Abstract
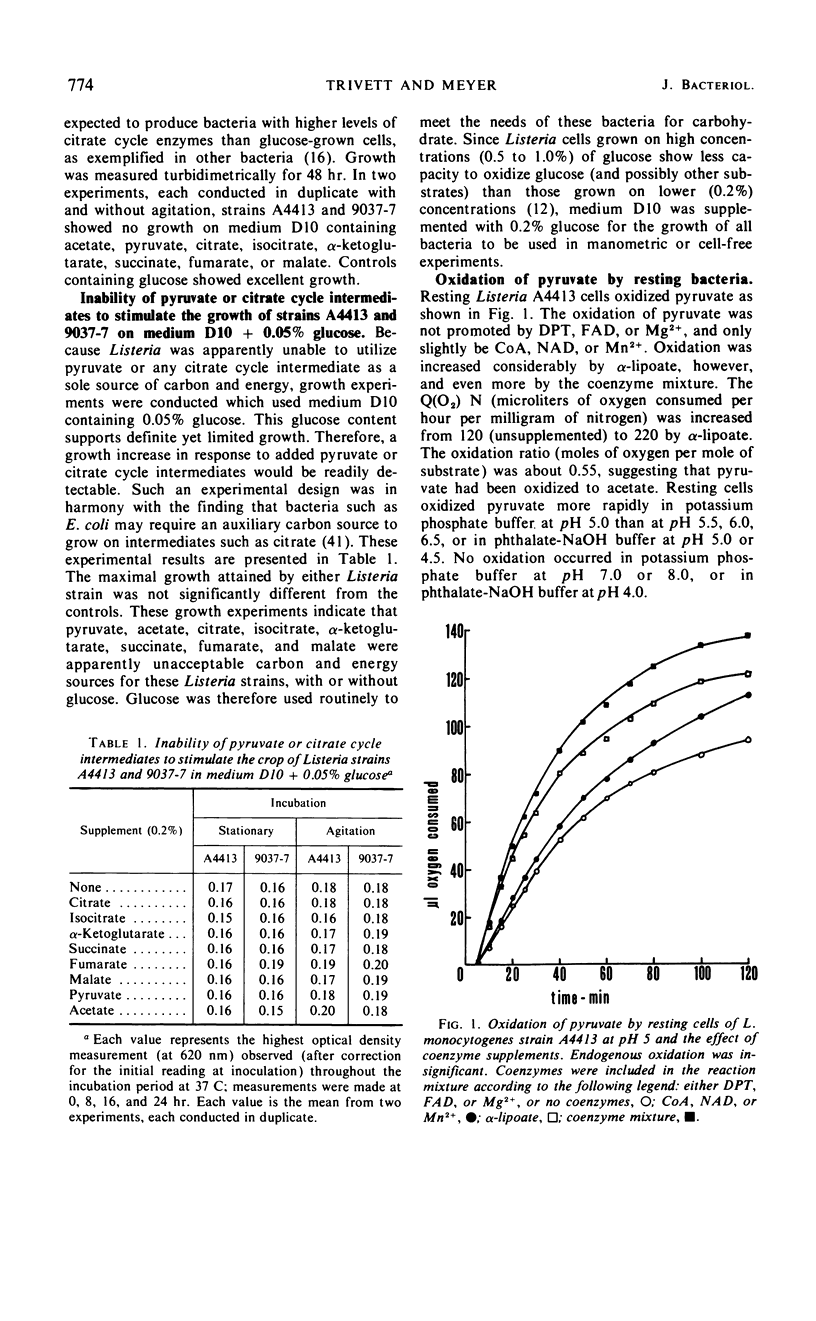
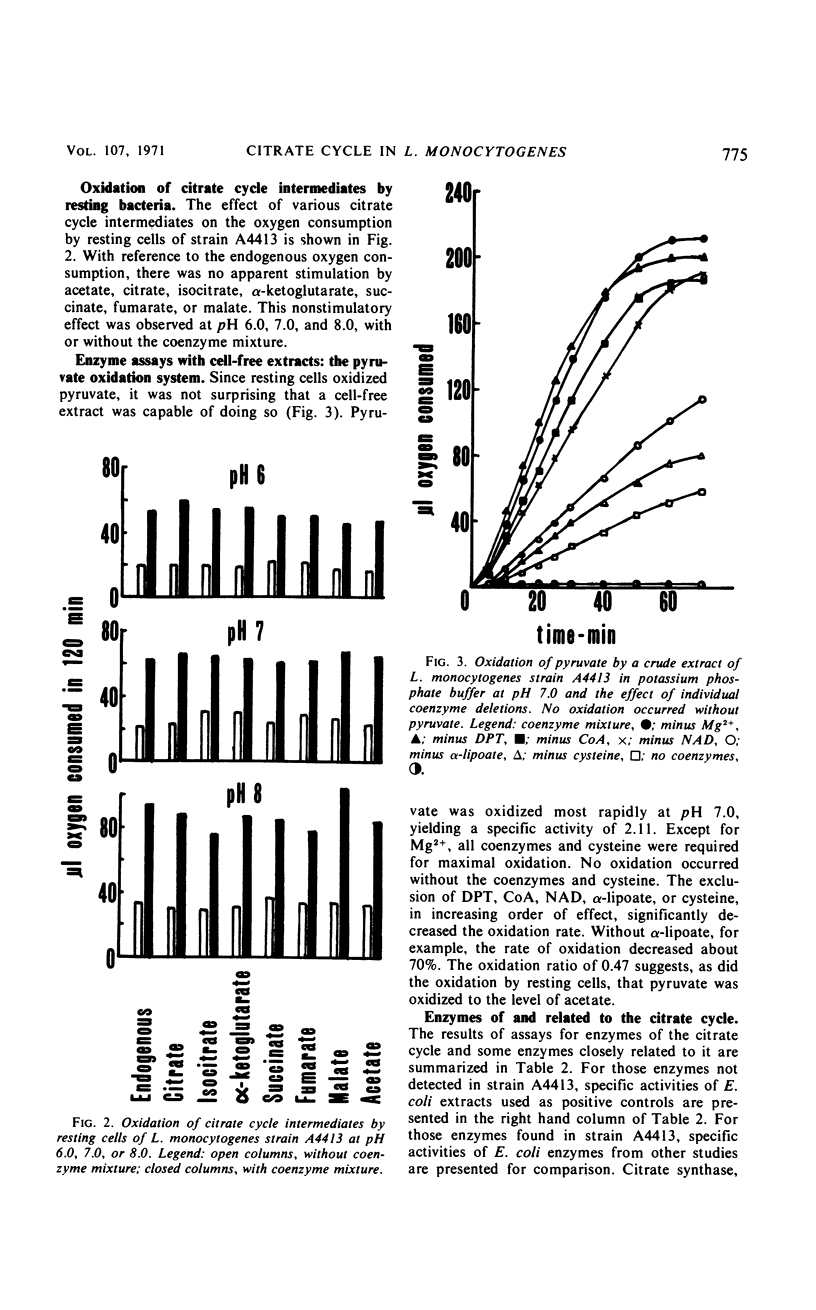
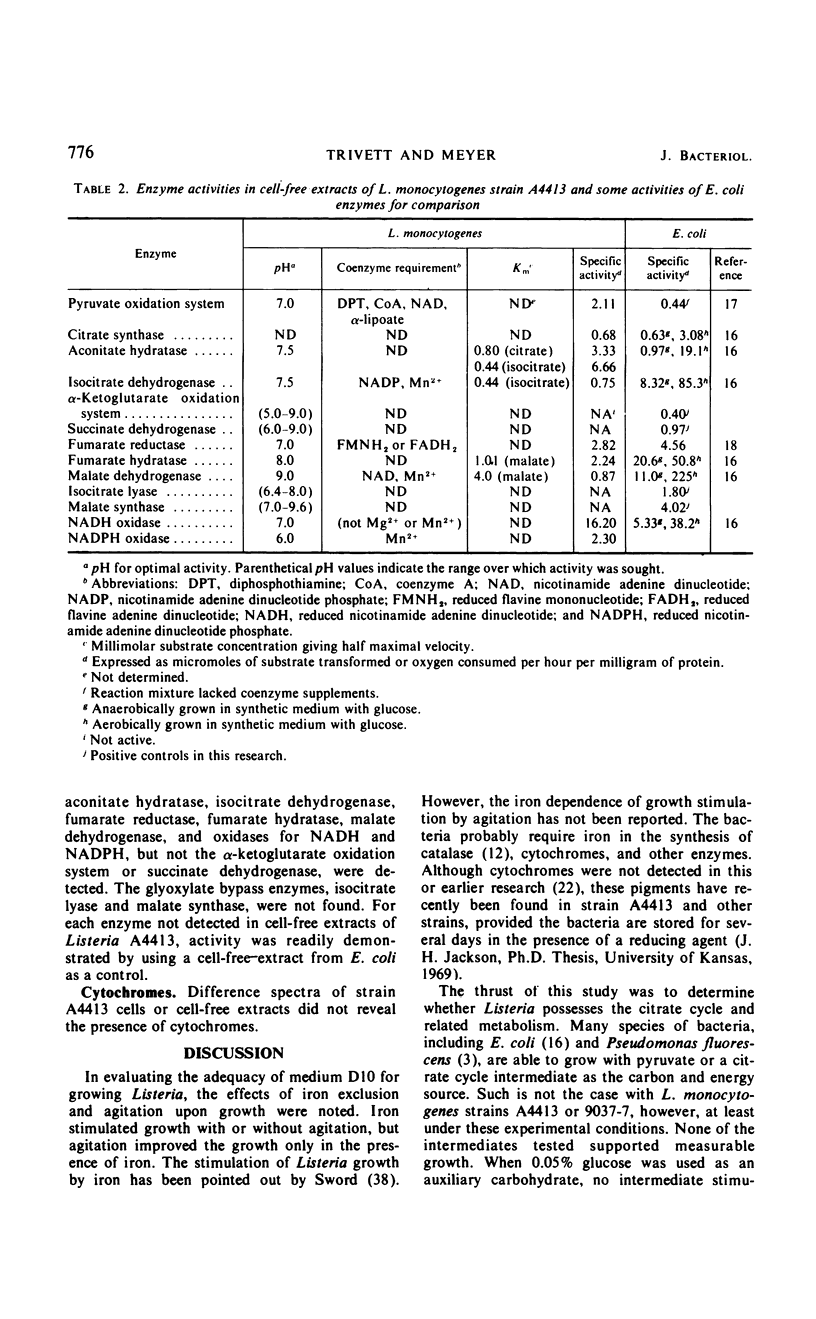
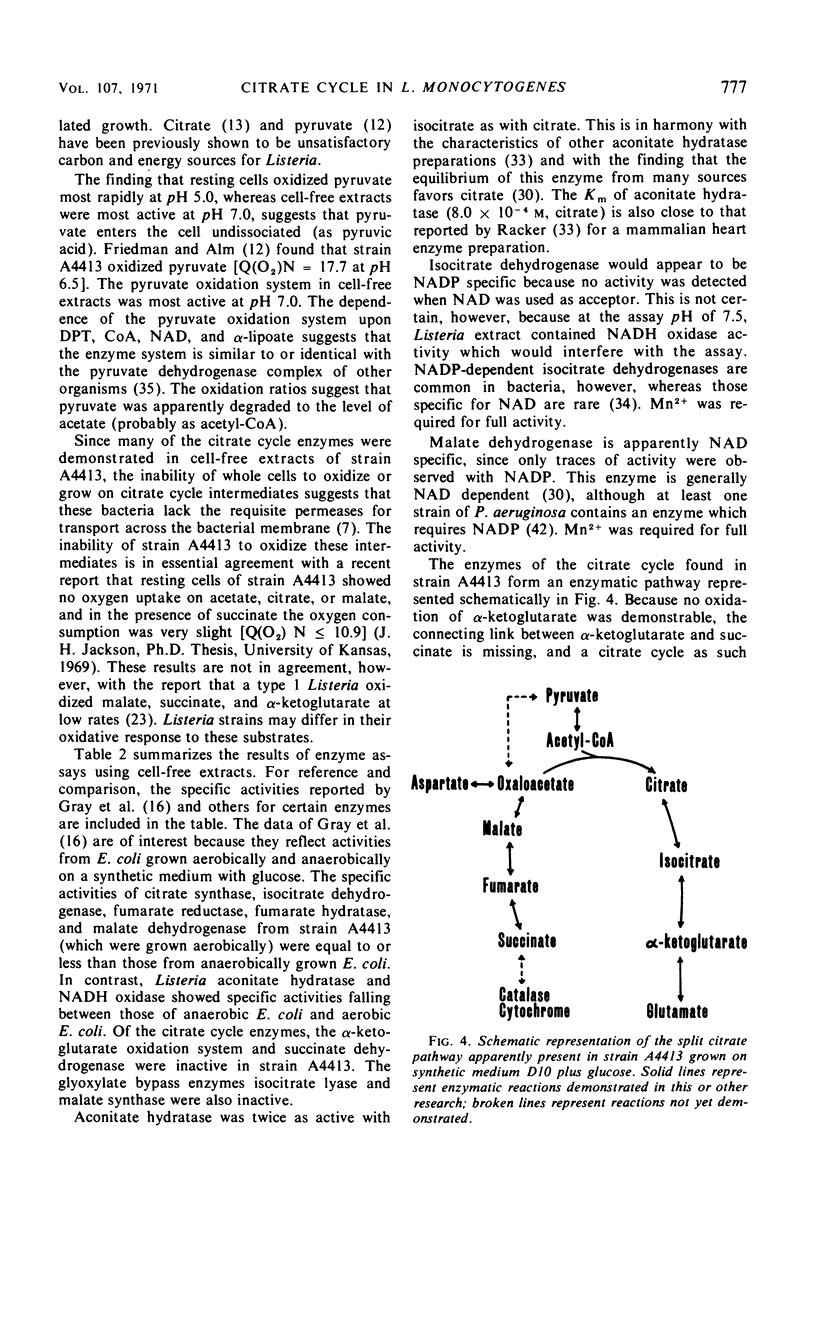
The growth response of Listeria monocytogenes strains A4413 and 9037-7 to carbohydrates was determined in a defined medium. Neither pyruvate, acetate, citrate, isocitrate, α-ketoglutarate, succinate, fumarate, nor malate supported growth. Furthermore, inclusion of any of these carbohydrates in the growth medium with glucose did not increase the growth of Listeria over that observed on glucose alone. Resting cell suspensions of strain A4413 oxidized pyruvate but not acetate, citrate, isocitrate, α-ketoglutarate, succinate, fumarate, or malate. Cell-free extracts of strain A4413 contained active citrate synthase, aconitate hydratase, isocitrate dehydrogenase, malate dehydrogenase, fumarate hydratase, fumarate reductase, pyruvate dehydrogenase system, and oxidases for reduced nicotinamide adenine dinucleotide and reduced nicotinamide adenine dinucleotide phosphate. The α-ketoglutarate oxidation system, succinate dehydrogenase, isocitrate lyase, and malate synthase were not detected. Cytochromes were not detected. The data suggest that strain A4413, under these conditions, utilizes a split noncyclic citrate pathway which has an oxidative portion (citrate synthase, aconitate