Abstract
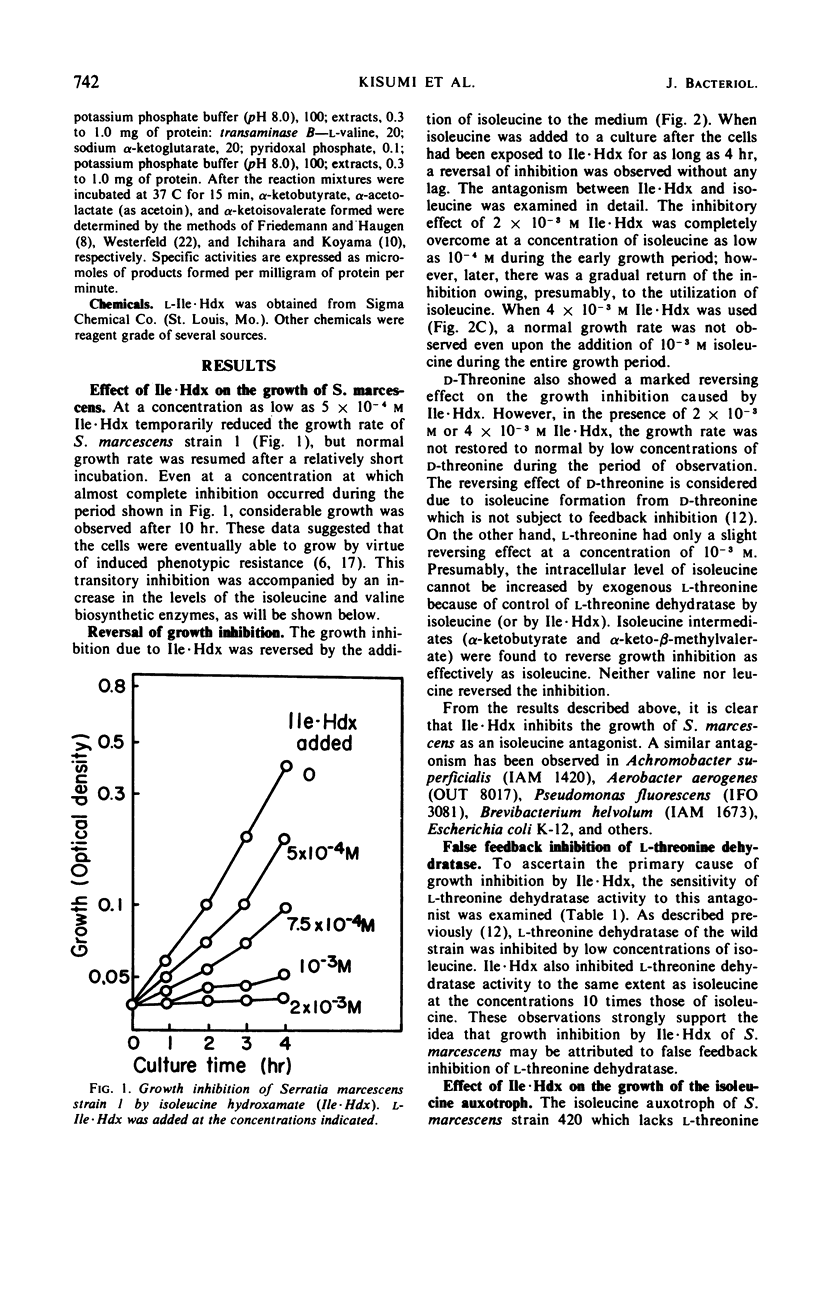
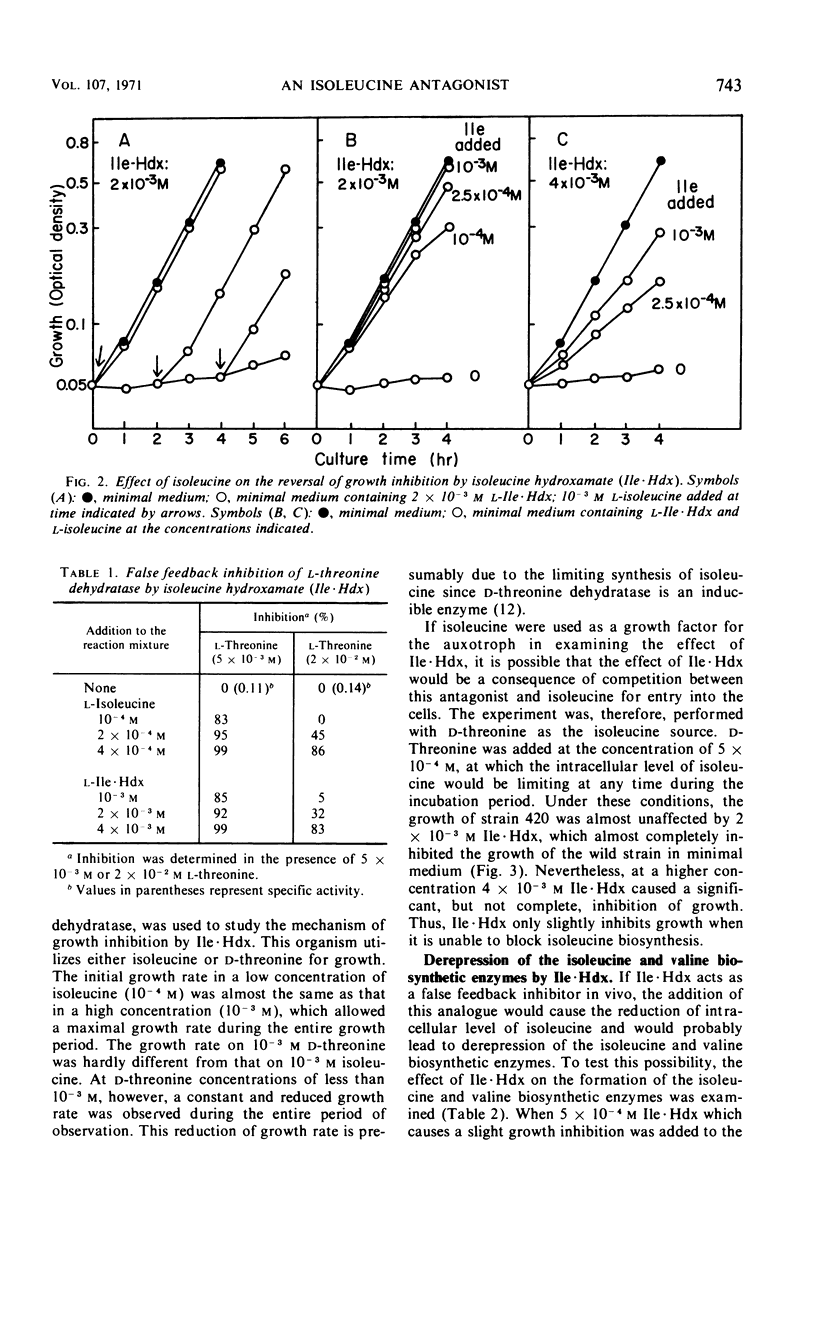
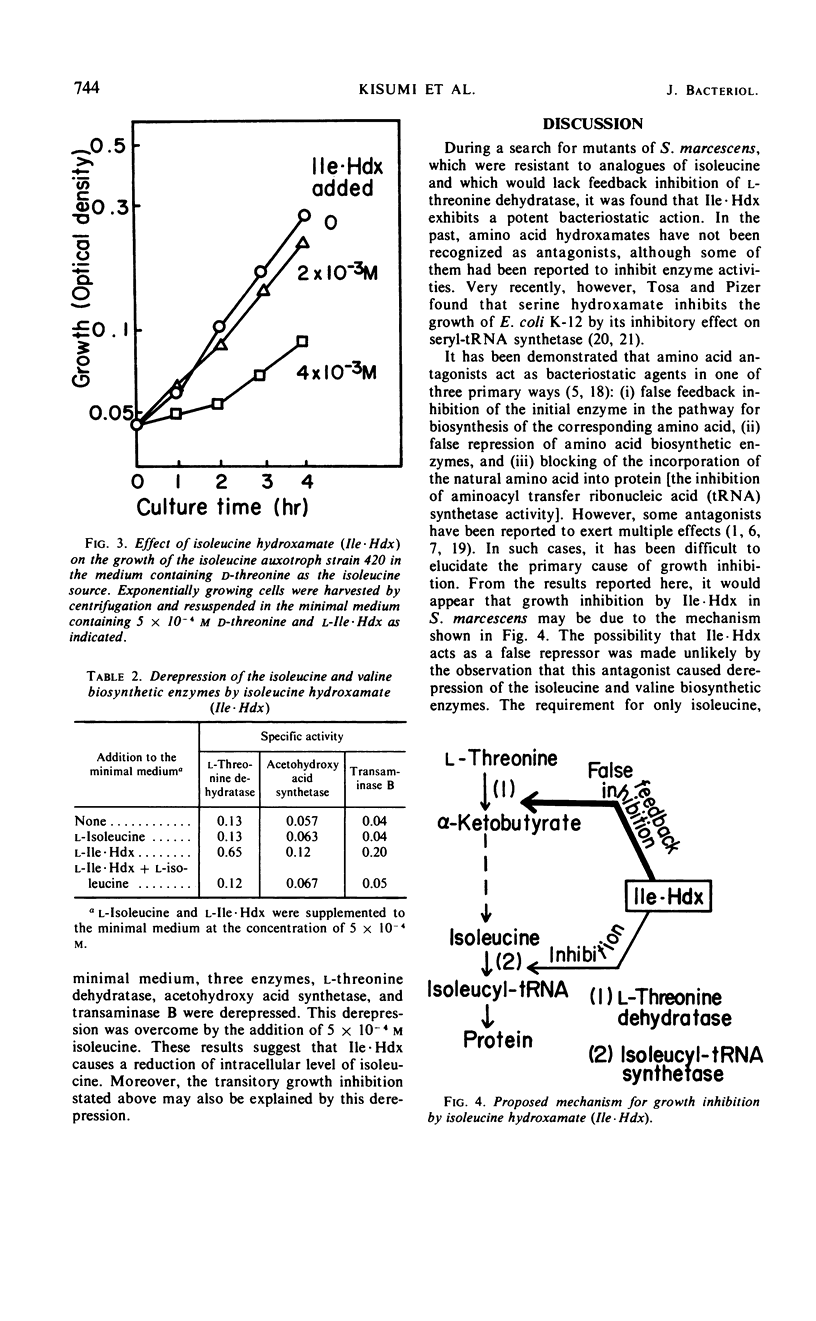
Isoleucine hydroxamate (Ile·Hdx) was found to inhibit the growth of Serratia marcescens and to antagonize isoleucine. At a low concentration of Ile·Hdx, at which the growth of the wild strain was completely inhibited, the growth of an isoleucine auxotroph was not inhibited in the medium containing a limiting amount of d-threonine as the isoleucine source. At a higher concentration, this antagonist exhibited a considerable inhibitory effect on the growth of the auxotroph. Ile·Hdx showed the same inhibitory effect as isoleucine on l-threonine dehydratase activity at the concentrations 10 times those of isoleucine. Ile·Hdx caused also derepression of isoleucine-valine biosynthetic enzymes and the derepression was overcome by isoleucine. These results indicate the Ile·Hdx causes growth inhibition by its effects on isoleucine metabolism.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calvo J. M., Freundlich M., Umbarger H. E. Regulation of branched-chain amino acid biosynthesis in Salmonella typhimurium: isolation of regulatory mutants. J Bacteriol. 1969 Mar;97(3):1272–1282. doi: 10.1128/jb.97.3.1272-1282.