Abstract
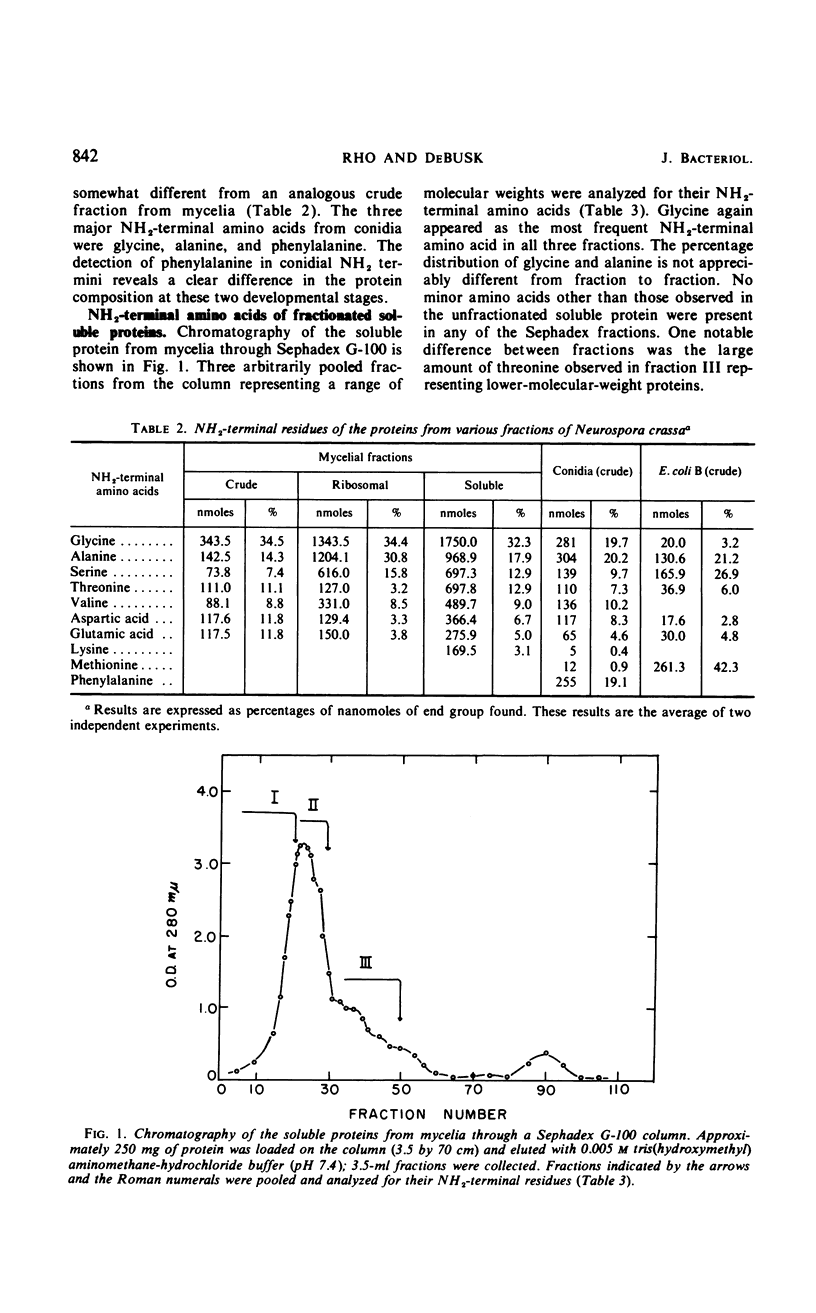
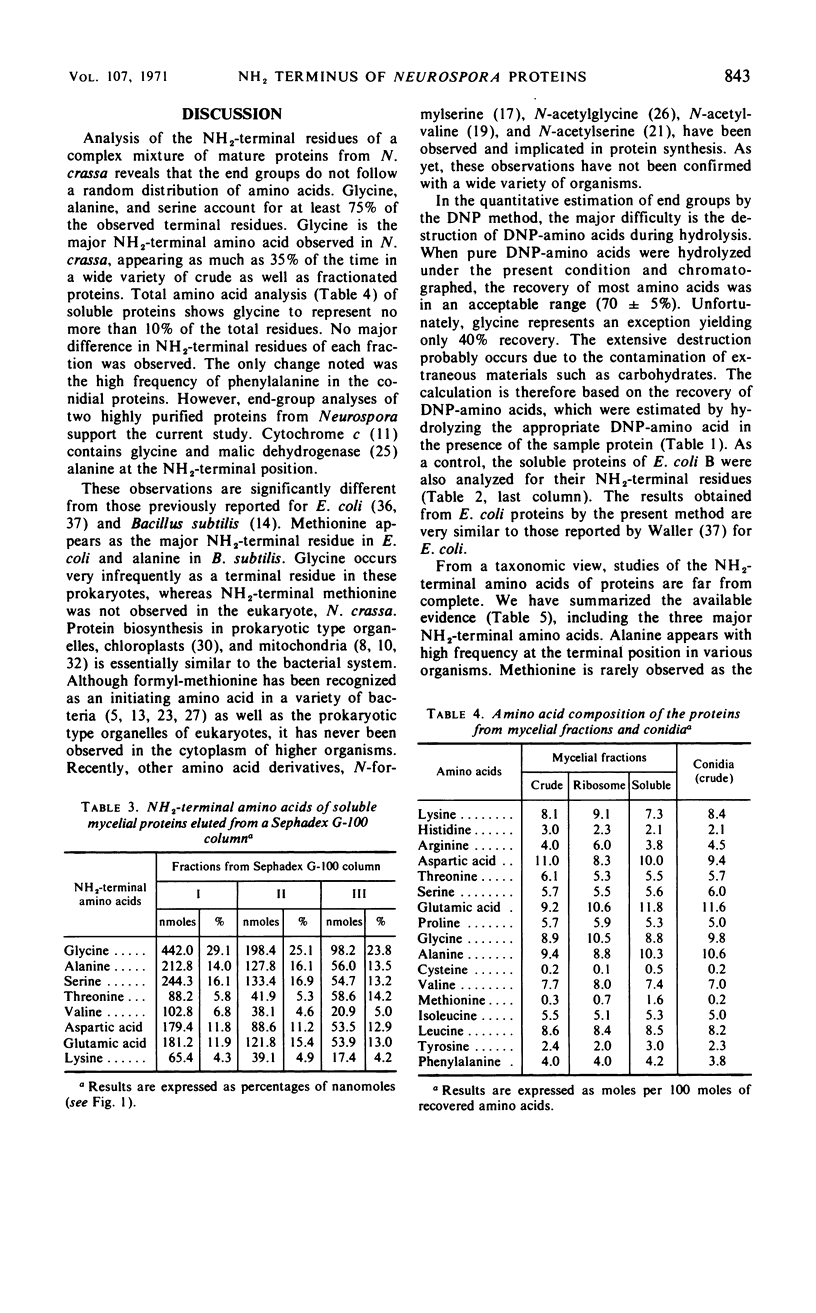
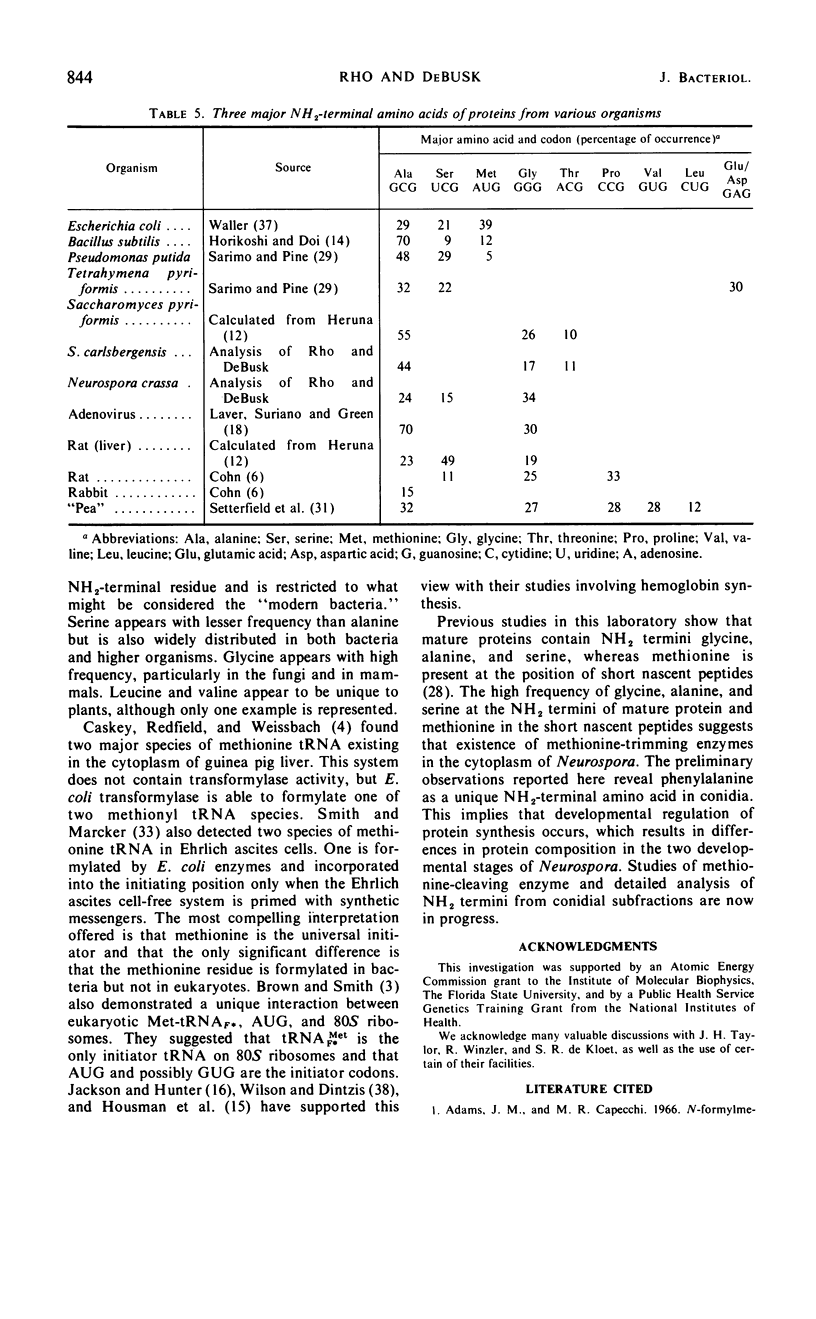
The NH2-terminal amino acid composition of the soluble and ribosomal proteins from Neurospora crassa mycelia and conidia was determined by the dinitrophenyl method. A nonrandom distribution of NH2-terminal amino acids was observed in the complex protein mixtures. Glycine, alanine, and serine accounted for 75% of the NH2-terminal amino acids, and glycine appeared most frequently in mature proteins of mycelia. The appearance of phenylalanine as one of the major NH2-termini in crude conidial fraction suggests that the composition of proteins may vary in different developmental stages.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Capecchi M. R. N-formylmethionyl-sRNA as the initiator of protein synthesis. Proc Natl Acad Sci U S A. 1966 Jan;55(1):147–155. doi: 10.1073/pnas.55.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J., Leahy J., Schweet R. FORMATION OF THE PEPTIDE CHAIN OF HEMOGLOBIN. Proc Natl Acad Sci U S A. 1960 Aug;46(8):1030–1038. doi: 10.1073/pnas.46.8.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. C., Smith A. E. Initiator codons in eukaryotes. Nature. 1970 May 16;226(5246):610–612. doi: 10.1038/226610a0. [DOI] [PubMed] [Google Scholar]
- Caskey C. T., Redfield B., Weissbach H. Formylation of guinea pig liver methionyl-sRNA. Arch Biochem Biophys. 1967 Apr;120(1):119–123. doi: 10.1016/0003-9861(67)90605-4. [DOI] [PubMed] [Google Scholar]
- Clark B. F., Marcker K. A. The role of N-formyl-methionyl-sRNA in protein biosynthesis. J Mol Biol. 1966 Jun;17(2):394–406. doi: 10.1016/s0022-2836(66)80150-x. [DOI] [PubMed] [Google Scholar]