hydratase, and isocitrate dehydrogenase) and a reductive portion (malate dehydrogenase, fumarate hydratase, and fumarate reductase). This pathway is probably important in biosynthesis but not for a net gain in energy.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARRIGONI O., SINGER T. P. Limitations of the phenazine methosulphate assay for succinic and related dehydrogenases. Nature. 1962 Mar 31;193:1256–1258. doi: 10.1038/1931256a0. [DOI] [PubMed] [Google Scholar]
- Amarasingham C. R., Davis B. D. Regulation of alpha-ketoglutarate dehydrogenase formation in Escherichia coli. J Biol Chem. 1965 Sep;240(9):3664–3668. [PubMed] [Google Scholar]
- BARRETT J. T., KALLIO R. E. Terminal respiration in Pseudomonas fluorescens: component enzymes of the tricarboxylic acid cycle. J Bacteriol. 1953 Nov;66(5):517–525. doi: 10.1128/jb.66.5.517-525.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B. Spectrophotometry of intracellular respiratory pigments. Science. 1954 Nov 12;120(3124):767–775. doi: 10.1126/science.120.3124.767. [DOI] [PubMed] [Google Scholar]
- CLARKE P. H., MEADOW P. M. Evidence for the occurrence of Permeases for tricarboxylic acid cycle intermediates in Pseudomonas aeruginosa. J Gen Microbiol. 1959 Feb;20(1):144–155. doi: 10.1099/00221287-20-1-144. [DOI] [PubMed] [Google Scholar]