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowden L., Lewis D., Tristram H. Toxic amino acids: their action as antimetabolites. Adv Enzymol Relat Areas Mol Biol. 1967;29:89–163. doi: 10.1002/9780470122747.ch3. [DOI] [PubMed] [Google Scholar]
- Freundlich M., Clarke L. P. Control of isoleucine, valine and leucine biosynthesis. V. Dual effect of alpha-aminobutyric acid on repression and endproduct inhibition in Escherichia coli. Biochim Biophys Acta. 1968 Dec 23;170(2):271–281. doi: 10.1016/0304-4165(68)90007-x. [DOI] [PubMed] [Google Scholar]
- Freundlich M., Trela J. M. Control of isoleucine, valine, and leucine biosynthesis. VI. Effect of 5',5',5'-trifluoroleucine on repression in Salmonella typhimurium. J Bacteriol. 1969 Jul;99(1):101–106. doi: 10.1128/jb.99.1.101-106.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARDING W. M., SHIVE W. Cyclopentaneglycine, an inhibitory analogue of isoleucine. J Biol Chem. 1954 Jan;206(1):401–410. [PubMed] [Google Scholar]
- Ichihara A., Koyama E. Transaminase of branched chain amino acids. I. Branched chain amino acids-alpha-ketoglutarate transaminase. J Biochem. 1966 Feb;59(2):160–169. doi: 10.1093/oxfordjournals.jbchem.a128277. [DOI] [PubMed] [Google Scholar]