- Cohn P. Properties of ribosomal proteins from two mammalian sources. Biochem J. 1967 Mar;102(3):735–741. doi: 10.1042/bj1020735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DINTZIS H. M. Assembly of the peptide chains of hemoglobin. Proc Natl Acad Sci U S A. 1961 Mar 15;47:247–261. doi: 10.1073/pnas.47.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epler J. L., Shugart L. R., Barnett W. E. N-formylmethionyl transfer ribonucleic acid in mitochondria from Neurospora. Biochemistry. 1970 Sep 1;9(18):3575–3579. doi: 10.1021/bi00820a011. [DOI] [PubMed] [Google Scholar]
- FRAENKEL-CONRAT H., HARRIS J. I., LEVY A. L. Recent developments in techniques for terminal and sequence studies in peptides and proteins. Methods Biochem Anal. 1955;2:359–425. doi: 10.1002/9780470110188.ch12. [DOI] [PubMed] [Google Scholar]
- Galper J. B., Darnell J. E. The presence of N-formyl-methionyl-tRNA in HeLa cell mitochondria. Biochem Biophys Res Commun. 1969 Jan 27;34(2):205–214. doi: 10.1016/0006-291x(69)90633-0. [DOI] [PubMed] [Google Scholar]
- HARUNA I. Comparative studies of amino acid composition and N-terminal amino acids of the protein from ribosomes of Escherichia coli, yeast and rat liver. Biochim Biophys Acta. 1963 Jul 30;72:451–455. [PubMed] [Google Scholar]
- Heller J., Smith E. L. Neurospora crassa cytochrome c. II. Chymotryptic peptides, tryptic peptides, cyanogen bromide peptides, and the complete amino acid sequence. J Biol Chem. 1966 Jul 10;241(13):3165–3180. [PubMed] [Google Scholar]