- DIXON G. H., KORNBERG H. L., LUND P. Purification and properties of malate synthetase. Biochim Biophys Acta. 1960 Jul 1;41:217–233. doi: 10.1016/0006-3002(60)90004-4. [DOI] [PubMed] [Google Scholar]
- ELLS H. A. A colorimetric method for the assay of soluble succinic dehydrogenase and pyridinenucleotide-linked dehydrogenases. Arch Biochem Biophys. 1959 Dec;85:561–562. doi: 10.1016/0003-9861(59)90527-2. [DOI] [PubMed] [Google Scholar]
- ENGLESBERG E., LEVY J. B. Induced synthesis of tricarboxylic acid cycle enzymes as correlated with the oxidation of acetate and glucose by Pasteurella pestis. J Bacteriol. 1955 Apr;69(4):418–431. doi: 10.1128/jb.69.4.418-431.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEDMAN M. E., ALM W. L. Effect of glucose concentration in the growth medium on some metabolic activities of Listeria monocytogenes. J Bacteriol. 1962 Aug;84:375–376. doi: 10.1128/jb.84.2.375-376.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEDMAN M. E., KAUTTER D. A. Effect of nutrition on the respiratory virulence of Listeria monocytogenes. J Bacteriol. 1962 Mar;83:456–462. doi: 10.1128/jb.83.3.456-462.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEDMAN M. E., ROESSLER W. G. Growth of Listeria monocytogenes in defined media. J Bacteriol. 1961 Oct;82:528–533. doi: 10.1128/jb.82.4.528-533.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh B. K., Carroll K. K. Isolation, composition, and structure of membrane of Listeria monocytogenes. J Bacteriol. 