDT P., BLACK S. H. Permeability of bacterial spores. II. Molecular variables affecting solute permeation. J Bacteriol. 1961 Nov;82:750–760. doi: 10.1128/jb.82.5.750-760.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERHARDT P., JUDGE J. A. POROSITY OF ISOLATED CELL WALLS OF SACCHAROMYCES CEREVISIAE AND BACILLUS MEGATERIUM. J Bacteriol. 1964 Apr;87:945–951. doi: 10.1128/jb.87.4.945-951.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROTTE G. Passage of dextran molecules across the blood-lymph barrier. Acta Chir Scand Suppl. 1956;211:1–84. [PubMed] [Google Scholar]
- HESS E. L., LAGG S. E. Some physical aspects of the bacterial cell. Science. 1958 Aug 15;128(3320):356–358. doi: 10.1126/science.128.3320.356. [DOI] [PubMed] [Google Scholar]
- KEDEM O., KATCHALSKY A. Thermodynamic analysis of the permeability of biological membranes to non-electrolytes. Biochim Biophys Acta. 1958 Feb;27(2):229–246. doi: 10.1016/0006-3002(58)90330-5. [DOI] [PubMed] [Google Scholar]
- MACDONALD R. E., GERHARDT P. Bacterial permeability: the uptake and oxidation of citrate by Escherichia coli. Can J Microbiol. 1958 Apr;4(2):109–124. doi: 10.1139/m58-013. [DOI] [PubMed] [Google Scholar]
- MARQUIS R. E., GERHARDT P. RESPIRATION-COUPLED AND PASSIVE UPTAKE OF ALPHA-AMINOISOBUTYRIC ACID, A METABOLICALLY INERT TRANSPORT ANALOGUE, BY BACILLUS MEGATERIUM. J Biol Chem. 1964 Oct;239:3361–3371. [PubMed] [Google Scholar]
- Marquis R. E. Osmotic sensitivity of bacterial protoplasts and the response of their limiting membrane to stretching. Arch Biochem Biophys. 1967 Feb;118(2):323–331. doi: 10.1016/0003-9861(67)90356-6. [DOI] [PubMed] [Google Scholar]
- Marquis R. E. Osmotic stability of bacterial protoplasts related to molecular size of stabilizing solutes. Biochem Biophys Res Commun. 1965 Sep 8;20(5):580–585. doi: 10.1016/0006-291x(65)90438-9. [DOI] [PubMed] [Google Scholar]
- Marquis R. E. Salt-induced contraction of bacterial cell walls. J Bacteriol. 1968 Mar;95(3):775–781. doi: 10.1128/jb.95.3.775-781.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAPPENHEIMER J. R., RENKIN E. M., BORRERO L. M. Filtration, diffusion and molecular sieving through peripheral capillary membranes; a contribution to the pore theory of capillary permeability. Am J Physiol. 1951 Oct;167(1):13–46. doi: 10.1152/ajplegacy.1951.167.1.13. [DOI] [PubMed] [Google Scholar]
- PHELPS C. F. THE PHYSICAL PROPERTIES OF INULIN SOLUTIONS. Biochem J. 1965 Apr;95:41–47. doi: 10.1042/bj0950041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHERRER R., GERHARDT P. MOLECULAR SIEVING BY CELL MEMBRANES OF BACILLUS MEGATERIUM. Nature. 1964 Nov 14;204:649–650. doi: 10.1038/204649a0. [DOI] [PubMed] [Google Scholar]
- SCHULTZ S. G., SOLOMON A. K. Determination of the effective hydrodynamic radii of small molecules by viscometry. J Gen Physiol. 1961 Jul;44:1189–1199. doi: 10.1085/jgp.44.6.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salton M. R. Structure and function of bacterial cell membranes. Annu Rev Microbiol. 1967;21:417–442. doi: 10.1146/annurev.mi.21.100167.002221. [DOI] [PubMed] [Google Scholar]
- Soll A. H. A new approach to molecular configuration applied to aqueous pore transport. J Gen Physiol. 1967 Dec;50(11):2565–2578. doi: 10.1085/jgp.50.11.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon A. K. Characterization of biological membranes by equivalent pores. J Gen Physiol. 1968 May;51(5 Suppl):335S+–335S+. [PubMed] [Google Scholar]
- Trevithick J. R., Metzenberg R. L. Genetic alteration of pore size and other properties of the Neurospora cell wall. J Bacteriol. 1966 Oct;92(4):1016–1020. doi: 10.1128/jb.92.4.1016-1020.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Heijenoort J., Elbaz L., Dezélée P., Petit J. F., Bricas E., Ghuysen J. M. Structure of the meso-diaminopimelic acid containing peptidoglycans in Escherichia coli B and Bacillus megaterium KM. Biochemistry. 1969 Jan;8(1):207–213. doi: 10.1021/bi00829a030. [DOI] [PubMed] [Google Scholar]
- WALLENIUS G. [Renal clearance of dextran as a measure of glomerular permeability]. Acta Soc Med Ups Suppl. 1954 Apr 8;59(4):1–91. [PubMed] [Google Scholar]
- WEIBULL C. The isolation of protoplasts from Bacillus megaterium by controlled treatment with lysozyme. J Bacteriol. 1953 Dec;66(6):688–695. doi: 10.1128/jb.66.6.688-695.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudkin M. D. Isolation and analysis of the protoplast membrane of Bacillus megaterium. Biochem J. 1966 Mar;98(3):923–928. doi: 10.1042/bj0980923. [DOI] [PMC free article] [PubMed] [Google Scholar]