- Horikoshi K., Doi R. H. The NH2-terminal residues of Bacillus subtilis proteins. J Biol Chem. 1968 May 10;243(9):2381–2384. [PubMed] [Google Scholar]
- Housman D., Jacobs-Lorena M., Rajbhandary U. L., Lodish H. F. Initiation of haemoglobin synthesis by methionyl-tRNA. Nature. 1970 Aug 29;227(5261):913–918. doi: 10.1038/227913a0. [DOI] [PubMed] [Google Scholar]
- Jackson R., Hunter T. Role of methionine in the initiation of haemoglobin synthesis. Nature. 1970 Aug 15;227(5259):672–676. doi: 10.1038/227672a0. [DOI] [PubMed] [Google Scholar]
- Kim W. S. N-formylseryl-transfer RNA. Science. 1969 Feb 28;163(3870):947–949. doi: 10.1126/science.163.3870.947. [DOI] [PubMed] [Google Scholar]
- LEVY A. L. A paper chromatographic method for the quantitative estimation of amino-acids. Nature. 1954 Jul 17;174(4420):126–127. doi: 10.1038/174126a0. [DOI] [PubMed] [Google Scholar]
- LOWTHER A. G. Identification of N-2: 4-dinitrophenylamino-acids. Nature. 1951 May 12;167(4254):767–768. doi: 10.1038/167767b0. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Suriano J. R., Green M. Adenovirus proteins. II. N-terminal amino acid analysis. J Virol. 1967 Aug;1(4):723–728. doi: 10.1128/jvi.1.4.723-728.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laycock D. G., Hunt J. A. Synthesis of rabbit globin by a bacterial cell free system. Nature. 1969 Mar 22;221(5186):1118–1122. doi: 10.1038/2211118a0. [DOI] [PubMed] [Google Scholar]
- Liew C. C., Haslett G. W., Allfrey V. G. N-acetyl-seryl-tRNA and polypeptide chain initiation during histone biosynthesis. Nature. 1970 May 2;226(5244):414–417. doi: 10.1038/226414a0. [DOI] [PubMed] [Google Scholar]
