Abstract
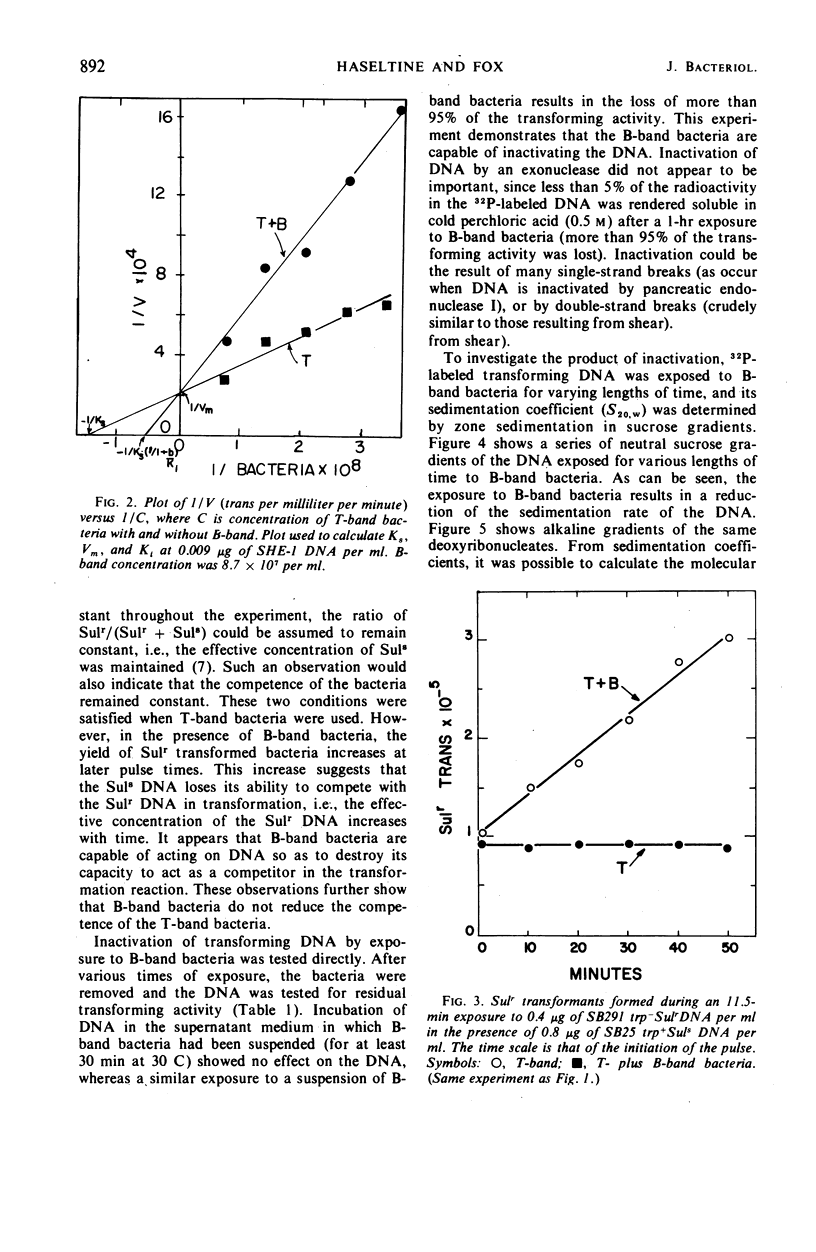
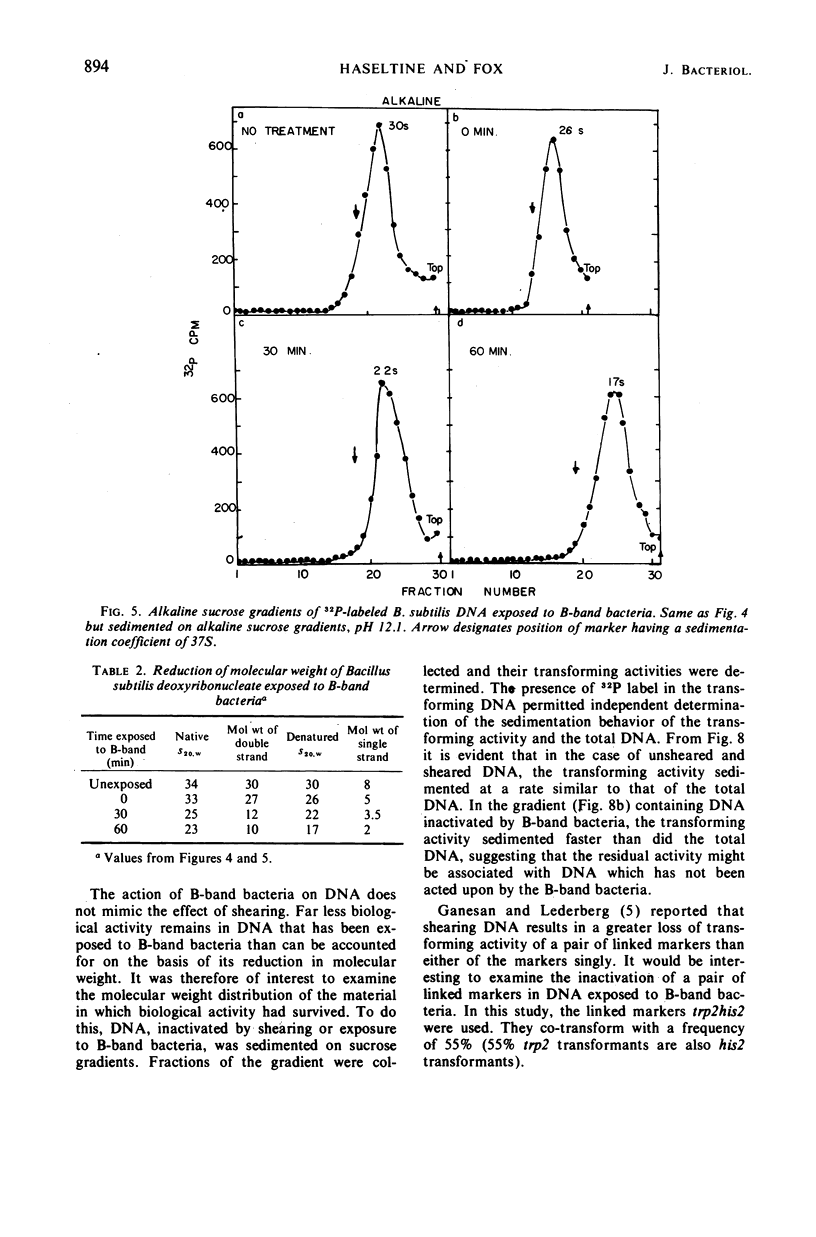
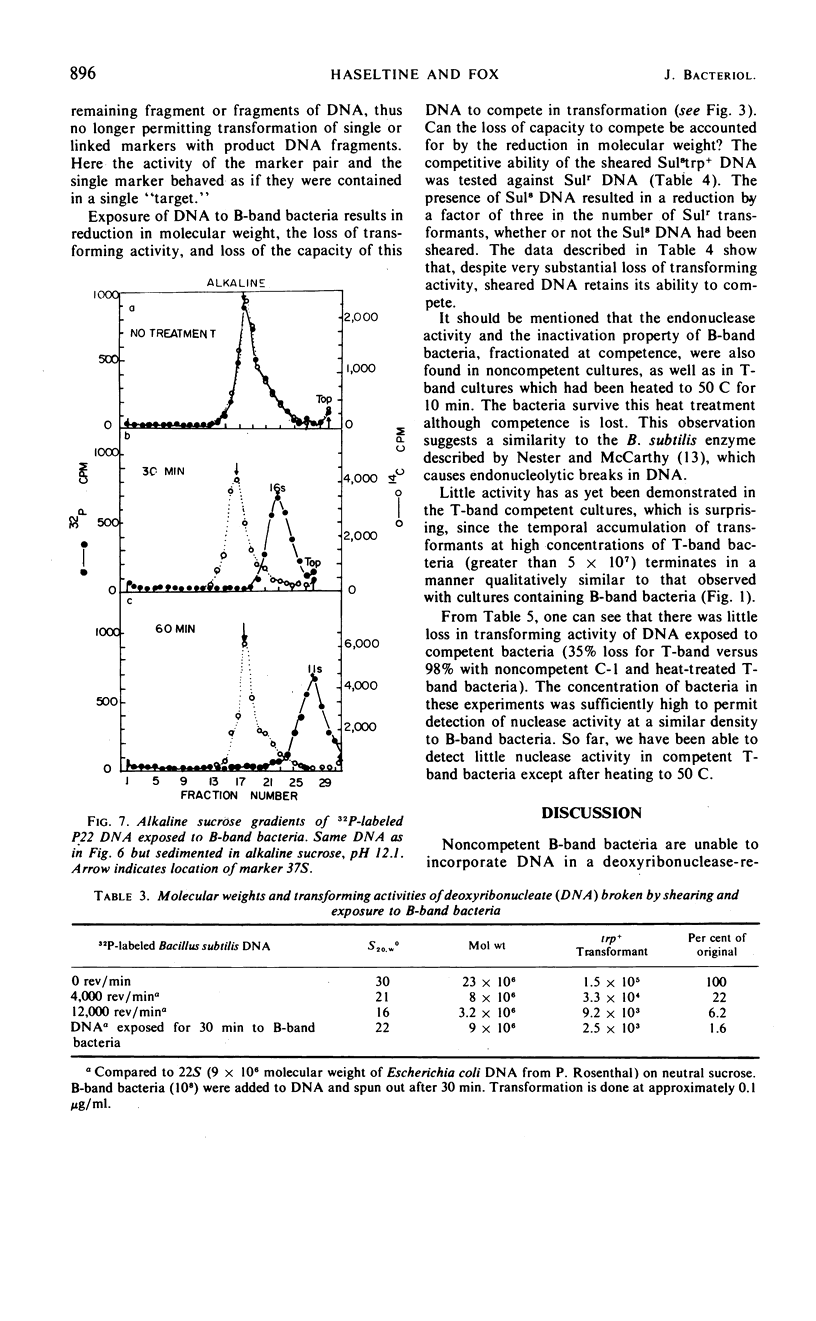
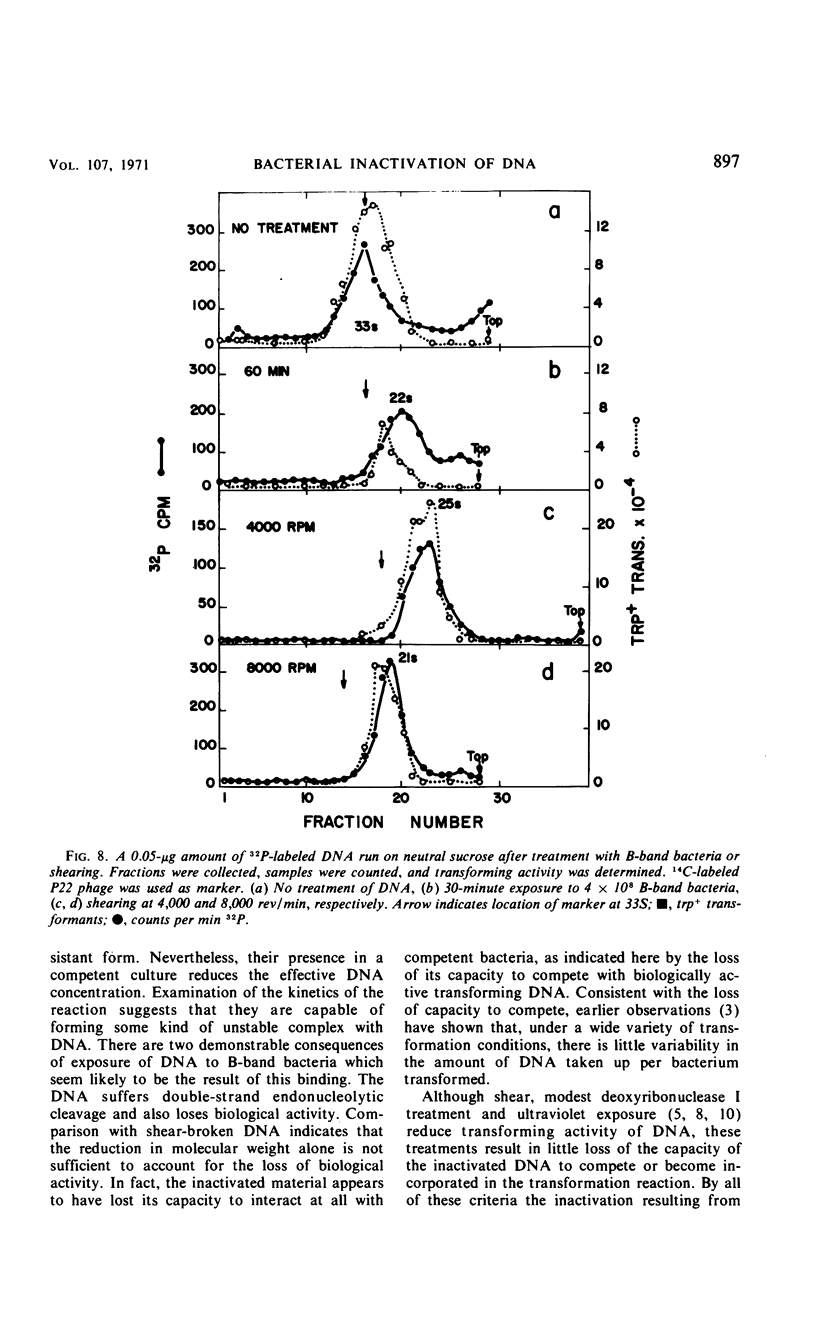
Competent and noncompetent bacteria are able to bind deoxyribonucleate (DNA). The consequence of this binding is different for the two types of bacteria. Competent bacteria are able to utilize the DNA for transformation. DNA exposed to noncompetent bacteria loses its biological activity with a coincident reduction in the double-stranded molecular weight. The reduction in molecular weight does not appear to be sufficient to account for the loss of biological activity observed.