1968 Feb;95(2):688–699. doi: 10.1128/jb.95.2.688-699.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray C. T., Wimpenny J. W., Mossman M. R. Regulation of metabolism in facultative bacteria. II. Effects of aerobiosis, anaerobiosis and nutrition on the formation of Krebs cycle enzymes in Escherichia coli. Biochim Biophys Acta. 1966 Mar 28;117(1):33–41. doi: 10.1016/0304-4165(66)90149-8. [DOI] [PubMed] [Google Scholar]
- HALPERN Y. S., EVEN-SHOSHAN A., ARTMAN M. EFFECT OF GLUCOSE ON THE UTILIZATION OF SUCCINATE AND THE ACTIVITY OF TRICARBOXYLIC ACID-CYCLE ENZYMES IN ESCHERICHIA COLI. Biochim Biophys Acta. 1964 Nov 8;93:228–236. doi: 10.1016/0304-4165(64)90370-8. [DOI] [PubMed] [Google Scholar]
- HIRSCH C. A., RASMINSKY M., DAVIS B. D., LIN E. C. A FUMARATE REDUCTASE IN ESCHERICHIA COLI DISTINCT FROM SUCCINATE DEHYDROGENASE. J Biol Chem. 1963 Nov;238:3770–3774. [PubMed] [Google Scholar]
- Hooper A. B. Biochemical basis of obligate autotrophy in Nitrosomonas europaea. J Bacteriol. 1969 Feb;97(2):776–779. doi: 10.1128/jb.97.2.776-779.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEELER R. F., GRAY M. L. Antigenic and related biochemical properties of Listeria monocytogenes. I. Preparation and composition of cell wall material. J Bacteriol. 1960 Nov;80:683–692. doi: 10.1128/jb.80.5.683-692.