Abstract
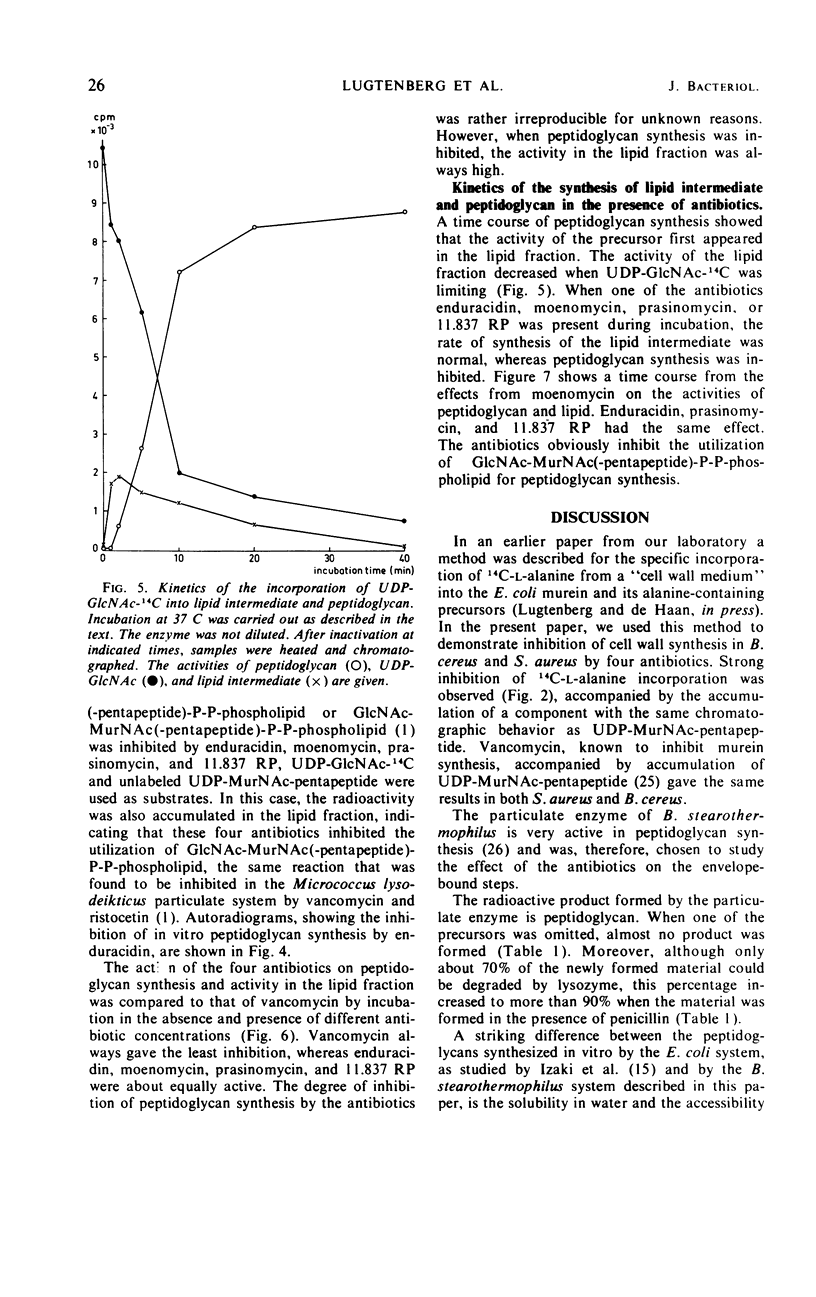
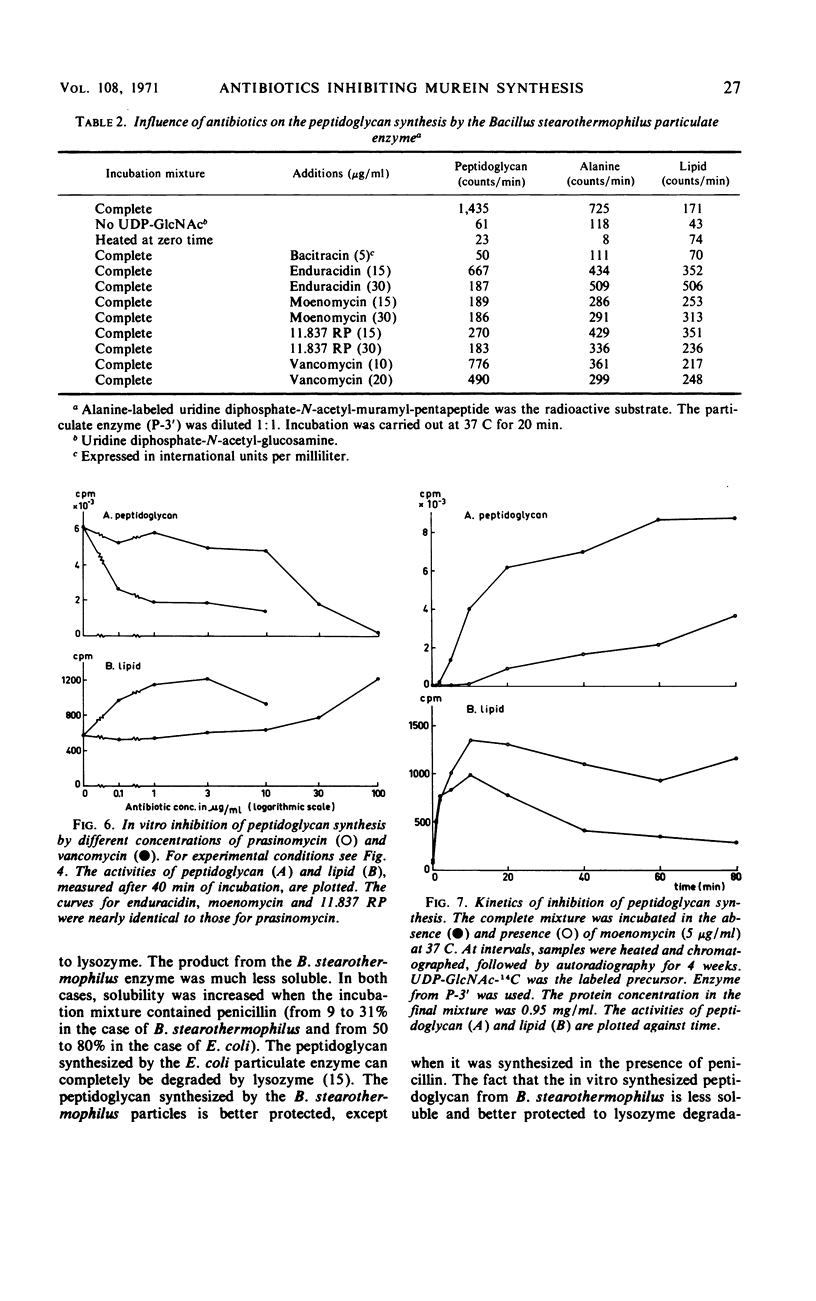
Recent literature on the antibiotics enduracidin, moenomycin, prasinomycin, and 11.837 RP suggested an interaction with murein synthesis. Incubation of sensitive strains from Bacillus cereus and Staphylococcus aureus in a “wall medium” containing labeled l-alanine showed that all four antibiotics inhibited the incorporation of alanine into murein and gave rise to accumulation of radioactive uridine diphosphate-N-acetyl-muramyl (UDP-MurNAc)-pentapeptide. Peptidoglycan was synthesized when the particulate enzyme of B. stearothermophilus was incubated with the murein precursors UDP-N-acetyl-glucosamine (UDP-GlcNAc) and UDP-MurNAc-pentapeptide. The newly formed polymer was less accessible for lysozyme and more strongly bound to the acceptor than the same product from the Escherichia coli particulate enzyme. After incubation in the presence of penicillin, a greater part of the peptidoglycan was lysozyme sensitive and more loosely bound to the acceptor. The antibiotics enduracidin, moenomycin, prasinomycin, and 11.837 RP inhibited peptidoglycan synthesis by the B. stearothermophilus particulate enzyme. The rate of synthesis of GlcNAc-MurNAc(-pentapeptide)-P-P-phospholipid was independent from the addition of these antibiotics, but its utilization was strongly inhibited. With the present results, it is not possible to distinguish the mechanisms of action of enduracidin, moenomycin, prasinomycin, and 11.837 RP from the mechanisms of action of vancomycin and ristocetin.