- MILLS G. L. Observations on the application of fluorodinitrobenzene to the quantitative analysis of proteins. Biochem J. 1952 Mar;50(5):707–712. doi: 10.1042/bj0500707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUNKRES K. D., RICHARDS F. M. THE PURIFICATION AND PROPERTIES OF NEUROSPORA MALATE DEHYDROGENASE. Arch Biochem Biophys. 1965 Mar;109:466–479. doi: 10.1016/0003-9861(65)90391-7. [DOI] [PubMed] [Google Scholar]
- Narita K., Tsuchida I., Tsunazawa S., Ogata K. Formation of acetylglypuromycin by the incubation of hen's oviduct minces with puromycin. Biochem Biophys Res Commun. 1969 Oct 8;37(2):327–332. doi: 10.1016/0006-291x(69)90738-4. [DOI] [PubMed] [Google Scholar]
- Pine M. J., Gordon B., Sarimo S. S. Protein initiation without folate in Streptococcus faecium. Biochim Biophys Acta. 1969 Apr 22;179(2):439–447. doi: 10.1016/0005-2787(69)90052-5. [DOI] [PubMed] [Google Scholar]
- Rho H. M., DeBusk A. G. NH2-terminal methionine in nascent peptides from Neurospora crassa. Biochem Biophys Res Commun. 1971 Jan 22;42(2):319–325. doi: 10.1016/0006-291x(71)90105-7. [DOI] [PubMed] [Google Scholar]
- SETTERFIELD G., NEELIN J. M., NEELIN E. M., BAYLEY S. T. Studies on basic proteins from ribosomes of buds of pea seedlings. J Mol Biol. 1960 Dec;2:416–424. doi: 10.1016/s0022-2836(60)80051-4. [DOI] [PubMed] [Google Scholar]
- Sarimo S. S., Pine M. J. Taxonomic comparison of the amino termini of microbial cell proteins. J Bacteriol. 1969 May;98(2):368–374. doi: 10.1128/jb.98.2.368-374.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J. H., Meyer R., Eisenstadt J. M., Brawerman G. Involvement of N-formylmethionine in initiation of protein synthesis in cell-free extracts of Euglena gracilis. J Mol Biol. 1967 May 14;25(3):571–574. doi: 10.1016/0022-2836(67)90210-0. [DOI] [PubMed] [Google Scholar]