- KISUMI M., ASHIKAGA Y., KATO J., CHIBATA I. Studies on the isoleucine fermentation. II. On the mechanism of isoleucine formation and threonine dehydrase. J Biochem. 1962 Dec;52:400–408. doi: 10.1093/oxfordjournals.jbchem.a127636. [DOI] [PubMed] [Google Scholar]
- KISUMI M. Studies on the isoleucine fermentation. I. Screening of organisms and investigation of cultural conditions. J Biochem. 1962 Dec;52:390–399. doi: 10.1093/oxfordjournals.jbchem.a127635. [DOI] [PubMed] [Google Scholar]
- Kisumi M., Kato J., Komatsubara S., Chibata I. Increase in isoleucine accumulation by alpha-aminobutyric acid-resistant mutants of Serratia marcescens. Appl Microbiol. 1971 Apr;21(4):569–574. doi: 10.1128/am.21.4.569-574.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisumi M., Komatsubara S., Chibata I. Multivalent repression and genetic depression of isoleucine-valine biosynthetic enzymes in Serratia marcescens. J Bacteriol. 1971 Sep;107(3):824–827. doi: 10.1128/jb.107.3.824-827.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisumi M., Komatsubara S., Chibata I. Valine accumulation by alpha-aminobutyric acid-resistant mutants of Serratia marcescens. J Bacteriol. 1971 May;106(2):493–499. doi: 10.1128/jb.106.2.493-499.