Full text
PDF

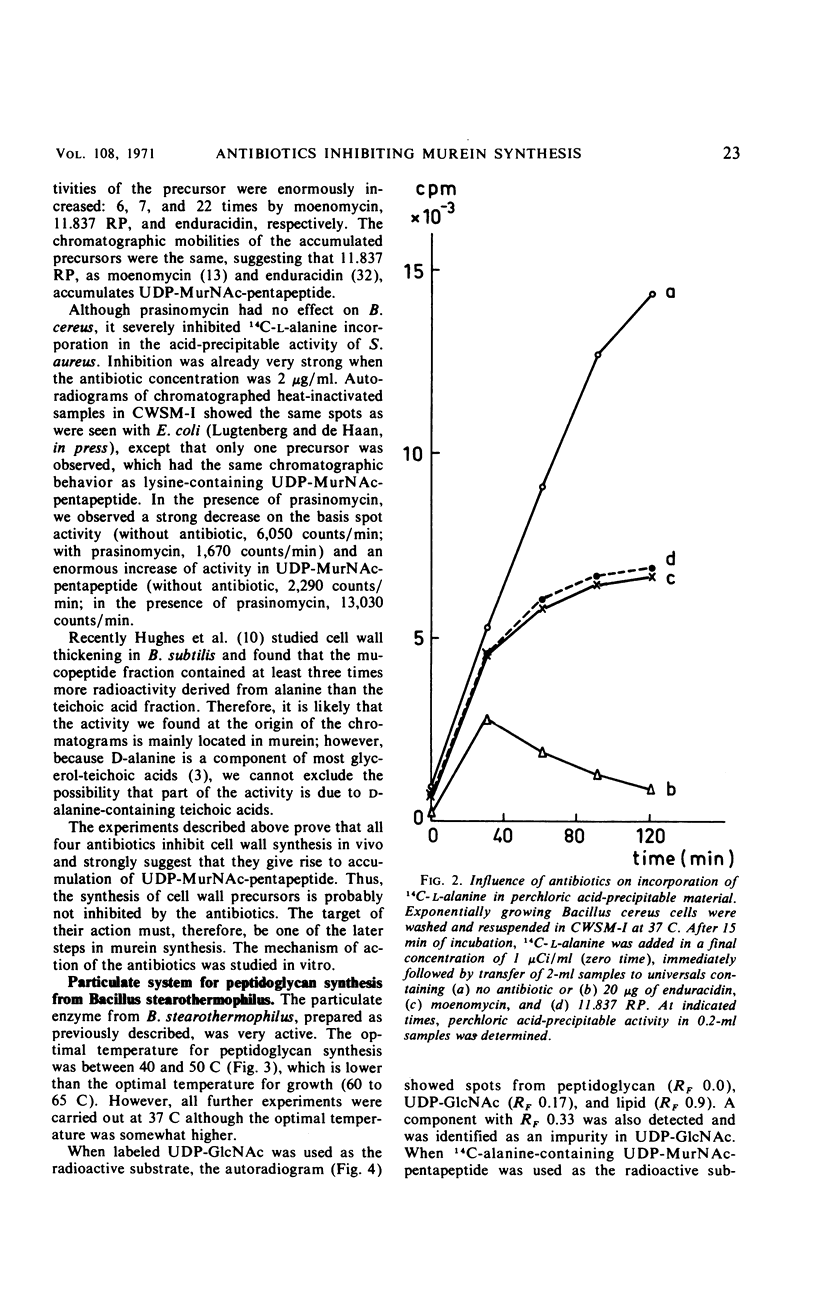
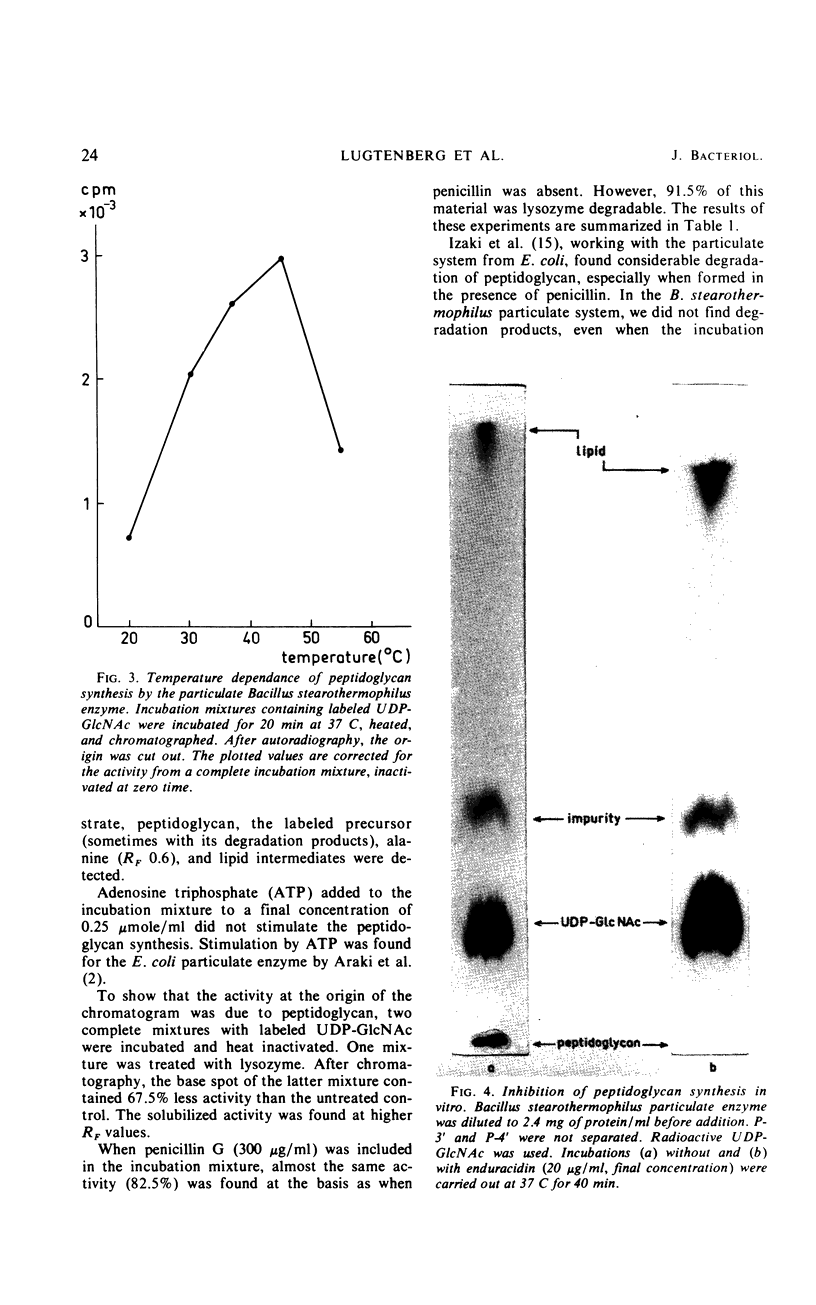



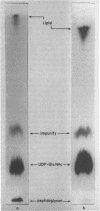
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. S., Matsuhashi M., Haskin M. A., Strominger J. L. Biosythesis of the peptidoglycan of bacterial cell walls. II. Phospholipid carriers in the reaction sequence. J Biol Chem. 1967 Jul 10;242(13):3180–3190. [PubMed] [Google Scholar]
- Araki Y., Shirai R., Shimada A., Ishimoto N., Ito E. Enzymatic synthesis of cell wall mucopeptide in a particulate preparation of Escherichia coli. Biochem Biophys Res Commun. 1966 May 25;23(4):466–472. doi: 10.1016/0006-291x(66)90751-0. [DOI] [PubMed] [Google Scholar]
- Archibald A. R., Baddiley J., Blumsom N. L. The teichoic acids. Adv Enzymol Relat Areas Mol Biol. 1968;30:223–253. doi: 10.1002/9780470122754.ch5. [DOI] [PubMed] [Google Scholar]
- BEST G. K., DURHAM N. N. EFFECT OF VANCOMYCIN ON BACILLUS SUBTILIS. Arch Biochem Biophys. 1964 Apr;105:120–125. doi: 10.1016/0003-9861(64)90242-5. [DOI] [PubMed] [Google Scholar]
- Best G. K., Durham N. N. Adsorption of the ristocetins to Bacillus subtilis cell walls. Antimicrob Agents Chemother (Bethesda) 1965;5:334–338. [PubMed] [Google Scholar]
- Best G. K., Durham N. N. Vancomycin adsorption to Bacillus subtilis cell walls. Arch Biochem Biophys. 1965 Sep;111(3):685–691. doi: 10.1016/0003-9861(65)90250-x. [DOI] [PubMed] [Google Scholar]
- Best G. K., Grastie M. K., McConnell R. D. Relative affinity of vancomycin and ristocetin for cell walls and uridine diphosphate-N-acetylmuramyl pentapeptide. J Bacteriol. 1970 May;102(2):476–482. doi: 10.1128/jb.102.2.476-482.