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURGI E., HERSHEY A. D. Sedimentation rate as a measure of molecular weight of DNA. Biophys J. 1963 Jul;3:309–321. doi: 10.1016/s0006-3495(63)86823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botstein D. Synthesis and maturation of phage P22 DNA. I. Identification of intermediates. J Mol Biol. 1968 Jun 28;34(3):621–641. doi: 10.1016/0022-2836(68)90185-x. [DOI] [PubMed] [Google Scholar]
- Cahn F. H., Fox M. S. Fractionation of transformable bacteria from ocompetent cultures of Bacillus subtilis on renografin gradients. J Bacteriol. 1968 Mar;95(3):867–875. doi: 10.1128/jb.95.3.867-875.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOX M. S., HOTCHKISS R. D. Initiation of bacterial transformation. Nature. 1957 Jun 29;179(4574):1322–1325. doi: 10.1038/1791322a0. [DOI] [PubMed] [Google Scholar]
- GANESAN A. T., LEDERBERG J. PHYSICAL AND BIOLOGICAL STUDIES ON TRANSFORMING DNA. J Mol Biol. 1964 Sep;9:683–695. doi: 10.1016/s0022-2836(64)80175-3. [DOI] [PubMed] [Google Scholar]
- Hadden C., Nester E. W. Purification of competent cells in the Bacillus subtilis transformation system. J Bacteriol. 1968 Mar;95(3):876–885. doi: 10.1128/jb.95.3.876-885.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LERMAN L. S., TOLMACH L. J. Genetic transformation. I. Cellular incorporation of DNA accompanying transformation in Pneumococcus. Biochim Biophys Acta. 1957 Oct;26(1):68–82. doi: 10.1016/0006-3002(57)90055-0. [DOI] [PubMed] [Google Scholar]
- LEVINE M. Mutations in the temperate phage P22 and lysogeny in Salmonella. Virology. 1957 Feb;3(1):22–41. doi: 10.1016/0042-6822(57)90021-1. [DOI] [PubMed] [Google Scholar]
- Litt M., Marmur J., Ephrussi-Taylor H., Doty P. THE DEPENDENCE OF PNEUMOCOCCAL TRANSFORMATION ON THE MOLECULAR WEIGHT OF DEOXYRIBOSE NUCLEIC ACID. Proc Natl Acad Sci U S A. 1958 Feb;44(2):144–152. doi: 10.1073/pnas.44.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy C., Nester E. W. Heat-activated endonuclease in Bacillus subtilis. J Bacteriol. 1969 Mar;97(3):1426–1430. doi: 10.1128/jb.97.3.1426-1430.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]