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MCCORD T. J., HOWELL D. C., THARP D. L., DAVIS A. L. 2-AMINO-3-METHYLTHIOBUTYRIC ACID, AN ISOLEUCINE ANTAGONIST. J Med Chem. 1965 May;8:290–292. doi: 10.1021/jm00327a004. [DOI] [PubMed] [Google Scholar]
- MOYED H. S. Interference with the feed-back control of histidine biosynthesis. J Biol Chem. 1961 Aug;236:2261–2267. [PubMed] [Google Scholar]
- RICHMOND M. H. The effect of amino acid analogues on growth and protein synthesis in microorganisms. Bacteriol Rev. 1962 Dec;26:398–420. doi: 10.1128/br.26.4.398-420.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentirmai A., Umbarger H. E. Isoleucine and valine metabolism of Escherichia coli. XIV. Effect of thiaisoleucine. J Bacteriol. 1968 May;95(5):1666–1671. doi: 10.1128/jb.95.5.1666-1671.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosa T., Pizer L. I. Biochemical bases for the antimetabolite action of L-serine hydroxamate. J Bacteriol. 1971 Jun;106(3):972–982. doi: 10.1128/jb.106.3.972-982.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosa T., Pizer L. I. Effect of serine hydroxamate on the growth of Escherichia coli. J Bacteriol. 1971 Jun;106(3):966–971. doi: 10.1128/jb.106.3.966-971.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]