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordet C., Perkins H. R. Iodinated vancomycin and mucopeptide biosynthesis by cell-free preparations from Micrococcus lysodeikticus. Biochem J. 1970 Oct;119(5):877–883. doi: 10.1042/bj1190877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi Y., Strominger J. L., Sweeley C. C. Structure of a lipid intermediate in cell wall peptidoglycan synthesis: a derivative of a C55 isoprenoid alcohol. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1878–1884. doi: 10.1073/pnas.57.6.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber G. Moenomycin, IV. Säurehydrolyse und Charakterisierung der Spaltprodukte. Justus Liebigs Ann Chem. 1967;707:170–176. doi: 10.1002/jlac.19677070124. [DOI] [PubMed] [Google Scholar]
- Huber G., Nesemann G. Moenomycin, an inhibitor of cell wall synthesis. Biochem Biophys Res Commun. 1968 Jan 11;30(1):7–13. doi: 10.1016/0006-291x(68)90704-3. [DOI] [PubMed] [Google Scholar]
- Huber G., Schacht U., Weidenmüller H. L., Schmidt-Thomé J., Duphorn J., Tschesche R. Meonomycin, a new antibiotic. II. Characterization and chemistry. Antimicrob Agents Chemother (Bethesda) 1965;5:737–742. [PubMed] [Google Scholar]
- Hughes R. C., Tanner P. J., Stokes E. Cell-wall thickening in Bacillus subtilis. Comparison of thickened and normal walls. Biochem J. 1970 Nov;120(1):159–170. doi: 10.1042/bj1200159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaki K., Matsuhashi M., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. 8. Peptidoglycan transpeptidase and D-alanine carboxypeptidase: penicillin-sensitive enzymatic reaction in strains of Escherichia coli. J Biol Chem. 1968 Jun 10;243(11):3180–3192. [PubMed] [Google Scholar]