- Smith A. E., Marcker K. A. Cytoplasmic methionine transfer RNAs from eukaryotes. Nature. 1970 May 16;226(5246):607–610. doi: 10.1038/226607a0. [DOI] [PubMed] [Google Scholar]
- Smith A. E., Marcker K. A. N-formylmethionyl transfer RNA in mitochondria from yeast and rat liver. J Mol Biol. 1968 Dec 14;38(2):241–243. doi: 10.1016/0022-2836(68)90409-9. [DOI] [PubMed] [Google Scholar]
- Steitz J. A. Polypeptide chain initiation: nucleotide sequences of the three ribosomal binding sites in bacteriophage R17 RNA. Nature. 1969 Dec 6;224(5223):957–964. doi: 10.1038/224957a0. [DOI] [PubMed] [Google Scholar]
- VOGEL M., WEINSTEIN L., DELAGI E., ABRAMSON A. S. The physiologic basis of electrodiagnosis. Phys Ther Rev. 1956 Jan;36(1):42–44. doi: 10.1093/ptj/36.1.42. [DOI] [PubMed] [Google Scholar]
- WALLER J. P., HARRIS J. I. Studies on the composition of the protein from Escherichia coli ribosomes. Proc Natl Acad Sci U S A. 1961 Jan 15;47:18–23. doi: 10.1073/pnas.47.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALLER J. P. THE NH2-TERMINAL RESIDUES OF THE PROTEINS FROM CELL-FREE EXTRACTS OF E. COLI. J Mol Biol. 1963 Nov;7:483–496. doi: 10.1016/s0022-2836(63)80096-0. [DOI] [PubMed] [Google Scholar]
- Wilson D. B., Dintzis H. M. Protein chain initiation in rabbit reticulocytes. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1282–1289. doi: 10.1073/pnas.66.4.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]