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORNBERG A., PRICER W. E., Jr Di- and triphosphopyridine nucleotide isocitric dehydrogenases in yeast. J Biol Chem. 1951 Mar;189(1):123–136. [PubMed] [Google Scholar]
- Kraus P. A simple modified micromethod for the determination of citrate. Clin Chim Acta. 1965 Oct;12(4):462–465. doi: 10.1016/0009-8981(65)90137-3. [DOI] [PubMed] [Google Scholar]
- RACKER E. Spectrophotometric measurements of the enzymatic formation of fumaric and cis-aconitic acids. Biochim Biophys Acta. 1950 Jan;4(1-3):211–214. doi: 10.1016/0006-3002(50)90026-6. [DOI] [PubMed] [Google Scholar]
- REED L. J., FERNANDEZ-MORAN H., KOIKE M., WILLMS C. R. ELECTRON MICROSCOPIC AND BIOCHEMICAL STUDIES OF PYRUVATE DEHYDROGENASE COMPLEX OF ESCHERICHIA COLI. Science. 1964 Aug 28;145(3635):930–932. doi: 10.1126/science.145.3635.930. [DOI] [PubMed] [Google Scholar]
- Ragland T. E., Kawasaki T., Lowenstein J. M. Comparative aspects of some bacterial dehydrogenases and transhydrogenases. J Bacteriol. 1966 Jan;91(1):236–244. doi: 10.1128/jb.91.1.236-244.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRICKER F., FISERA J., KRCMERY V., FERENCIK M. Comparison of virulence and activity of some enzymes of Listeria monocytogenes. Folia Microbiol (Praha) 1963 Mar;8:89–92. doi: 10.1007/BF02877229. [DOI] [PubMed] [Google Scholar]