- Izaki K., Matsuhashi M., Strominger J. L. Glycopeptide transpeptidase and D-alanine carboxypeptidase: penicillin-sensitive enzymatic reactions. Proc Natl Acad Sci U S A. 1966 Mar;55(3):656–663. doi: 10.1073/pnas.55.3.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaki K., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. XIV. Purification and properties of two D-alanine carboxypeptidases from Escherichia coli. J Biol Chem. 1968 Jun 10;243(11):3193–3201. [PubMed] [Google Scholar]
- Katz W., Martin H. H. Peptide crosslinkage in cell wall murein of Proteus mirabilis and its penicillin-induced unstable L-form. Biochem Biophys Res Commun. 1970 May 22;39(4):744–749. doi: 10.1016/0006-291x(70)90268-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laskin A. I., Chan W. M., Smith D. A., Meyers E. Mode of action of prasinomycin. Antimicrob Agents Chemother (Bethesda) 1967;7:251–256. [PubMed] [Google Scholar]
- Leyh-Bouille M., Ghuysen J. M., Nieto M., Perkins H. R., Schleifer K. H., Kandler O. On the Streptomyces albus G DD carboxypeptidase mechanism of action of penicillin, vancomycin, and ristocetin. Biochemistry. 1970 Jul 21;9(15):2971–2975. doi: 10.1021/bi00817a006. [DOI] [PubMed] [Google Scholar]
- Perkins H. R., Nieto M. The preparation of iodinated vancomycin and its distribution in bacteria treated with the antibiotic. Biochem J. 1970 Jan;116(1):83–92. doi: 10.1042/bj1160083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins H. R. Specificity of combination between mucopeptide precursors and vancomycin or ristocetin. Biochem J. 1969 Jan;111(2):195–205. doi: 10.1042/bj1110195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REISSIG J. L., STORMINGER J. L., LELOIR L. F. A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem. 1955 Dec;217(2):959–966. [PubMed] [Google Scholar]
- REYNOLDS P. E. Studies on the mode of action of vancomycin. Biochim Biophys Acta. 1961 Sep 16;52:403–405. doi: 10.1016/0006-3002(61)90698-9. [DOI] [PubMed] [Google Scholar]
- Reynolds P. E. Synthesis of cell-wall mucopeptide by particulate preparations from Bacillus megaterium and Bacillus stearothermophilus. J Gen Microbiol. 1968 Aug;53(1 Suppl):iv–iv. [PubMed] [Google Scholar]
- Schwarz U., Asmus A., Frank H. Autolytic enzymes and cell division of Escherichia coli. J Mol Biol. 1969 May 14;41(3):419–429. doi: 10.1016/0022-2836(69)90285-x. [DOI] [PubMed] [Google Scholar]
- Siewert G., Strominger J. L. Bacitracin: an inhibitor of the dephosphorylation of lipid pyrophosphate, an intermediate in the biosynthesis of the peptidoglycan of bacterial cell walls. Proc Natl Acad Sci U S A. 1967 Mar;57(3):767–773. doi: 10.1073/pnas.57.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. K., Neuhaus R. C. Reversal of the vancomycin inhibition of peptidoglycan synthesis by cell walls. J Bacteriol. 1968 Aug;96(2):374–382. doi: 10.1128/jb.96.2.374-382.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya K., Takeuchi Y. Enduracidin, an inhibitor of cell wall synthesis. J Antibiot (Tokyo) 1968 Jun;21(6):426–428. doi: 10.7164/antibiotics.21.426. [DOI] [PubMed] [Google Scholar]
- WEIDEL W., PELZER H. BAGSHAPED MACROMOLECULES--A NEW OUTLOOK ON BACTERIAL CELL WALLS. Adv Enzymol Relat Areas Mol Biol. 1964;26:193–232. doi: 10.1002/9780470122716.ch5. [DOI] [PubMed] [Google Scholar]
- Weisenborn F. L., Bouchard J. L., Smith D., Pansy F., Maestrone G., Miraglia G., Meyers E. The prasinomycins: antibiotics containing phosphorus. Nature. 1967 Mar 18;213(5081):1092–1094. doi: 10.1038/2131092a0. [DOI] [PubMed] [Google Scholar]
- Wise E. M., Jr, Park J. T. Penicillin: its basic site of action as an inhibitor of a peptide cross-linking reaction in cell wall mucopeptide synthesis. Proc Natl Acad Sci U S A. 1965 Jul;54(1):75–81. doi: 10.1073/pnas.54.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]