- Smith A. J., London J., Stanier R. Y. Biochemical basis of obligate autotrophy in blue-green algae and thiobacilli. J Bacteriol. 1967 Oct;94(4):972–983. doi: 10.1128/jb.94.4.972-983.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sword C. P. Mechanisms of pathogenesis in Listeria monocytogenes infection. I. Influence of iron. J Bacteriol. 1966 Sep;92(3):536–542. doi: 10.1128/jb.92.3.536-542.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivett T. L., Meyer E. A. Effect of erythritol on the in vitro growth and respiration of Listeria monocytogenes. J Bacteriol. 1967 Mar;93(3):1197–1198. doi: 10.1128/jb.93.3.1197-1198.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAUGHN R. H., OSBORNE J. T., WEDDING G. T., TABACHNICK J., BEISEL C. G., BRAXTON T. The utilization of citrate by Escherichia coli. J Bacteriol. 1950 Aug;60(2):119–127. doi: 10.1128/jb.60.2.119-127.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Tigerstrom M., Campbell J. J. The tricarboxylic acid cycle, the glyoxylate cycle, and the enzymes of glucose oxidation in Pseudomonas aeruginosa. Can J Microbiol. 1966 Oct;12(5):1015–1022. doi: 10.1139/m66-136. [DOI] [PubMed] [Google Scholar]
- WELSHIMER H. J. VITAMIN REQUIREMENTS OF LISTERIA MONOCYTOGENES. J Bacteriol. 1963 May;85:1156–1159. doi: 10.1128/jb.85.5.1156-1159.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]


